Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Treatments, and Management Conditions
2.2. Sample Collection, Analyses, and Measurements
2.3. Statistical Analyses
3. Results and Discussion
3.1. Carnitine Concentrations in Blood
3.2. Rectal Temperature and Respiratory Rate
3.3. Feed Intake and Feed Sorting
3.4. Milk Yield and Composition
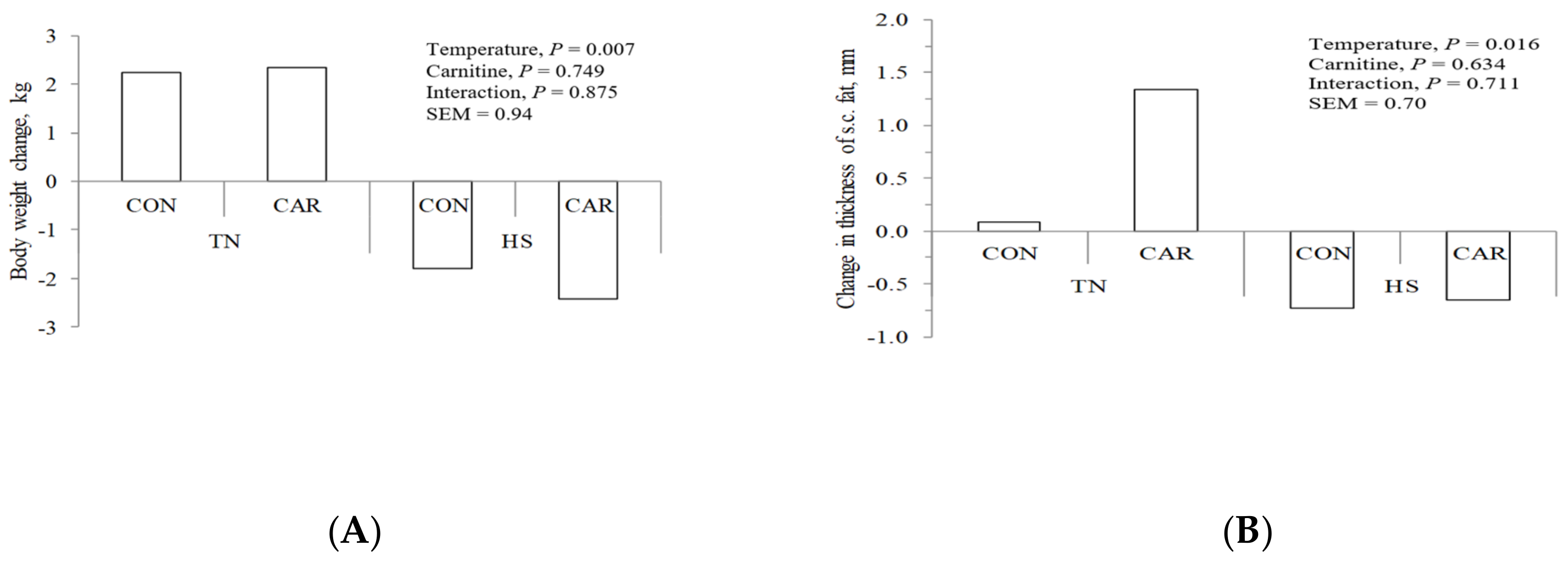
3.5. Body Weight and Subcutaneous Fat Assessment
3.6. Blood Metabolites
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baumgard, L.H.; Rhoads, R.P. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.A.K.; Caja, G.; Hamzaoui, S.; Such, X.; Albanell, E.; Badaoui, B.; Loor, J.J. Thermal stress in ruminants: Responses and strategies for alleviation. In Animal Welfare in Extensive Production Systems, 1st ed.; Villalba, J.J., Manteca, X., Eds.; 5M Publishing: Sheffield, UK, 2016; pp. 11–36. [Google Scholar]
- Salama, A.A.K.; Caja, G.; Hamzaoui, S.; Badaoui, B.; Castro-Costa, A.; Façanha, D.E.; Guilhermino, M.M.; Bozzi, R. Different levels of response to heat stress in dairy goats. Small Rumin. Res. 2014, 121, 73–79. [Google Scholar] [CrossRef]
- Hamzaoui, S.; Salama, A.A.K.; Albanell, E.; Such, X.; Caja, G. Physiological responses and lactational performances of late-lactation dairy goats under heat stress conditions. J. Dairy Sci. 2013, 96, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Contreras-Jodar, A.; Salama, A.A.K.; Hamzaoui, S.; Vailati-Riboni, M.; Caja, G.; Loor, J.J. Effects of chronic heat stress on lactational performance and the transcriptomic profile of blood cells in lactating dairy goats. J. Dairy Res. 2018, 85, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Salvado, L.; Barroso, E.; Vazques-Carrera, M. An overview of the crosstalk and metabolic dysregulation during diabetic cardiomyopathy. Int. J. Cardiol. 2013, 168, 3160–3172. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.; Wanders, R. Carnitine biosynthesis in mammals. Biochem. J. 2002, 429, 417–429. [Google Scholar] [CrossRef]
- Randle, P.J. Regulatory interactions between lipids and carbohydrates: The glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 1998, 14, 263–283. [Google Scholar] [CrossRef]
- Carlson, D.B.; McFadden, J.W.; D’Angelo, A.; Woodworth, J.C.; Drackley, J.K. Dietary L-carnitine affects periparturient nutrient metabolism and lactation in multiparous cows. J. Dairy Sci. 2007, 90, 3422–3441. [Google Scholar] [CrossRef]
- National Research Council (NRC). A Guide to Environmental Research on Animals; National Academy of Sciences: Washington, DC, USA, 1971. [Google Scholar]
- Silanikove, N.; Koluman, N.D. Impact of climate change on the dairy industry intemperate zones: Predications on the overall negative impact and on the positive role of dairy goats in adaptation to earth warming. Small Ruminant Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- LaCount, D.W.; Ruppert, L.D.; Drackley, J.K. Ruminal degradation and dose response of dairy cows to dietary L-carnitine. J. Dairy Sci. 1996, 79, 260–269. [Google Scholar] [CrossRef]
- Institut National de la Recherche Agronomique (INRA). INRA Feeding System for Ruminants; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2003; Volume I. [Google Scholar]
- Heinrichs, J.; Kononoff, P. Evaluating Particle Size of Forages and TMRs Using the New Penn State Forage Particle Separator; Cooperative Extension DAS; College of Agriculture Science, Pennsylvania State University: University Park, TX, USA, 2002; pp. 2–42. [Google Scholar]
- Teixeira, A.; Joy, M.; Delfa, R. In vivo estimation of goat carcass composition and body fat partition by real-time ultrasonography. J. Anim. Sci. 2008, 86, 2369–2376. [Google Scholar] [CrossRef]
- Hirche, F.; Fischer, M.; Keller, J.; Eder, K. Determination of carnitine, its short chain acyl esters and metabolic precursors trimethyllysine and γ-butyrobetaine by quasi-solid phase extraction and MS/MS detection. J. Chromatogr. B 2009, 877, 2158–2162. [Google Scholar] [CrossRef] [PubMed]
- LaCount, D.W.; Drackley, J.K.; Weigel, D.J. Responses of dairy cows during early lactation to ruminal or abomasal administration of L-carnitine. J. Dairy Sci. 1995, 78, 1824–1836. [Google Scholar] [CrossRef]
- Greenwood, R.H.; Titgemeyer, E.C.; Stokka, G.L.; Drouillard, J.S.; Loest, C.A. Effects of L-carnitine on nitrogen retention and blood metabolites of growing steers and performance of finishing steers. J. Anim. Sci. 2001, 79, 254–260. [Google Scholar] [CrossRef]
- Thomson, E.M.; Snoswell, A.M.; Clarke, P.L.; Thompson, G.E. Effect of cold exposure on mammary gland uptake of fat precursors and secretion of milk fat and carnitine in the goat. Q. J. Exp. Physiol. 1979, 64, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.V.N.; Singh, G.; Varshney, V.P. Antioxidants supplementation on acid base balance during heat stress in goats. Asian-australas. J. Anim. Sci. 2010, 23, 1462–1468. [Google Scholar] [CrossRef]
- Castro-Costa, A.; Salama, A.A.K.; Moll, X.; Aguiló, J.; Caja, G. Using wireless rumen sensors for evaluating the effects of diet and ambient temperature in non-lactating dairy goats. J. Dairy Sci. 2015, 98, 4646–4658. [Google Scholar] [CrossRef]
- Hamzaoui, S.; Salama, A.A.K.; Caja, G.; Albanell, E.; Such, X. Effects of supplementation with propylene glycol in heat-stressed dairy goats. J. Dairy Sci. 2014, 97 (Suppl. 1), 736–737. [Google Scholar]
- Carlson, D.B.; Litherland, N.B.; Dann, H.M.; Woodworth, J.C.; Drackley, J.K. Metabolic effects of abomasal L-carnitine infusion and feed restriction in lactating Holstein cows. J. Dairy Sci. 2006, 89, 4819–4834. [Google Scholar] [CrossRef]
- Pirestani, A.; Aghakhani, M. The effects of rumen-protected choline and L-carnitine supplementation in the transition period on reproduction, production, and some metabolic diseases of dairy cattle. J. Applied Anim. Res. 2018, 46, 435–440. [Google Scholar] [CrossRef]
- Chapa, A.M.; Fernandez, J.M.; White, T.W.; Bunting, L.D.; Gentry, L.R.; Lovejoy, J.C.; Owen, K.Q. Influence of dietary carnitine in growing sheep fed diets containing non-protein nitrogen. Small Rumin. Res. 2001, 40, 13–28. [Google Scholar] [CrossRef]
- Hajilou, M.; Dehghan-Banadaky, M.; Zali, A.; Rezayazdi, K. The effects of dietary L-carnitine and rumen protected choline on growth performance, carcass characteristics and blood and rumen metabolites of Holstein young bulls. J. Appl. Anim. Res. 2014, 42, 89–96. [Google Scholar] [CrossRef]
- Mendizabal, J.A.; Delfa, R.; Arana, A.; Eguinoa, P.; Purroy, A. Lipogenic activity in goats (Blancaceltibérica) with different body condition scores. Small Rumin. Res. 2007, 67, 285–290. [Google Scholar] [CrossRef]
- Owen, K.Q.; Ji, H.; Maxwell, C.V.; Nelssen, J.L.; Goodband, R.D.; Tokach, M.D.; Tremblay, G.C.; Koo, S.I. Dietary L-carnitine suppresses mitochondrial branched-chain keto acid dehydrogenase activity and enhances protein accretion and carcass characteristics of swine. J. Anim. Sci. 2001, 79, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Scholz, H.; Kecman, J.; Von Heimendahl, E.; Menn, F.; Ahrens, A. Application of protected L-carnitine in dairy cows during transition and high lactation period. Global J. Sci. Frontier Res. 2014, 14, 41–46. [Google Scholar]
- Citil, M.; Karapehlivan, M.; Erdogan, H.M.; Yucayurt, R.; Atakisi, E.; Atakisi, O. Effect of orally administered L-carnitine on selected biochemical indicators of lactating Tuj-ewes. Small Rumin. Res. 2009, 81, 174–177. [Google Scholar] [CrossRef]
- Srikandakumar, A.; Johnson, E.H. Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian milking Zebu cows. Trop. Anim. Health Prod. 2004, 36, 685–692. [Google Scholar] [CrossRef]
- Collier, R.J.; Dahl, G.E.; VanBaale, M.J. Major advances associated with environmental effects on dairy cattle. J. Dairy Sci. 2006, 89, 1244–1253. [Google Scholar] [CrossRef]
| Item | Total Mixed Ration |
|---|---|
| Component, % | |
| Dry matter | 88.2 |
| Organic matter | 88.1 |
| Crude protein | 17.7 |
| Ether extract | 1.79 |
| Neutral detergent fiber | 39.3 |
| Acid detergent fiber | 28.6 |
| Nutritive value1 | |
| UEL,2 /kg | 1.08 |
| UFL,3 /kg | 0.76 |
| PDI,4 g/kg | 94.2 |
| PDIA,5 g/kg | 45.5 |
| RPB,6 g/kg | 31.6 |
| Caabs, g/kg | 2.73 |
| Pabs, g/kg | 0.84 |
| L-Carnitine | TN | HS | SEM | Effect 1 (p =) | ||||
|---|---|---|---|---|---|---|---|---|
| CON | CAR | CON | CAR | T | C | T × C | ||
| Free | 20.42 | 61.23 | 18.42 | 50.50 | 4.06 | 0.13 | 0.01 | 0.30 |
| Acetyl-carnitine | 6.79 | 17.49 | 6.90 | 21.24 | 2.08 | 0.35 | 0.01 | 0.38 |
| Total | 26.78 | 78.30 | 24.90 | 71.30 | 4.32 | 0.32 | 0.01 | 0.56 |
| Item | TN | HS | SEM | Effect 1 (p =) | ||||
|---|---|---|---|---|---|---|---|---|
| CON | CAR | CON | CAR | T | C | T × C | ||
| Rectal temperature, °C | ||||||||
| 0800 h | 38.5 | 38.5 | 39.1c | 39.2c | 0.05 | 0.001 | 0.290 | 0.146 |
| 1200 h | 38.6 | 38.5 | 39.7b | 39.7b | 0.05 | 0.001 | 0.520 | 0.863 |
| 1700 h | 38.7 | 38.6 | 39.9a | 39.9a | 0.05 | 0.001 | 0.222 | 0.648 |
| Average | 38.6 | 38.5 | 39.6 | 39.6 | 0.04 | 0.001 | 0.762 | 0.464 |
| Respiratory rate, breaths/min | ||||||||
| 0800 h | 36 | 35 | 88b | 88b | 3.0 | 0.001 | 0.939 | 0.933 |
| 1200 h | 36 | 36 | 126a | 121a | 3.0 | 0.001 | 0.455 | 0.282 |
| 1700 h | 40 | 39 | 133a | 127a | 3.0 | 0.001 | 0.247 | 0.144 |
| Average | 37 | 37 | 116 | 112 | 2.4 | 0.001 | 0.430 | 0.542 |
| Item | TN | HS | SEM | Effect 1 (p =) | ||||
|---|---|---|---|---|---|---|---|---|
| CON | CAR | CON | CAR | T | C | T × C | ||
| DM intake, kg/d | 2.60 | 2.56 | 1.85 | 1.95 | 0.16 | 0.007 | 0.976 | 0.859 |
| Orts average particle size, mm | 5.31 | 7.92 | 3.99 | 5.02 | 1.10 | 0.057 | 0.238 | 0.674 |
| Milk yield, kg/d | 1.90 | 1.80 | 1.59 | 1.69 | 0.14 | 0.059 | 0.955 | 0.730 |
| FCM, L/d2 | 2.28 | 2.15 | 1.81 | 1.90 | 0.19 | 0.028 | 0.880 | 0.765 |
| Milk composition, % | ||||||||
| Total solids | 8.89 | 8.91 | 8.37 | 8.46 | 0.19 | 0.005 | 0.926 | 0.961 |
| Fat | 4.33 | 4.21 | 4.02 | 3.96 | 0.20 | 0.076 | 0.729 | 0.984 |
| Protein | 3.51 | 3.54 | 3.14 | 3.22 | 0.18 | 0.049 | 0.951 | 0.989 |
| Lactose | 4.64 | 4.65 | 4.47 | 4.47 | 0.06 | 0.006 | 0.991 | 0.788 |
| Fat yield, g/d | 85.8 | 80.7 | 66.5 | 69.3 | 7.8 | 0.015 | 0.864 | 0.844 |
| Protein yield, g/d | 69.1 | 64.6 | 50.3 | 54.5 | 6.0 | 0.008 | 0.991 | 0.737 |
| Somatic cell count, Log | 5.97 | 6.00 | 6.30 | 6.22 | 0.23 | 0.276 | 0.873 | 0.842 |
| Item | TN | HS | SEM | Effect 1 (p =) | ||||
|---|---|---|---|---|---|---|---|---|
| CON | CAR | CON | CAR | T | C | T × C | ||
| Na, mmol/L | 148.0 | 147.6 | 147.0 | 147.0 | 0.52 | 0.349 | 0.881 | 0.299 |
| K, mmol/L | 3.47 | 3.47 | 3.84 | 3.87 | 0.15 | 0.042 | 0.754 | 0.243 |
| Ionized Ca, mmol/L | 1.28 | 1.27 | 1.27 | 1.31 | 0.02 | 0.066 | 0.378 | 0.310 |
| Cl, mmol/L | 103.8 | 103.6 | 107.9 | 107.8 | 0.67 | 0.007 | 0.109 | 0.497 |
| TCO2, mmol/L | 25.7 | 25.9 | 20.9 | 21.2 | 0.69 | 0.009 | 0.678 | 0.871 |
| Anion gap | 23.0 | 22.7 | 22.8 | 23.1 | 0.54 | 0.707 | 0.247 | 0.869 |
| Hematocrit, % PCV | 17.6 | 18.6 | 17.7 | 18.4 | 0.92 | 0.770 | 0.609 | 0.899 |
| Hemoglobin, g/dL | 5.98 | 6.31 | 6.05 | 6.26 | 0.31 | 0.778 | 0.599 | 0.891 |
| Glucose, mg/dL | 59.1 | 59.6 | 59.9 | 58.6 | 1.71 | 0.743 | 0.962 | 0.733 |
| Urea, mg/dL | 23.2 | 24.5 | 18.3 | 19.8 | 1.85 | 0.008 | 0.710 | 0.971 |
| Creatinine, mg/dL | 0.47 | 0.52 | 0.57 | 0.54 | 0.02 | 0.007 | 0.906 | 0.211 |
| Triglycerides, mg/dL | 17.6 | 18.0 | 17.1 | 17.1 | 1.3 | 0.878 | 0.660 | 0.501 |
| Cholesterol, mg/dL | 74.7 | 76.2 | 85.5 | 79.9 | 5.8 | 0.219 | 0.911 | 0.733 |
| non-esterified fatty acids, mmol/L | 0.08 | 0.13 | 0.15 | 0.12 | 0.02 | 0.291 | 0.333 | 0.276 |
| ß-hydroxybutyrate, mmol/L | 0.72 | 0.70 | 0.80 | 0.85 | 0.11 | 0.187 | 0.972 | 0.932 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehaba, N.; Salama, A.A.K.; Such, X.; Albanell, E.; Caja, G. Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation. Animals 2019, 9, 567. https://doi.org/10.3390/ani9080567
Mehaba N, Salama AAK, Such X, Albanell E, Caja G. Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation. Animals. 2019; 9(8):567. https://doi.org/10.3390/ani9080567
Chicago/Turabian StyleMehaba, Nabil, Ahmed A. K. Salama, Xavier Such, Elena Albanell, and Gerardo Caja. 2019. "Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation" Animals 9, no. 8: 567. https://doi.org/10.3390/ani9080567
APA StyleMehaba, N., Salama, A. A. K., Such, X., Albanell, E., & Caja, G. (2019). Lactational Responses of Heat-Stressed Dairy Goats to Dietary L-Carnitine Supplementation. Animals, 9(8), 567. https://doi.org/10.3390/ani9080567





