Short-Term Oral UMP/UR Administration Regulates Lipid Metabolism in Early-Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Samples Collection
2.4. Serum Biochemical Indices
2.5. Plasma Free Fatty Acids
2.6. Liver Fatty Acids
2.7. RNA Extraction and cDNA Synthesis
2.8. Real-Time Quantitative PCR (RT-qPCR)
2.9. Total Protein Extraction and Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Serum Biochemical Indices
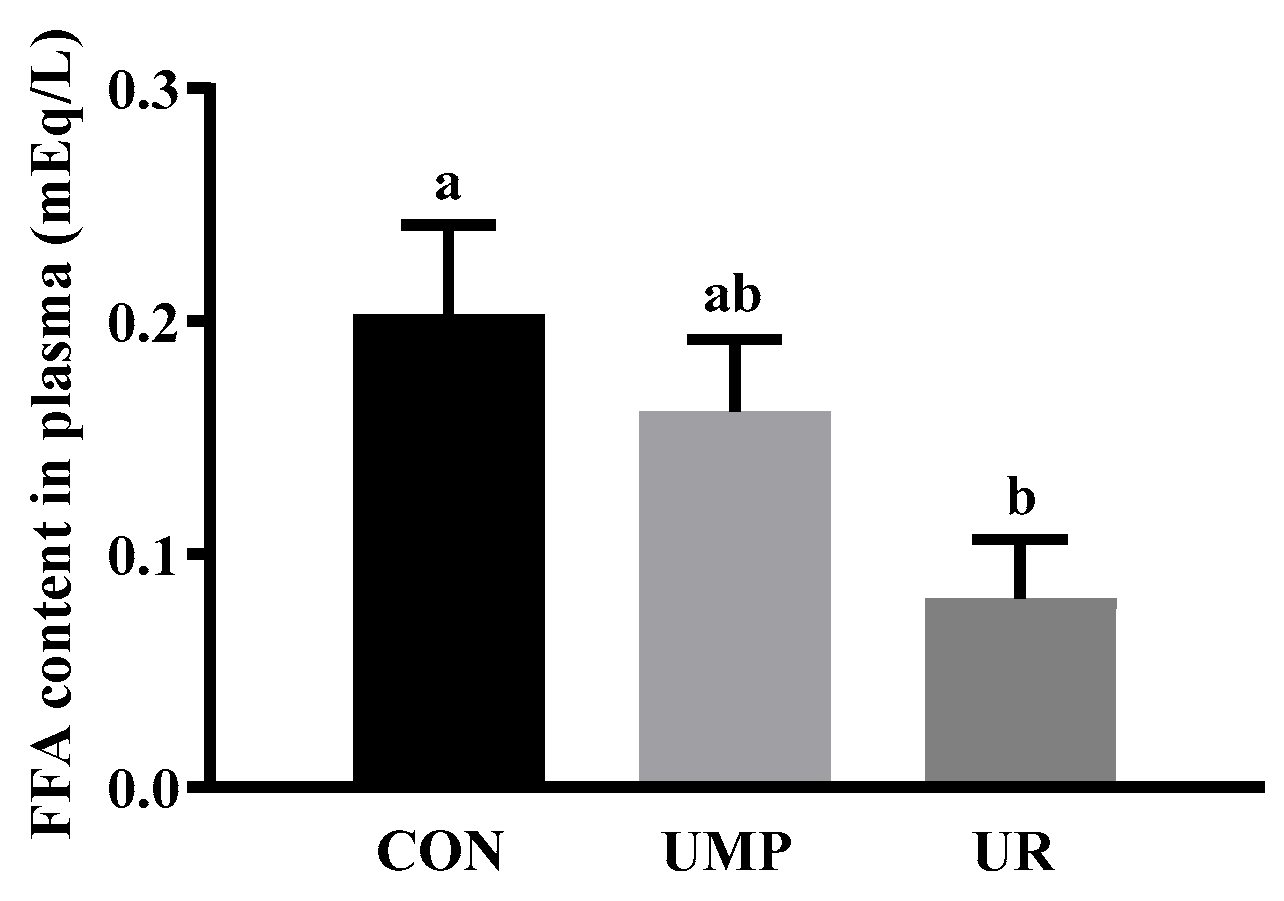
3.2. Plasma Free Fatty Acid
3.3. Liver Fatty Acid Profile
3.4. Relative Gene Expression of Lipid Metabolism
3.5. Expression of Proteins Involved in Energy Metabolism
4. Discussion
5. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, B.; Zhou, H.; Wu, X.; Chen, Z.; Yao, J.; Yin, Y.L. Effects of dietary supplementation with uridine monophosphate on performance and intestinal morphology of weanling piglets. J. Anim. Sci. 2016, 94, 82–86. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, H.S.; Wang, X.C.; Hu, Q.; Liu, C.X.; Wu, X.; Deng, D.; Hou, Y.Q.; Nyachoti, C.M.; Xiao, D.F.; et al. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.; Touchette, K.; Coalson, J.; Whisnant, C.; Brown, J.; Oliver, S.M.; Odle, J.; Harrell, R. Pigs weaned from the sow at 10 days of age respond to dietary energy source of manufactured liquid diets and exogenous porcine somatotropin. J. Anim. Sci. 2005, 83, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhao, M.; Yang, R.; Zhang, Z.; Li, Y.; Wang, J. Effect of dietary nucleotides on immune function in Balb/C mice. Int. Immunopharmacol. 2013, 17, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, Q.; Li, G.; Fan, Z.; Wang, H.; Wu, X. Dietary supplement with nucleotides in the form of uridine monophosphate or uridine stimulate the intestinal development and promote nucleotide transport in weaned piglets. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Peters, D.; Stein, H.H. Nucleotides in sow colostrum and milk at different stages of lactation. J. Anim. Sci. 2004, 82, 1339–1342. [Google Scholar] [CrossRef]
- Mateo, C.; Dave, R.; Stein, H. Effects of supplemental nucleosides for newly weaned pigs. J. Anim. Sci. 2004, 82, 71. [Google Scholar]
- Le, T.T.; Urasaki, Y.; Pizzorno, G. Uridine prevents fenofibrate-induced fatty liver. PLoS ONE 2014, 9, e87179. [Google Scholar] [CrossRef]
- Le, T.T.; Urasaki, Y.; Pizzorno, G. Uridine prevents tamoxifen-induced liver lipid droplet accumulation. Bmc Pharm. Toxicol. 2014, 15, 27. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.V.; Gordillo, R.; An, Y.; Zhang, C.; Liang, Q.; Yoshino, J.; Cautivo, K.M.; De Brabander, J.; Elmquist, J.K.; et al. An adipo-biliary-uridine axis that regulates energy homeostasis. Science 2017, 355, 1124. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Wang, Q.; Wan, D.; Zhang, Y.; Wu, X.; Yin, Y. Uridine/UMP metabolism and their function on the gut in segregated early weaned piglets. Food Funct. 2019, 10, 4081–4089. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Pizzorno, G.; Le, T.T. Chronic Uridine Administration Induces Fatty Liver and Pre-Diabetic Conditions in Mice. PLoS ONE 2016, 11, e0146994. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.S.; Ji, W.; Wang, J.L.; Li, B.; Hu, J.P.; Wu, X. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wan, D.; Zhang, Y.; Zhang, Y.; Long, C.; Chen, S.; He, L.; Tan, B.; Wu, X.; Yin, Y.L.; et al. Diurnal variations in polyunsaturated fatty acid contents and expression of genes involved in their de novo synthesis in pigs. Biochem. Biophys. Res. Commun. 2017, 483, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wu, X.; Guo, X.; Long, C.; Li, S.; Hu, C.-A.A.; Yin, Y.L. Maternal chitosan oligosaccharide supplementation affecting expression of circadian clock genes, and possible association with hepatic cholesterol accumulation in suckling piglets. Biol. Rhythm Res. 2016, 47, 253–265. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Zhang, Y.; Zhang, J.; Deng, Z.; Wu, X.; Yin, Y. Dynamic oral administration of uridine affects the diurnal rhythm of bile acid and cholesterol metabolism-related genes in mice. Biol. Rhythm Res. 2019, 50, 543–552. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yin, J.; Ruan, Z.; Wu, X.; Yin, Y. Uridine dynamic administration affects circadian variations in lipid metabolisms in the liver of high-fat-diet-fed mice. Chronobiol. Int. 2019, 36, 1258–1267. [Google Scholar] [CrossRef]
- Gao, L.; Lin, X.; Xie, C.; Zhang, T.; Wu, X.; Yin, Y. The time of Calcium Feeding Affects the Productive Performance of Sows. Animal (Basel) 2019, 9, 337. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Q.; Wang, J.; Tan, B.; Fan, Z.; Deng, Z.Y.; Wu, X.; Yin, Y.L. Developmental changes in hepatic glucose metabolism in a newborn piglet model: A comparative analysis for suckling period and early weaning period. Biochem. Biophys. Res. Commun. 2016, 470, 824–830. [Google Scholar] [CrossRef]
- Xie, C.; Long, C.; Wu, X.; Yang, H.; Fan, Z.; Xiao, D.; Wang, Y.; Yin, Y.L. Effect of maternal supplementation with chitosan oligosaccharide on the antioxidant capacity of suckling piglets. J. Anim. Sci. 2016, 94, 453–456. [Google Scholar] [CrossRef]
- Xie, C.; Wu, X.; Long, C.; Wang, Q.; Fan, Z.; Li, S.; Yin, Y. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. Bmc. Vet. Res. 2016, 12, 243. [Google Scholar] [CrossRef]
- Le, T.T.; Ziemba, A.; Urasaki, Y.; Hayes, E.; Brotman, S.; Pizzorno, G. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J. Lipid Res. 2013, 54, 1044–1057. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Koyama, H.; Kurajoh, M.; Shoji, T.; Tsutsumi, Z.; Moriwaki, Y. Biochemistry of uridine in plasma. Clin. Chim. Acta. 2011, 412, 1712–1724. [Google Scholar] [CrossRef]
- Musunuru, K.; Orho-Melander, M.; Caulfield, M.P.; Li, S.; Salameh, W.A.; Reitz, R.E.; Berglund, G.; Hedblad, B.; Engström, G.; Williams, P.T. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1975–1980. [Google Scholar] [CrossRef]
- Maki, K.C. The fat of the matter: Lipoprotein effects of dietary fatty acids vary by body weight status. Am. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Ooi, L.G.; Ahmad, R.; Yuen, K.H.; Liong, M.T. Lactobacillus gasseri [corrected] CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J. Dairy Sci. 2010, 93, 5048–5058. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, F.; Liu, J.; Wang, H.; Han, X.; Zhang, Y.; Yang, Z. Selection of Cholesterol-Lowering Lactic Acid Bacteria and its Effects on Rats Fed with High-Cholesterol Diet. Curr. Microbiol. 2017, 74, 623–631. [Google Scholar] [CrossRef]
- Zhang, Y.H.; An, T.; Zhang, R.C.; Zhou, Q.; Huang, Y.; Zhang, J. Very high fructose intake increases serum LDL-cholesterol and total cholesterol: A meta-analysis of controlled feeding trials. J. Nutr. 2013, 143, 1391–1398. [Google Scholar] [CrossRef]
- Pellerin, L. Food for thought: The importance of glucose and other energy substrates for sustaining brain function under varying levels of activity. Diabetes Metab. 2010, 36, S59–S63. [Google Scholar] [CrossRef]
- Yamamoto, T.; Inokuchi, T.; Ka, T.; Yamamoto, A.; Takahashi, S.; Tsutsumi, Z.; Tamada, D.; Okuda, C.; Moriwaki, Y. Relationship between plasma uridine and insulin resistance in patients with non-insulin-dependent diabetes mellitus. Nucleosides Nucleotides Nucleic Acids 2010, 29, 504–508. [Google Scholar] [CrossRef]
- Lallès, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Clouard, C.; Val-Laillet, D. Impact of sensory feed additives on feed intake, feed preferences, and growth of female piglets during the early postweaning period. J. Anim. Sci. 2014, 92, 2133–2140. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wojtczak, L. Short-and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- Su, Y.B.; Bi, X.H.; Ma, X.K.; Huang, Q.; Li, Z.C.; Liu, L.; Piao, X.S.; Li, D.F.; Lai, C.H. Determination and prediction of the digestible and metabolizable energy content of lipid sources fed to growing pigs. Anim. Feed Sci. Technol. 2015, 209, 119–127. [Google Scholar] [CrossRef]
- Yecies, J.L.; Zhang, H.H.; Menon, S.; Liu, S.; Yecies, D.; Lipovsky, A.I.; Gorgun, C.; Kwiatkowski, D.J.; Hotamisligil, G.S.; Lee, C.H.; et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011, 14, 21–32. [Google Scholar] [CrossRef]
- Amemiya-Kudo, M.; Oka, J.; Takeuchi, Y.; Okazaki, H.; Yamamoto, T.; Yahagi, N.; Matsuzaka, K.; Okazaki, S.; Osuga, J.; Yamada, N.; et al. Suppression of the Pancreatic Duodenal Homeodomain Transcription Factor-1 (Pdx-1) Promoter by Sterol Regulatory Element-binding Protein-1c. J. Biol. Chem. 2011, 286, 27902–27914. [Google Scholar] [CrossRef]
- Qin, Y.; Dalen, K.T.; Gustafsson, J.A.; Nebb, H.I. Regulation of hepatic fatty acid elongase 5 by LXRalpha-SREBP-1c. Biochim. Biophys Acta. 2009, 1791, 140–147. [Google Scholar] [CrossRef]
- Davidson, E.A.; Pickens, C.A.; Fenton, J.I. Increasing dietary EPA and DHA influence estimated fatty acid desaturase activity in systemic organs which is reflected in the red blood cell in mice. Int. J. Food Sci. Nutr. 2018, 69, 183–191. [Google Scholar] [CrossRef]
- Jianhua, L.; Xueqin, M.; Jifen, H. Expression and clinical significance of LXRalpha and SREBP-1c in placentas of preeclampsia. Open Med. (Wars) 2016, 11, 292–296. [Google Scholar] [CrossRef]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysisa highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Marcelin, G.; Liu, S.M.; Li, X.; Schwartz, G.J.; Chua, S. Genetic control of ATGL-mediated lipolysis modulates adipose triglyceride stores in leptin-deficient mice. J. Lipid Res. 2012, 53, 964–972. [Google Scholar] [CrossRef] [Green Version]
- Olsen, R.K.; Cornelius, N.; Gregersen, N. Redox signalling and mitochondrial stress responses; lessons from inborn errors of metabolism. J. Inherit. Metab. Dis. 2015, 38, 703–719. [Google Scholar] [CrossRef] [Green Version]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N.; et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]



| Ingredient Composition | (%) | Nutrient Levels | (%) |
|---|---|---|---|
| Skimmed milk powder | 85.0 | DE a (MJ/kg) | 14.65 |
| Dried whey | 5.0 | CP b | 20.50 |
| Glucose | 2.5 | Calcium | 0.70 |
| Plasma proteins | 3.5 | Total phosphorus | 0.60 |
| Premix * | 4.0 | Lysine | 1.45 |
| Total | 100.0 | Methionine | 0.48 |
| Tryptophan | 0.29 |
| Gene | Accession No. | Nucleotide Sequence of Primers (5’-3′) | Product Size (bp) |
|---|---|---|---|
| LXRα | NM_001101814.1 | F: GTAGATGGCTGAGGCGTGAC | 96 |
| R: TTCCCAACCCTTTGACTCTTT | |||
| SREBP1c | NM_214157.1 | F: CCTCTGTCTCTCCTGCAACC | 229 |
| R: GACCGGCTCTCCATAGACAA | |||
| ELOVL2 | XM_013977421.1 | F: ATTCTTCACCACCAGCGAGG | 131 |
| R: TGCCTGGCTGTTATCACTCG | |||
| ELOVL5 | XM_013986754.1 | F: TACCACCATGCCACTATGCT | 102 |
| R: GACGTGGATGAAGCTGTTGA | |||
| CPT-1α | NM_001129805.1 | F: CCATCAAAACTGCCTTCCTTAG | 118 |
| R: AGCGAGTGTGCCAGATACAAA | |||
| FADS1 | NM_001113041.1 | F: GTCACTGCCTGGCTCATTCT | 155 |
| R: AGGTGGTTCCACGTAGAGGT | |||
| FADS2 | NM_001171750.1 | F: ACGGCCTTCATCCTTGCTAC | 144 |
| R: GTTGGCAGAGGCACCCTTTA | |||
| ATGL | NM_001098605.1 | F: ATGGTGCCCTACACGCTG | 111 |
| R: GCCTGTCTGCTCCTTTATCC | |||
| HSL | HM591297.1 | F: GAAGGGAGAGCTATGGCACC | 130 |
| R: CTCACACTCTCCAAGCCCAG | |||
| β-actin | XM_003357928.2 | F: CGTTGGCTGGTTGAGAATC | 132 |
| R: CGGCAAGACAGAAATGACAA |
| Items | CON | UMP | UR | SEM | p-Value |
|---|---|---|---|---|---|
| TG (mmol/L) | 0.357 | 0.337 | 0.341 | 0.219 | 0.933 |
| TC (mmol/L) | 2.346 ab | 2.630 a | 2.114 b | 0.092 | 0.062 |
| Glu (mmol/L) | 5.359 | 4.577 | 4.553 | 0.207 | 0.193 |
| LDL (mmol/L) | 0.801 b | 1.049 a | 0.790 b | 0.048 | 0.039 |
| TBA (umol/L) | 18.050 a | 12.271 b | 7.317 c | 1.493 | 0.008 |
| Fatty Acid Composition, % | CON | UMP | UR | SEM | p-Value |
|---|---|---|---|---|---|
| C12:0 | 0.195 a | 0.116 b | 0.095 b | 0.015 | 0.007 |
| C14:0 | 0.788 a | 0.603 b | 0.578 b | 0.036 | 0.031 |
| C16:0 | 15.340 | 15.507 | 15.121 | 0.226 | 0.793 |
| C17:0 | 0.398 | 0.411 | 0.395 | 0.013 | 0.865 |
| C18:0 | 21.237 | 21.656 | 21.922 | 0.276 | 0.631 |
| C16:1 | 0.907 | 0.768 | 0.760 | 0.095 | 0.802 |
| C18:1n9c | 17.734 | 17.846 | 17.856 | 0.380 | 0.991 |
| C20:1 | 0.313 | 0.302 | 0.300 | 0.012 | 0.903 |
| C18:2n6c | 15.954 | 16.406 | 16.196 | 0.200 | 0.686 |
| C20:3n6 | 2.449 | 2.297 | 2.675 | 0.129 | 0.491 |
| C20:4n6 | 17.668 | 17.623 | 17.459 | 0.239 | 0.938 |
| C22:6n6 | 6.401 | 5.923 | 6.043 | 0.138 | 0.375 |
| SFA | 38.108 | 38.438 | 38.273 | 0.201 | 0.821 |
| MUFA | 19.080 | 19.006 | 19.034 | 0.395 | 0.997 |
| PUFA | 42.812 | 42.556 | 42.681 | 0.291 | 0.945 |
| EPA | 16.123 | 16.543 | 16.338 | 0.200 | 0.722 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, S.; Xie, C.; Wang, R.; Zhang, Y.; Zhou, X.; Wu, X. Short-Term Oral UMP/UR Administration Regulates Lipid Metabolism in Early-Weaned Piglets. Animals 2019, 9, 610. https://doi.org/10.3390/ani9090610
Zhang Y, Guo S, Xie C, Wang R, Zhang Y, Zhou X, Wu X. Short-Term Oral UMP/UR Administration Regulates Lipid Metabolism in Early-Weaned Piglets. Animals. 2019; 9(9):610. https://doi.org/10.3390/ani9090610
Chicago/Turabian StyleZhang, Yumei, Songge Guo, Chunyan Xie, Ruxia Wang, Yan Zhang, Xihong Zhou, and Xin Wu. 2019. "Short-Term Oral UMP/UR Administration Regulates Lipid Metabolism in Early-Weaned Piglets" Animals 9, no. 9: 610. https://doi.org/10.3390/ani9090610
APA StyleZhang, Y., Guo, S., Xie, C., Wang, R., Zhang, Y., Zhou, X., & Wu, X. (2019). Short-Term Oral UMP/UR Administration Regulates Lipid Metabolism in Early-Weaned Piglets. Animals, 9(9), 610. https://doi.org/10.3390/ani9090610







