Clinical Effects of the Extract of the Seeds of the Indian Celery—Apium graveolens—In Horses Affected by Chronic Osteoarthritis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. CSE Powder and Dosages
2.2. Horses
2.3. Clinical Evaluation and Scoring System
2.4. Laboratory Analysis and Questionnaire
2.5. Statistical Analysis
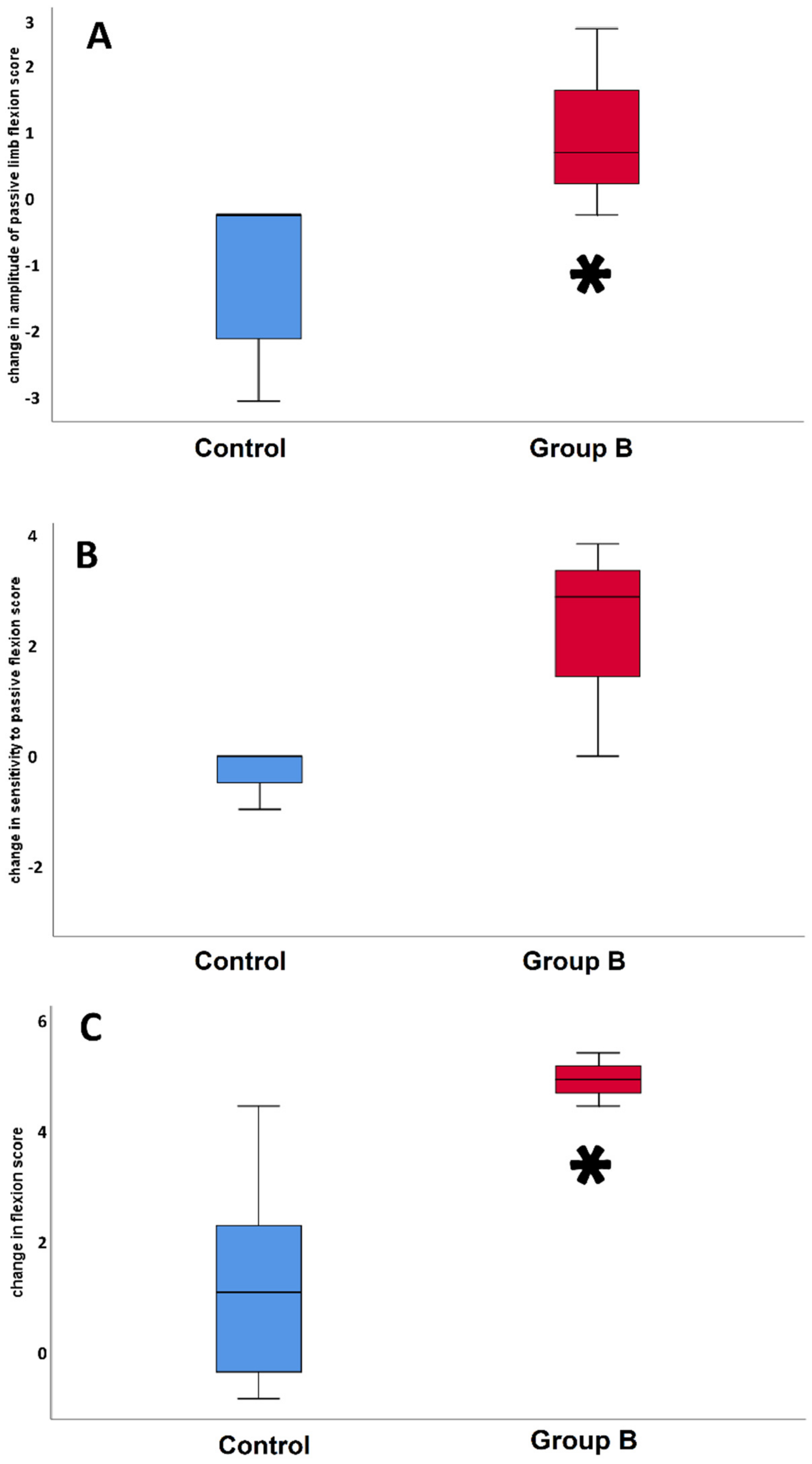
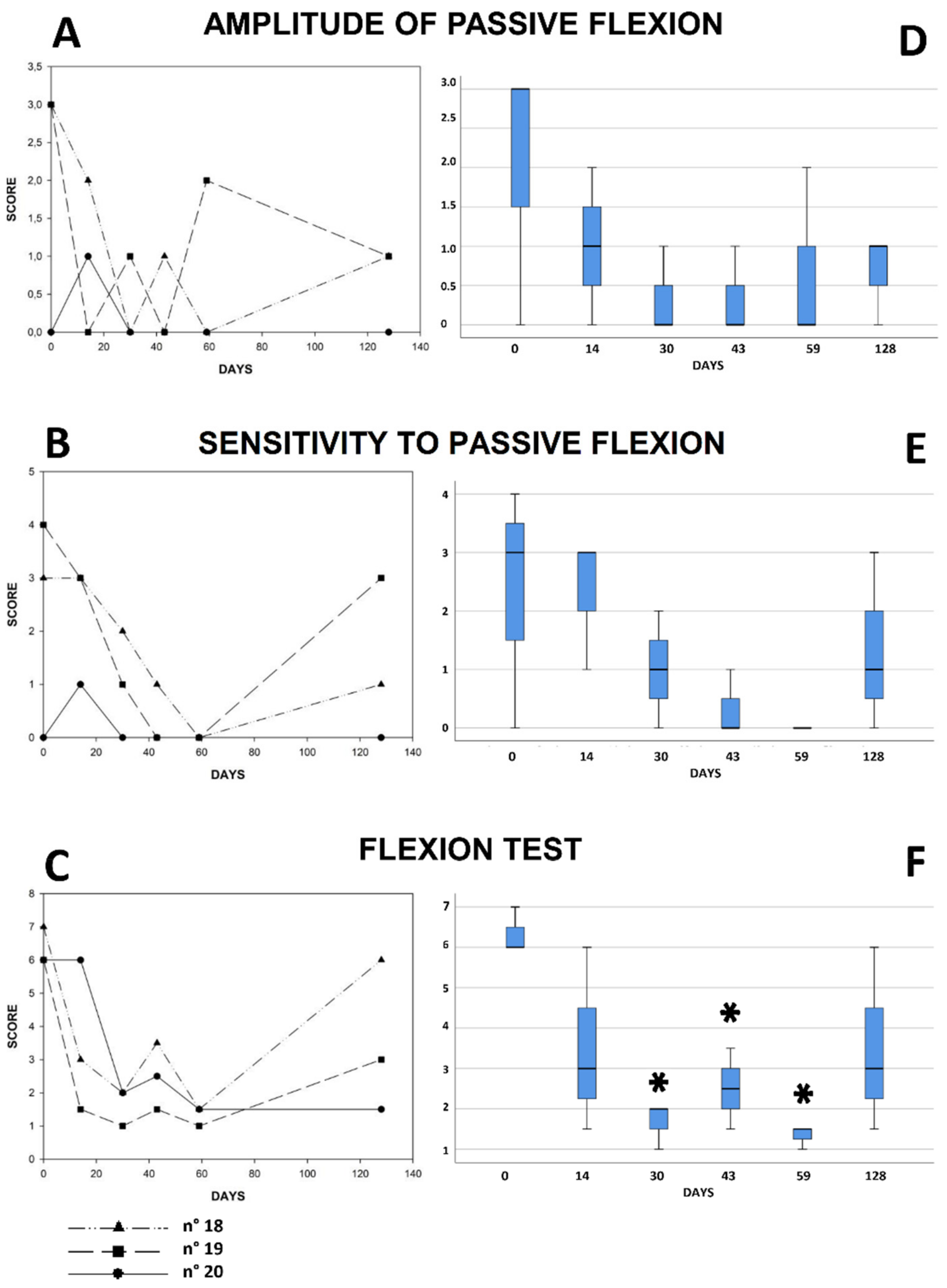
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caron, J.P.; Genovese, R.L. Principles and practices of joint disease treatment. In Diagnosis and Management of Lameness in the Horse, 2nd ed.; Ross, M.W., Dyson, S.J., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 746–764. [Google Scholar]
- Goldring, S.R.; Goldring, M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal Interact. 2006, 6, 376–378. [Google Scholar]
- Curtiss, J.; Paul, H. The pathophysiology of joint infections. Clin. Orthop. Relat. Res. 1973, 96, 129–135. [Google Scholar] [CrossRef]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of osteoarthritis. Med. Clin. N. Am. 2009, 93, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, L.R.; Nixon, A.J. Medical treatment of osteoarthritis in the horse: A review. Vet. J. 2006, 171, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, A. The Encyclopedia of Medicinal Plant; No. C/581.63403 C4; Dorling Kindersley: London, UK, 1996. [Google Scholar]
- Gaby, A.R. Natural treatments for osteoarthritis. Altern. Med. Rev. 1999, 4, 330–341. [Google Scholar] [PubMed]
- Trumble, T.N. The use of nutraceuticals for osteoarthritis in horses. Vet. Clin. N. Am. Equine Pract. 2005, 21, 575–597. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 2012, 1, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Powanda, M.C.; Rainsford, K.D. A toxicological investigation of a celery seed extract having anti-inflammatory activity. Inflammopharmacology 2011, 19, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.W.; Roberts, M.S.; Brooks, P.M. Over the counter (OTC) oral remedies for arthritis and rheumatism: How effective are they? Inflammopharmacology 1999, 7, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.H.; Bao, T.H.; Deng, Y.; Li, H.; Chen, L.X. Constituents from Apium graveolens and their anti-inflammatory effects. J. Asian Nat. Prod. Res. 2017, 19, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, H.B. Chemistry, Technology, and Nutraceutical Functions of Celery (Apium graveolens L.) An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, M.W.; Butters, D.E.; Clarcke, M.L.; Rainsford, K.D. NSAID gastropathy: Prevention by celery seed extracts in disease-stressed rats. Inflammopharmacology 2001, 1, 201–209. [Google Scholar] [CrossRef]
- Kester, W.O. Definition and classification of lameness. In Guide for Veterinary Services and Judging of Equestrian Events, 4th ed.; American Association of Equine Practitioners: Lexington, KY, USA, 1991. [Google Scholar]
- MacAllister, C.G.; Morgan, S.J.; Borne, A.T.; Pollet, R.A. Comparison of adverse effects of phenylbutazone, flunixin meglumine, and ketoprofen in horses. J. Am. Vet. Med. Assoc. 1993, 202, 71–77. [Google Scholar] [PubMed]
- Noble, G.; Edwards, S.; Lievaart, J.; Pippia, J.; Boston, R.; Raidal, S.L. Pharmacokinetics and safety of single and multiple oral doses of meloxicam in adult horses. J. Vet. Intern. Med. 2012, 5, 1192–1201. [Google Scholar] [CrossRef]
- Tamzali, Y.; Guelfi, J.F.; Braun, J.P. Plasma fibrinogen measurement in the horse: Comparison of Millar’s technique with a chronometric technique and the QBC-Vet Autoreader™. Res. Vet. Sci. 2001, 71, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Crisman, M.V.; Scarratt, W.K.; Zimmerman, K.L. Blood proteins and inflammation in the horse. Vet. Clin. N. Am. Equine Pract. 2008, 4, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.S.; Divers, T.J.; Stokol, T.; Mohammed, O.H. Serum iron and plasma fibrinogen concentrations as indicators of systemic inflammatory diseases in horses. J. Vet. Intern. Med. 2007, 21, 489–494. [Google Scholar] [CrossRef]
- Kusano, K.; Yamasaki, M.; Kiuchi, M.; Kaneko, K.; Koyama, K. Reference range of blood biomarkers for oxidative stress in Thoroughbred racehorses (2–5 years old). J. Equine Sci. 2016, 27, 125–129. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power: The FRAP assay”. Anal. Biochem. 1996, 2391, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.M. Adams and Stashak’s Lameness in Horses, 6th ed.; Wiley-Blackwell: Ames, IA, USA, 2011. [Google Scholar]
- Blumenthal, M.; Busse, W.R.; Goldberg, A.; Gruenwald, J.; Hall, T.; Riggins, C.W.; Klein, S.; Rister, R.S. The Complete German Commission E Monographs—Therapeutic Guide to Herbal Medicines; Integrative Medicine Communication; American Botanical Council: Austin, TX, USA, 1998. [Google Scholar]
- De Grauw, J.C.; Van De Lest, C.H.; Van Weeren, R.; Brommer, H.; Brama, P.A. Arthrogenic lameness of the fetlock: Synovial fluid markers of inflammation and cartilage turnover in relation to clinical joint pain. Equine Vet. J. 2006, 38, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Beluche, L.A.; Bertone, A.L.; Anderson, D.E.; Rohde, C. Effects of oral administration of phenylbutazone to horses on in vitro articular cartilage metabolism. Am. J. Vet. Res. 2001, 62, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Momin, R.A.; Nair, M.G. Antioxidant, cyclooxygenase and topoisomerase inhibitory compounds from Apium graveolens Linn. Seeds. Phytomedicine 2002, 9, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Mencherini, T.; Cau, A.; Bianco, G.; Della Loggia, R.; Aquino, R.P.; Autore, G. An extract of Apium graveolens var. dulce leaves: Structure of the major constituent, apiin and its anti-inflammatory properties. J. Pharm. Pharmacol. 2007, 59, 891–897. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battaglia, B.; Angelone, M.; Vera, E.; Basini, G.; Bussolati, S.; Paci, M.; Del Bue, M.; Aldigeri, R.; Grolli, S.; Quintavalla, F.; et al. Clinical Effects of the Extract of the Seeds of the Indian Celery—Apium graveolens—In Horses Affected by Chronic Osteoarthritis. Animals 2019, 9, 585. https://doi.org/10.3390/ani9080585
Battaglia B, Angelone M, Vera E, Basini G, Bussolati S, Paci M, Del Bue M, Aldigeri R, Grolli S, Quintavalla F, et al. Clinical Effects of the Extract of the Seeds of the Indian Celery—Apium graveolens—In Horses Affected by Chronic Osteoarthritis. Animals. 2019; 9(8):585. https://doi.org/10.3390/ani9080585
Chicago/Turabian StyleBattaglia, Beatrice, Mario Angelone, Elena Vera, Giuseppina Basini, Simona Bussolati, Massimiliano Paci, Maurizio Del Bue, Raffaella Aldigeri, Stefano Grolli, Fausto Quintavalla, and et al. 2019. "Clinical Effects of the Extract of the Seeds of the Indian Celery—Apium graveolens—In Horses Affected by Chronic Osteoarthritis" Animals 9, no. 8: 585. https://doi.org/10.3390/ani9080585
APA StyleBattaglia, B., Angelone, M., Vera, E., Basini, G., Bussolati, S., Paci, M., Del Bue, M., Aldigeri, R., Grolli, S., Quintavalla, F., & Ramoni, R. (2019). Clinical Effects of the Extract of the Seeds of the Indian Celery—Apium graveolens—In Horses Affected by Chronic Osteoarthritis. Animals, 9(8), 585. https://doi.org/10.3390/ani9080585





