Multi-Step Tail Biting Outbreak Intervention Protocols for Pigs Housed on Slatted Floors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
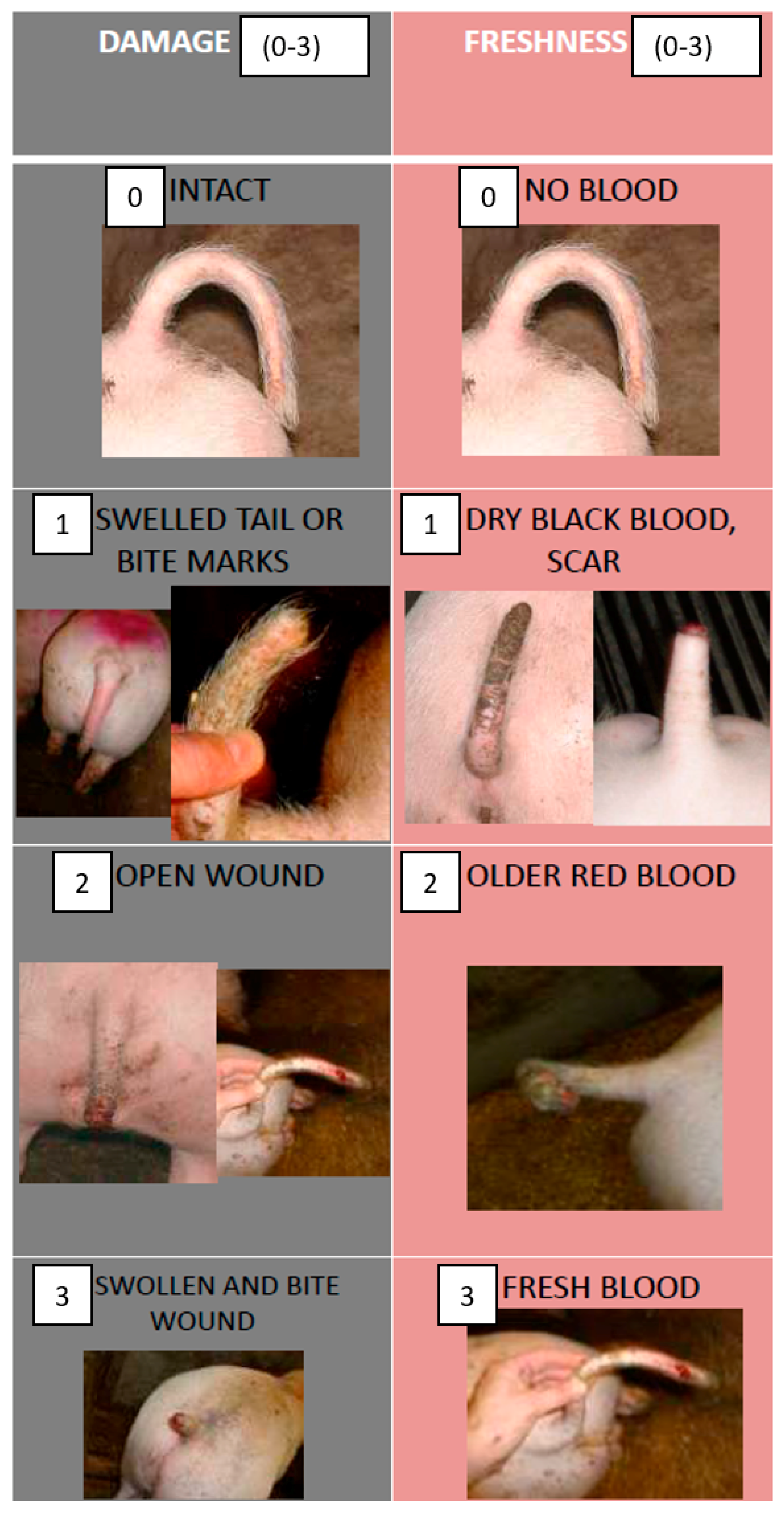
2.2. Recording of Tail Injuries and Tail Scores
2.3. Definition of a Tail Biting Outbreak
2.4. Intervention Methods
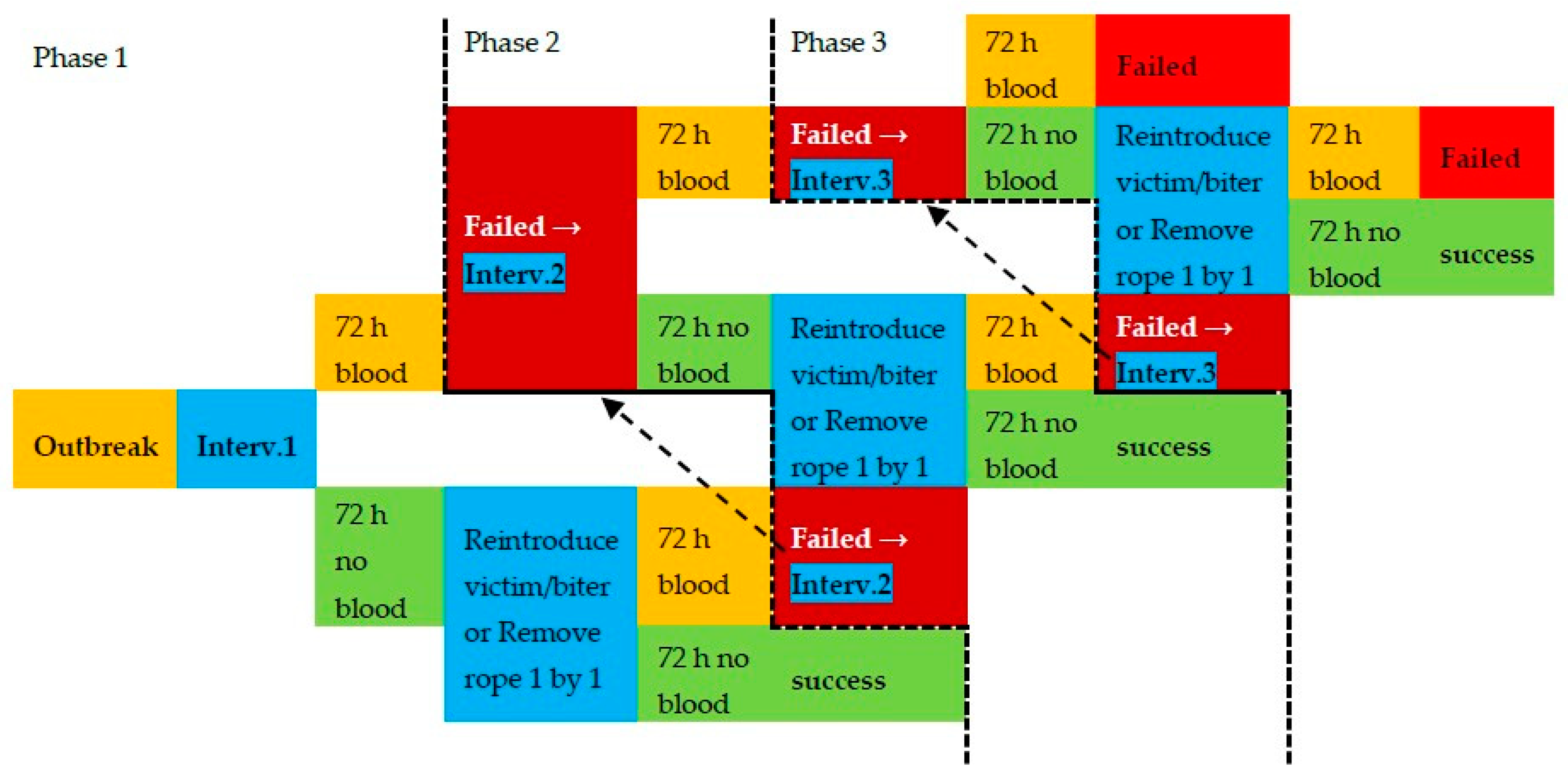
2.5. Intervention Protocol
2.6. Ethical Considerations
2.7. Data Analyses
3. Results
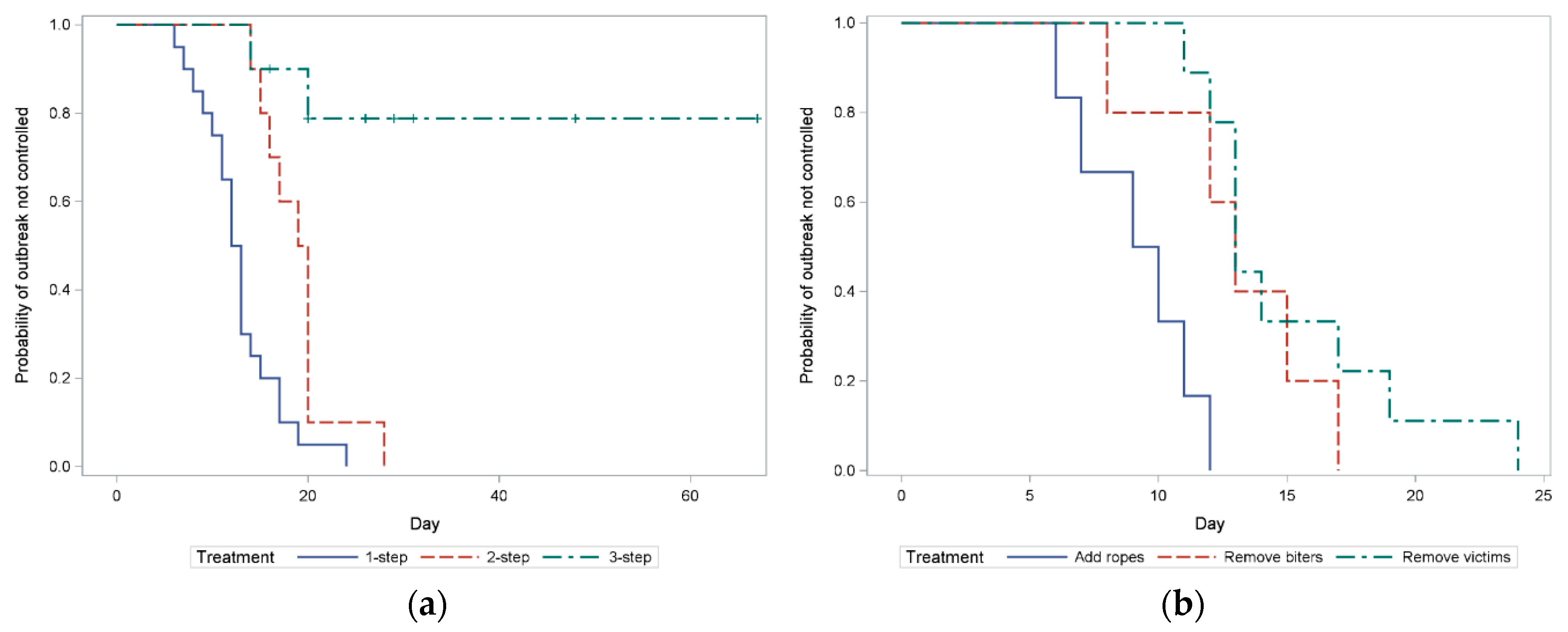
3.1. Intervention Success
3.2. Intervention Duration
3.3. Tail Lesion Scores
4. Discussion
4.1. Intervention Success and Duration
4.2. Intervention and Tail Lesion Scores
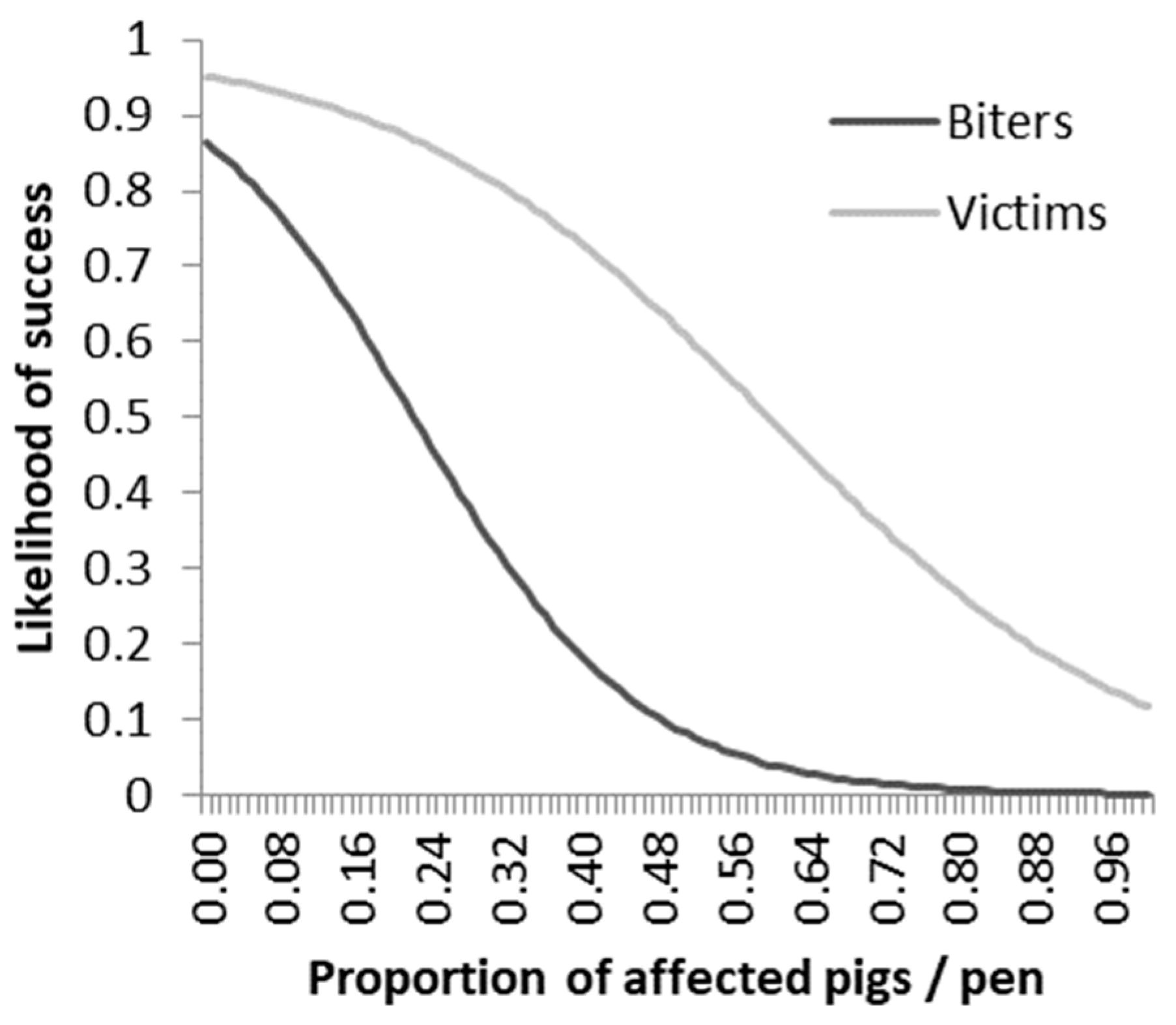
4.3. Reintroduction of Ex-Biters and Ex-Victims
4.4. Different Types of Outbreaks
4.5. Limitation in Data Collection
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- European Union. Council Directive 2008/120/EC of 18 December 2008 Laying Down Minimum Standards for the Protection of Pigs (Codified Version). Available online: https://eur-lex.europa.eu/eli/dir/2008/120/oj (accessed on 13 August 2019).
- De Briyne, N.; Berg, C.; Blaha, T.; Palzer, A.; Temple, D. Phasing out pig tail docking in the EU—Present state, challenges and possibilities. Porc. Health Manag. 2018, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- D’Eath, R.B.; Niemi, J.K.; Vosough Ahmadi, B.; Rutherford, K.M.D.; Ison, S.H.; Turner, S.P.; Anker, H.T.; Jensen, T.; Busch, M.E.; Jensen, K.K.; et al. Why are most EU pigs tail docked? Economic and ethical analysis of four pig housing and management scenarios in the light of EU legislation and animal welfare outcomes. Animal 2015, 10, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Nalon, E.; De Briyne, N. Efforts to Ban the Routine Tail Docking of Pigs and to Give Pigs Enrichment Materials via EU Law: Where do We Stand a Quarter of a Century on? Animals 2019, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- D’Eath, R.B.; Arnott, G.; Turner, S.P.; Jensen, T.; Lahrmann, H.P.; Busch, M.E.; Niemi, J.K.; Lawrence, A.B.; Sandøe, P. Injurious tail biting in pigs: How can it be controlled in existing systems without tail docking? Animal 2014, 8, 1479–1497. [Google Scholar] [CrossRef] [PubMed]
- Schrøder-Petersen, D.L.; Simonsen, H.B. Tail Biting in Pigs. Vet. J. 2001, 162, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, L.T.; Fels, M.; Oczak, M.; Vranken, E.; Ismayilova, G.; Guarino, M.; Viazzi, S.; Bahr, C.; Berck, D. Tail Biting in pigs—Causes and management intervention strategies to reduce the behavioural disorder. A review. Berliner und Münchener Tierärztliche Wochenschrift 2013, 126, 104–112. [Google Scholar] [PubMed]
- Taylor, N.R.; Main, D.C.J.; Mendl, M.; Edwards, S.A. Tail-biting: A new perspective. Vet. J. 2010, 186, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Bilkei, G. Tail-biting in outdoor pig production. Vet. J. 2006, 171, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S. What do we know about tail biting today? Pig J. 2011, 66, 81–86. [Google Scholar]
- Lahrmann, H.P. Tail Biting Outbreak in Pigs—Prevalence, Early Detection and Targeted Intervention. Ph.D. Thesis, University of Copenhagen, Kobenhavn, Denmark, 2018. [Google Scholar]
- Ewbank, R. Abnormal Behaviour and Pig Nutrition. An Unsuccessful attempt to Induce Tail Biting by Feeding a High Energy, Low Fibre Vegetable Protein Ration. Br. Vet. J. 1973, 129, 366–369. [Google Scholar] [CrossRef]
- Bracke, M. Rope test may indicate efficacy of tail-biting treatments in growing pigs. Anim. Welf. 2009, 18, 263–266. [Google Scholar]
- Zonderland, J.J.; Wolthuis-Fillerup, M.; van Reenen, C.G.; Bracke, M.B.M.; Kemp, B.; den Hartog, L.A.; Spoolder, H.A.M. Prevention and treatment of tail biting in weaned piglets. Appl. Anim. Behav. Sci. 2008, 110, 269–281. [Google Scholar] [CrossRef]
- Lahrmann, H.; Faustrup, J.F.; Hansen, C.F.; D’Eath, R.B.; Nielsen, J.P.; Forkman, B. The Effect of Straw, Rope, and Bite-Rite Treatment in Weaner Pens with a Tail Biting Outbreak. Animals 2019, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Statham, P.; Green, L.; Bichard, M.; Mendl, M. Predicting tail-biting from behaviour of pigs prior to outbreaks. Appl. Anim. Behav. Sci. 2009, 121, 157–164. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Jack, M.; Futro, A.; Talbot, D.; Zhu, Q.; Barclay, D.; Baxter, E.M. Automatic early warning of tail biting in pigs: 3D cameras can detect lowered tail posture before an outbreak. PLoS ONE 2018, 13, e0194524. [Google Scholar] [CrossRef]
- Wedin, M.; Baxter, E.M.; Jack, M.; Futro, A.; D’Eath, R.B. Early indicators of tail biting outbreaks in pigs. Appl. Anim. Behav. Sci. 2018, 208, 7–13. [Google Scholar] [CrossRef]
- Bracke, M.B.M.; De Lauwere, C.C.; Wind, S.M.M.; Zonerland, J.J. Attitudes of Dutch Pig Farmers Towards Tail Biting and Tail Docking. J. Agric. Environ. Ethics 2013, 26, 847–868. [Google Scholar] [CrossRef]
- Wallgren, T.; Westin, R.; Gunnarsson, S. A survey of straw use and tail biting in Swedish pig farms rearing undocked pigs. Acta Vet. Scand. 2016, 58, 84. [Google Scholar] [CrossRef]
- Valros, A.; Munsterhjelm, C.; Hänninen, L.; Kauppinen, T.; Heinonen, M. Managing undocked pigs–on-farm prevention of tail biting and attitudes towards tail biting and docking. Porc. Health Manag. 2016, 2, 2. [Google Scholar] [CrossRef]
- Haigh, A.; O’Driscoll, K. Irish pig farmer’s perceptions and experiences of tail biting. Animal 2019. submitted. [Google Scholar]
- Hunter, E.J.; Jones, T.A.; Guise, H.J.; Penny, R.H.C.; Hoste, S. The Relationship Between Tail Biting in Pigs, Docking Procedure and Other Management Practices. Vet. J. 2001, 161, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Peden, R.S.E.; Turner, S.P.; Boyle, L.A.; Camerlink, I. The translation of animal welfare research into practice: The case of mixing aggression between pigs. Appl. Anim. Behav. Sci. 2018, 204, 1–9. [Google Scholar] [CrossRef]
- Chou, J.-Y.; D’Eath, R.B.; Sandercock, D.A.; O’Driscoll, K. Can increased dietary fibre level and a single enrichment device reduce the risk of tail biting in undocked pigs on fully slatted systems? Animal 2019. submitted. [Google Scholar]
- Chou, J.-Y.; Sandercock, D.A.; D’Eath, R.B.; O’Driscoll, K. ENTAIL: Strategies to control tail biting in pigs on fully-slatted floor. In Proceedings of the Teagasc Pig Research Dissemination Day, Teagasc Pig Development Department: Horse & Jockey, Tipperary, Ireland, 1 May 2019. [Google Scholar]
- Chou, J.-Y.; Drique, C.; Sandercock, D.; D’Eath, R.; O’Driscoll, K. Rearing Undocked Pigs on Fully Slatted Floors Using Multiple Types and Variations of Enrichment. Animals 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Ursinus, W.W.; Van Reenen, C.G.; Kemp, B.; Bolhuis, J.E. Tail biting behaviour and tail damage in pigs and the relationship with general behaviour: Predicting the inevitable? Appl. Anim. Behav. Sci. 2014, 156, 22–36. [Google Scholar] [CrossRef]
- Valros, A. Tail biting. In Advances in Pig Welfare; Spinka, M., Ed.; The Advances in Farm Animal Welfare Series; Elsevier: Duxford, UK, 2018; pp. 137–166. [Google Scholar]
- van de Weerd, H.; Ison, S. Providing Effective Environmental Enrichment to Pigs: How Far Have We Come? Animals 2019, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Wallenbeck, A.; Keeling, L.J. Using data from electronic feeders on visit frequency and feed consumption to indicate tail biting outbreaks in commercial pig production1. J. Anim. Sci. 2013, 91, 2879–2884. [Google Scholar] [CrossRef]
- Larsen, M.L.V.; Andersen, H.M.-L.; Pedersen, L.J. Can tail damage outbreaks in the pig be predicted by behavioural change? Vet. J. 2016, 209, 50–56. [Google Scholar] [CrossRef]
- Larsen, M.L.V.; Andersen, H.M.-L.; Pedersen, L.J. Changes in activity and object manipulation before tail damage in finisher pigs as an early detector of tail biting. Animal 2019, 13, 1037–1044. [Google Scholar] [CrossRef]
- Sandercock, D.A.; Smith, S.H.; Di Giminiani, P.; Edwards, S.A. Histopathological Characterization of Tail Injury and Traumatic Neuroma Development after Tail Docking in Piglets. J. Comp. Pathol. 2016, 155, 40–49. [Google Scholar] [CrossRef]
- Turner, S.P.; Farnworth, M.J.; White, I.M.S.; Brotherstone, S.; Mendl, M.; Knap, P.; Penny, P.; Lawrence, A.B. The accumulation of skin lesions and their use as a predictor of individual aggressiveness in pigs. Appl. Anim. Behav. Sci. 2006, 96, 245–259. [Google Scholar] [CrossRef]
- Ewbank, R.; Meese, G. Aggressive behaviour in groups of domesticated pigs on removal and return of individuals. Anim. Sci. 1971, 13, 685–693. [Google Scholar] [CrossRef]
- Marchant-Forde, J.N.; Marchant-Forde, R.M. Minimizing inter-pig aggression during mixing. Pig News Inf. 2005, 26, 63N–71N. [Google Scholar]

| Day | D0 | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outbreak identified | Remove biters/+victims | Monitor | Monitor | Reintroduce + 3 ropes | Remove 1st rope | Remove 2nd rope | Remove 3rd rope | Monitor | Monitor | Monitor | Success |
| Add ropes × 3 | Remove 1st rope | Remove 2nd rope | Remove 3rd rope | Monitor | Monitor | Monitor | Success |
| Comparisons | Trial 1 | Trial 2 | Test | p-Value |
|---|---|---|---|---|
| Pens with outbreaks | 22 | 12 | X2 (1, N = 96) = 4.55 | 0.03 |
| Pens with recurring outbreaks | 4 (18.2%) | 2 (16.7%) | - | - |
| Pens with slow outbreaks (>72 h) | 5 (19.2%) | 3 (21.4%) | X2 (1, N = 40) = 0.03 | 0.87 |
| Mean duration of outbreaks (d) | 19.6 | 13.3 | t (34) = 2.28 | 0.03 |
| Successful interventions (%) | 76.92 | 85.71 | X2 (1, N = 40) = 0.44 | 0.51 |
| Interventions used (median) | 2 | 1 | U (N1 = 26, N2 = 14) = 267 | 0.58 |
| Method | 1st Step | Result | Count (Percentage) | 2nd Step | Result | Count (Percentage) | 3rd Step | Result | Count (Percentage) |
|---|---|---|---|---|---|---|---|---|---|
| B | 14 (35.0%) | Fail | 9 (22.5%) | 7 | Fail | 3 (15.0%) | 2 | Fail | 1 (10.0%) |
| Success | 5 (12.5%) | Success | 4 (20.0%) | Success | 1 (10.0%) | ||||
| V | 16 (40.0%) | Fail | 7 (17.5%) | 8 | Fail | 5 (25.0%) | 2 | Fail | 1 (10.0%) |
| Success | 9 (22.5%) | Success | 3 (15.0%) | Success | 1 (10.0%) | ||||
| R | 10 (25.0%) | Fail | 4 (10.0%) | 5 | Fail | 2 (10.0%) | 6 | Fail | 6 (60.0%) |
| Success | 6 (15.0%) | Success | 3 (15.0%) | Success | 0 (0.0%) | ||||
| Total | 40 | Fail | 20 (50.0%) | 20 | Fail | 10 (50.0%) | 10 | Fail | 8 (80.0%) |
| Success | 20 (50.0%) | Success | 10 (50.0%) | Success | 2 (20.0%) |
| Tail Damage | Before 1 | After 2 | Test | p-Value |
| Score 0 | 0.11 ± 0.02 | 0.15 ± 0.03 | U = 1115.0 | 0.481 |
| Score 2 & 3 | 0.26 ± 0.03 | 0.10 ± 0.02 | t = 4.00 | < 0.001 |
| Score 3 | 0.09 ± 0.02 | 0.02 ± 0.01 | U = 1397.0 | 0.006 |
| Blood Presence | Before 1 | After 2 | Test | p-Value |
| Score 0 | 0.21 ± 0.03 | 0.35 ± 0.04 | t = −2.77 | 0.007 |
| Score 2 & 3 | 0.38 ± 0.04 | 0.16 ± 0.03 | t = 4.67 | < 0.001 |
| Score 3 | 0.08 ± 0.02 | 0.03 ± 0.01 | U = 1334.5 | 0.048 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, J.-Y.; O’Driscoll, K.; D’Eath, R.B.; Sandercock, D.A.; Camerlink, I. Multi-Step Tail Biting Outbreak Intervention Protocols for Pigs Housed on Slatted Floors. Animals 2019, 9, 582. https://doi.org/10.3390/ani9080582
Chou J-Y, O’Driscoll K, D’Eath RB, Sandercock DA, Camerlink I. Multi-Step Tail Biting Outbreak Intervention Protocols for Pigs Housed on Slatted Floors. Animals. 2019; 9(8):582. https://doi.org/10.3390/ani9080582
Chicago/Turabian StyleChou, Jen-Yun, Keelin O’Driscoll, Rick B. D’Eath, Dale A. Sandercock, and Irene Camerlink. 2019. "Multi-Step Tail Biting Outbreak Intervention Protocols for Pigs Housed on Slatted Floors" Animals 9, no. 8: 582. https://doi.org/10.3390/ani9080582
APA StyleChou, J.-Y., O’Driscoll, K., D’Eath, R. B., Sandercock, D. A., & Camerlink, I. (2019). Multi-Step Tail Biting Outbreak Intervention Protocols for Pigs Housed on Slatted Floors. Animals, 9(8), 582. https://doi.org/10.3390/ani9080582






