Methane Emissions and the Use of Desmanthus in Beef Cattle Production in Northern Australia
Abstract
Simple Summary
Abstract
1. Introduction
2. Carbon Footprint from the Beef Industry in Queensland
2.1. The Australian Beef Cattle Market
2.2. The Different Sectors Included in the Carbon Footprint of the Beef Industry in Queensland
2.3. The Principal Causes Inducing Enteric Methane Emissions
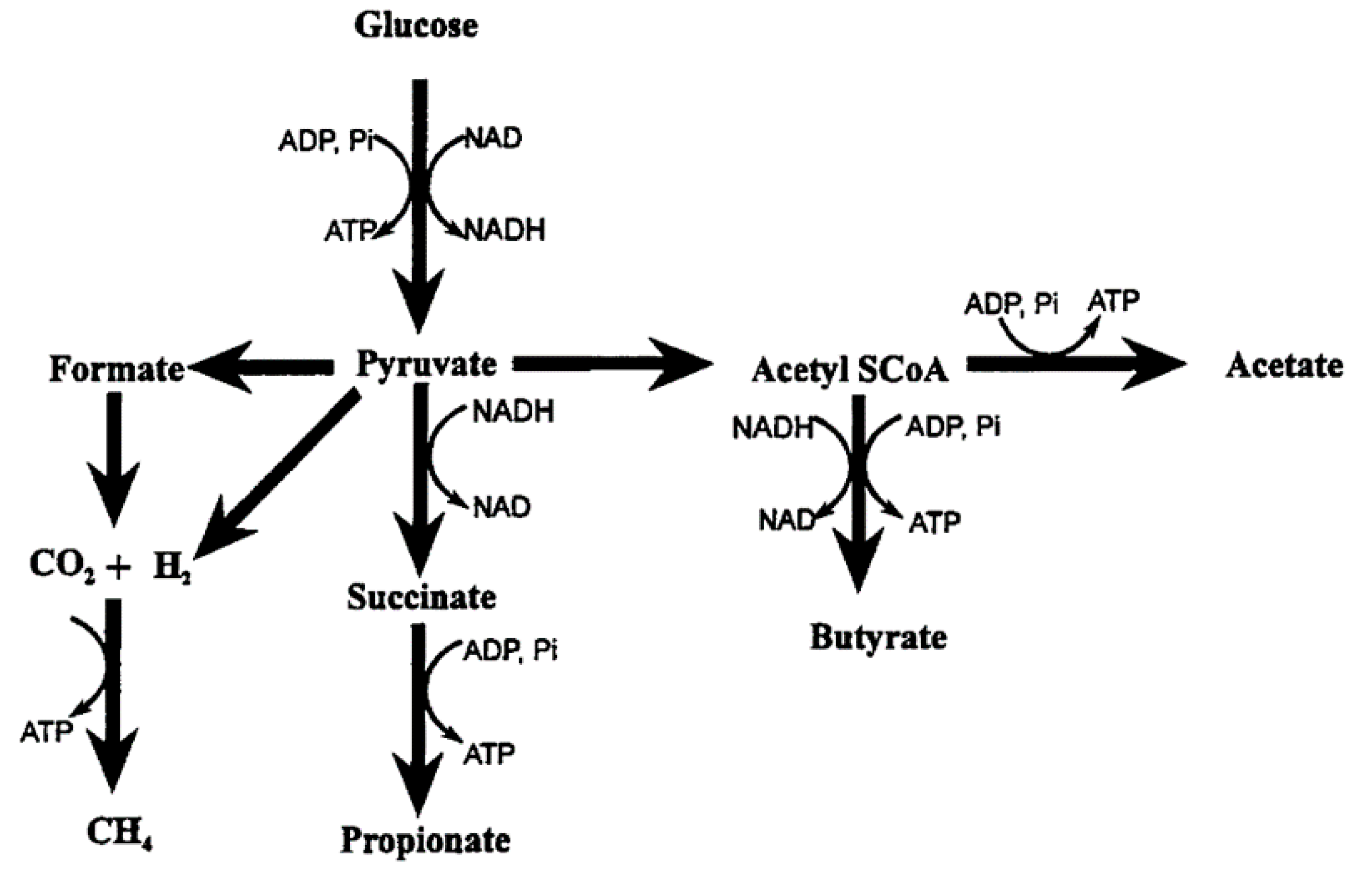
2.3.1. Rumen Microbial Fermentation
2.3.2. Low Animal Performance Increases Methane Production
2.3.3. Northern Australian Forage Diet Influences Rumen Microbiome and Methane Production
3. Mitigation Techniques against Methane Emission
3.1. The Use of Chemicals for Rumen Manipulation to Reduce Methane Production
3.1.1. The Use of Chemicals to Control Protozoa, the Main Hydrogen Producer
Defaunation
Ionophores
3.1.2. The Use of Chemicals to Control the Methanogen Numbers
3.2. The Use of Diet Manipulation to Reduce Methane Production
3.2.1. The Use of Concentrates to Reduce Methane Production
3.2.2. The Use of Legumes to Reduce Methane Production
4. The Use of Legumes to Increase Pasture Quality and Animal Performance in Northern Australia
4.1. The Use of Legumes to Increase Pasture quality
4.1.1. Ability to Fix Nitrogen
4.1.2. Ability to Extract Moisture and Nutrients from the Soil
4.2. The Use of Legumes to Increase Animal Productivity
4.3. Northern Australian Legumes
5. Desmanthus as a Potential Pasture Species for Ruminants
5.1. Performance Characteristics of Desmanthus
5.2. Desmanthus as a Potential Pasture to Reduce Methane Production
5.3. Desmanthus as a Potential Pasture to Increase Animal Production
6. Implications, Future Research and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change. Climate change 2007: The physical science basis. Agenda 2007, 6, 333. [Google Scholar]
- Australia’s National Greenhouse Accounts. Quarterly Update of Australia’s National Greenhouse Gas Inventory: June 2018; Australian Government Department of the Environment and Energy: Canberra, Australia, 2018. Available online: http://www.environment.gov.au/climate-change/climate-science-data/greenhouse-gas-measurement/publications/quarterly-update-australias-national-greenhouse-gas-inventory-june-2018 (accessed on 17 March 2019).
- Australian Greenhouse Emissions Information System. In National Greenhouse Gas Inventory—Kyoto Protocol Classifications; Australian Government Department of the Environment and Energy: Canberra, Australia, 2017. Available online: http://ageis.climatechange.gov.au/ (accessed on 26 July 2019).
- United Nations. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. 2017. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2017.html (accessed on 17 March 2019).
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Wright, I.A.; Tarawali, S.; Blümmel, M.; Gerard, B.; Teufel, N.; Herrero, M. Integrating crops and livestock in subtropical agricultural systems. J. Sci. Food Agric. 2012, 92, 1010–1015. [Google Scholar] [CrossRef]
- Winks, L. Townsville stylo research at Swan’s Lagoon. Trop. Grassl. 1973, 7, 201. [Google Scholar]
- Hall, T.J.; Walker, R.W. Pasture legume adaptation to six environments of the seasonally dry tropics of north Queensland. Trop. Grassl. 2005, 39, 182–196. [Google Scholar]
- Gardiner, C.P. Developing and commercializing new pasture legumes for clay soils in the semi-arid rangelands of northern Australia: The new Desmanthus cultivars JCU 1–5 and the Progardes story. In Tropical Forage Legumes: Harnessing the Potential of Desmanthus and Other Genera for Heavy Clay Soils; Lazier, J.R., Ahmad, N., Eds.; CABI: Wallingford, UK, 2016; pp. 283–304. [Google Scholar]
- MLA. Fast Facts Australia’s Beef Industry. 2018. Available online: https://www.mla.com.au/globalassets/mla-corporate/prices--markets/documents/trends--analysis/fast-facts--maps/mla_beef-fast-facts-2018.pdf (accessed on 17 March 2019).
- Commonwealth of Australia. Emissions Reduction Fund-Overview. 2019. Available online: http://www.environment.gov.au/system/files/resources/cef73480-9dea-4436-bb15-4882345ae9a5/files/erf-factsheet-overview.pdf (accessed on 17 March 2019).
- Bray, S.; Willcocks, J. Net Carbon Position of the Queensland Beef Industry; Queensland Department of Employment, Economic Development and Innovation: Brisbane, Australia, 2009. [Google Scholar]
- Eady, S.; Viner, J.; MacDonnell, J. On-farm greenhouse gas emissions and water use: Case studies in the Queensland beef industry. Anim. Prod. Sci. 2011, 51, 667–681. [Google Scholar] [CrossRef]
- Morgavi, D.; Forano, E.; Martin, C.; Newbold, C. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Hess, M. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol 2018, 9, 2161. [Google Scholar] [CrossRef]
- Czerkawski, J.W. An Introduction to Rumen Studies; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Immig, I. The rumen and hindgut as source of ruminant methanogenesis. Environ. Monit. Assess. 1996, 42, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Charmley, E.; Williams, S.R.O.; Moate, P.J.; Hegarty, R.S.; Herd, R.M.; Oddy, V.H.; Hannah, M.C. A universal equation to predict methane production of forage-fed cattle in Australia. Anim. Prod. Sci. 2016, 56, 169–180. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, O.; Leip, A.; Dong, H.; MacDonald, J.D.; Alfredo, C.; Bravo, G.; Widiawati, Y. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/index.html (accessed on 26 July 2019).
- Charmley, E.; Stephens, M.L.; Kennedy, P.M. Predicting livestock productivity and methane emissions in northern Australia: Development of a bio-economic modelling approach. Aust. J. Exp. Agric. 2008, 48, 109–113. [Google Scholar] [CrossRef]
- Arthur, P.; Archer, J.; Johnston, D.; Herd, R.; Richardson, E.; Parnell, P. Genetic and phenotypic variance and covariance components for feed intake, feed efficiency, and other postweaning traits in Angus cattle. J. Anim. Sci. 2001, 79, 2805–2811. [Google Scholar] [CrossRef] [PubMed]
- Exton, S.; Herd, R.; Davies, L.; Archer, J.; Arthur, P. Commerical Benefits to the Beef Industry from Genetic Improvement in Net Feed Efficiency. Asian Australas. J. Anim. 2000, 13, 338–341. [Google Scholar]
- Eady, S.J. Undertaking a Life Cycle Assessment for the Livestock Export Trade; Meat & Livestock Australia Limited: North Sydney, Australia, 2011. [Google Scholar]
- Pengelly, B.C.; Conway, M.J. Pastures on cropping soils: Which tropical pasture legume to use? Trop. Grassl. 2000, 34, 162–168. [Google Scholar]
- Tothill, J.C.; Gillies, C. The Pasture Lands of Northern Australia: Their Condition, Productivity and Sustainability; Tropical Grasslands Society of Australia: St Lucia, Australia, 1992. [Google Scholar]
- Hattersley, P. The distribution of C 3 and C 4 grasses in Australia in relation to climate. Oecologia 1983, 57, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Al Jassim, R.; Gaughan, J.; Tomkins, N. Effect of feeding forage characteristic of wet-or dry-season tropical C4 grass in northern Australia, on methane production, intake and rumen outflow rates in Bos indicus steers. Anim. Prod. Sci. 2017, 57, 2033–2041. [Google Scholar] [CrossRef]
- Hennessy, D.; Williamson, P.; Nolan, J.; Kempton, T.; Leng, R. The roles of energy-or protein-rich supplements in the subtropics for young cattle consuming basal diets that are low in digestible energy and protein. J. Agric. Sci.-Camb. 1983, 100, 657–666. [Google Scholar] [CrossRef]
- McLennan, S.R. Developing Profitable Strategies for Increasing Growth Rates of Cattle Grazing Tropical Pastures; Meat & Livestock Australia Limited: North Sydney, Australia, 1997; Project DAQ, 100. [Google Scholar]
- Shaw, N.; Bisset, W. Characteristics of a bunch spear grass (Heteropogon contortus (L). BEAUV.) pasture grazed by cattle in subtropical Queensland. Aust. J. Agric. Res. 1955, 6, 539–552. [Google Scholar] [CrossRef]
- Poppi, D.P.; McLennan, S.R. Nutritional research to meet future challenges. Anim. Prod. Sci. 2010, 50, 329–338. [Google Scholar] [CrossRef]
- Poppi, D.P.; McLennan, S.R. Protein and energy utilization by ruminants at pasture. J. Anim. Sci. 1995, 73, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Bortolussi, G.; McIvor, J.G.; Hodgkinson, J.; Coffey, S.; Holmes, C. The northern Australian beef industry, a snapshot. 3. Annual liveweight gains from pasture based systems. Aust. J. Agric. Res. 2005, 45, 1093–1108. [Google Scholar] [CrossRef]
- Poppi, D.P.; McLennan, S.R. Nutrition R&D: Past, present and future. In Proceedings of the Northern Beef Update Research Conference, Southbank Convention Centre, Townsville, Australia, 21–22 March 2007; Pattie, B., Restall, B., Eds.; [Google Scholar]
- Archimède, H.; Eugène, M.; Magdeleine, C.M.; Boval, M.; Martin, C.; Morgavi, D.; Doreau, M. Comparison of methane production between C3 and C4 grasses and legumes. Anim. Feed Sci. Technol. 2011, 166, 59–64. [Google Scholar] [CrossRef]
- Benchaar, C.; Pomar, C.; Chiquette, J. Evaluation of dietary strategies to reduce methane production in ruminants: A modelling approach. Can. J. Anim. Sci. 2001, 81, 563–574. [Google Scholar] [CrossRef]
- Joblin, K. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 1999, 50, 1307–1314. [Google Scholar] [CrossRef]
- Broucek, J. Options to methane production abatement in ruminants: A review. J. Anim. Plant Sci. 2018, 28, 348–364. [Google Scholar]
- Dohme, F.; Machmüller, A.; Estermann, B.; Pfister, P.; Wasserfallen, A.; Kreuzer, M. The role of the rumen ciliate protozoa for methane suppression caused by coconut oil. Lett. Appl. Microbiol. 1999, 29, 187–192. [Google Scholar] [CrossRef]
- Mathison, G.; Okine, E.; McAllister, T.; Dong, Y.; Galbraith, J.; Dmytruk, O. Reducing methane emissions from ruminant animals. J. Appl. Anim. Res. 1998, 14, 1–28. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Cheng, Y.-F.; Zhu, W.-Y.; Zeshan, B. Mitigation of ruminant methane production: current strategies, constraints and future options. World J. Microb. Biot. 2008, 24, 2747–2755. [Google Scholar] [CrossRef]
- Guan, H.; Wittenberg, K.; Ominski, K.; Krause, D. Efficacy of ionophores in cattle diets for mitigation of enteric methane. J. Anim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, W.; Wittenberg, K.; Corrigan, D. Methane production by steers on pasture. Can. J. Anim. Sci. 1997, 77, 519–524. [Google Scholar] [CrossRef]
- Russell, J.B.; Houlihan, A.J. Ionophore resistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol. Rev. 2003, 27, 65–74. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Veterinary Medicines and Inspections. Committee for Medical Products for Veterinary Use. 2007. Available online: https://www.ema.europa.eu/documents/mrl-report/monensin-cattle-including-dairy-cows-summary-report-committee-veterinary-medicinal-products_en.pdf (accessed on 17 March 2019).
- Newbold, C.; McIntosh, F.; Wallace, R. Changes in the microbial population of a rumen-simulating fermenter in response to yeast culture. Can. J. Anim. Sci. 1998, 78, 241–244. [Google Scholar] [CrossRef]
- McGinn, S.; Beauchemin, K.; Coates, T.; Colombatto, D. Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J. Anim. Sci. 2004, 82, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Morgavi, D.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, G.; Denman, S.E.; Yang, C.; Cheung, J.; Mitsumori, M.; McSweeney, C.S. Methane inhibition alters the microbial community, hydrogen flow, and fermentation response in the rumen of cattle. Front. Microbiol. 2016, 7, 1122. [Google Scholar] [CrossRef] [PubMed]
- McCrabb, G.; Berger, K.; Magner, T.; May, C.; Hunter, R. Inhibiting methane production in Brahman cattle by dietary supplementation with a novel compound and the effects on growth. Aust. J. Agric. Res. 1997, 48, 323–329. [Google Scholar] [CrossRef]
- Van Nevel, C.; Demeyer, D. Control of rumen methanogenesis. Environ. Monit. Assess. 1996, 42, 73–97. [Google Scholar] [CrossRef]
- Martinez Fernandez, G.; Duval, S.M.; Kindermann, M.; Schirra, H.J.; Denman, S.E.; McSweeney, C.S. 3-NOP vs. Halogenated compound: Methane production, ruminal fermentation and microbial community response in forage fed cattle. Front. Microbiol. 2018, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- Supplementary Feeding. 2019. Available online: https://www.mla.com.au/Research-and-development/Feeding-finishing-nutrition/Supplementary-feeding (accessed on 17 March 2019).
- Department of Agriculture Forestry and Fisheries. Supplementation Feeding: Some Basic Considerations. 2019. Available online: https://www.daf.qld.gov.au/business-priorities/agriculture/disaster-recovery/drought/managing/supplementation-feeding-considerations (accessed on 17 March 2019).
- McLennan, S.R.; Dunster, P.; O’rourke, P.; Murphy, G. Comparison of dry season urea supplements containing salt, sulfur or molasses for steers grazing native pasture in the dry tropics of northern Queensland. Aust. J. Exp. Agric. 1981, 21, 457–463. [Google Scholar] [CrossRef]
- Purnomoadi, A.; Rianto, E.; Kurihara, M. Reduction of methane production from Ongole Crossbreed cattle in Indonesia by increasing the concentrate feeding frequency. In Greenhouse Gas Control Technologies; Elsevier: Amsterdam, The Netherlands, 2005; Volume 7, pp. 2513–2516. [Google Scholar]
- Beauchemin, K.A.; Kreuzer, M.; O’mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- McSweeney, C.; Palmer, B.; McNeill, D.; Krause, D. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Techol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Woodward, S.; Waghorn, G.; Thomson, N. Supplementing dairy cows with oils to improve performance and reduce methane-does it work? In Proceedings of the New Zealand Society of Animal Production, Napier, New Zealand, 26–28 June 2006; Volume 66, pp. 176–181. [Google Scholar]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar]
- Hess, H.D.; Tiemann, T.T.; Noto, F.; Carulla, J.E.; Kreuzer, M. Strategic use of tannins as means to limit methane emission from ruminant livestock. In International Congress Series; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1293, pp. 164–167. [Google Scholar]
- Tiemann, T.T.; Lascano, C.E.; Wettstein, H.-R.; Mayer, A.C.; Kreuzer, M.; Hess, H.D. Effect of the tropical tannin-rich shrub legumes Calliandra calothyrsus and Flemingia macrophylla on methane emission and nitrogen and energy balance in growing lambs. Animal 2008, 2, 790–799. [Google Scholar] [CrossRef]
- Tan, H.; Sieo, C.; Abdullah, N.; Liang, J.; Huang, X.; Ho, Y. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim. Feed Sci. Techol. 2011, 169, 185–193. [Google Scholar] [CrossRef]
- Brewbaker, J.L. Leucaena: A multipurpose tree genus for tropical agroforestry. In Agroforestry: A Decade of Development; Steppler, H.A., Ramachandran Nair, P.K., Eds.; International Council for Research in Agroforestry: Nairobi, Kenya, 1987; pp. 289–323. [Google Scholar]
- Dalzell, S.; Burnett, D.; Dowsett, J.; Forbes, V.; Shelton, H. Prevalence of mimosine and DHP toxicity in cattle grazing Leucaena leucocephala pastures in Queensland, Australia. Anim. Prod. Sci. 2012, 52, 365–372. [Google Scholar] [CrossRef]
- Durmic, Z.; Ramírez-Restrepo, C.A.; Gardiner, C.; O’Neill, C.J.; Hussein, E.; Vercoe, P.E. Differences in the nutrient concentrations, in vitro methanogenic potential and other fermentative traits of tropical grasses and legumes for beef production systems in northern Australia. J. Sci. Food Agric. 2017, 97, 4075–4086. [Google Scholar] [CrossRef] [PubMed]
- Soltan, Y.A.; Morsy, A.S.; Sallam, S.M.; Lucas, R.C.; Louvandini, H.; Kreuzer, M.; Abdalla, A.L. Contribution of condensed tannins and mimosine to the methane mitigation caused by feeding Leucaena leucocephala. Arch. Anim. Nutr. 2013, 67, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.T.; McSweeney, C.; Tomkins, N.W.; Eckard, R.J. Improving greenhouse gas emissions intensities of subtropical and tropical beef farming systems using Leucaena leucocephala. Agric. Syst. 2015, 136, 138–146. [Google Scholar] [CrossRef]
- Soltan, Y.; Morsy, A.; Sallam, S.; Louvandini, H.; Abdalla, A. Comparative in vitro evaluation of forage legumes (prosopis, acacia, atriplex, and leucaena) on ruminal fermentation and methanogenesis. J. Anim. Feed Sci. 2012, 21, 759–772. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the condensed tannins of sainfoin (Onobrychis viciifolia Scop.) with fraction 1 leaf protein and with submaxillary mucoprotein, and their reversal by polyethylene glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- McSweeney, C.; Palmer, B.; Bunch, R.; Krause, D. In vitro quality assessment of tannin-containing tropical shrub legumes: Protein and fibre digestion. Anim. Feed Sci. Techol. 1999, 82, 227–241. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, K.; McGinn, S.; Martinez, T.; McAllister, T. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Field, J.; Kortekaas, S.; Lettinga, G. The tannin theory of methanogenic toxicity. Biol. Waste 1989, 29, 241–262. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Techol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Murdiati, T.B.; McSweeney, C.S.; Lowry, J. Complexing of toxic hydrolysable tannins of yellow-wood (Terminalia oblongata) and harendong (Clidemia hirta) with reactive substances: An approach to preventing toxicity. J. Appl. Toxicol. 1991, 11, 333–338. [Google Scholar] [CrossRef]
- Makkar, H.; Francis, G.; Becker, K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Cheng, K.-J. A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can. J. Anim. Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef]
- Vandermeulen, S.; Singh, S.; Ramírez-Restrepo, C.A.; Kinley, R.D.; Gardiner, C.P.; Holtum, J.A.; Bindelle, J. In vitro assessment of ruminal fermentation, digestibility and methane production of three species of Desmanthus for application in northern Australian grazing systems. Crop Pasture Sci. 2018, 69, 797–807. [Google Scholar] [CrossRef]
- Collins, J.; Gardiner, C.P.; Kempe, N.; Hannah, I. Successful Pasture Development at Cungelella: A grazier, a researcher and a seed company’s perspective. In Proceedings of the Northern Beef Research Update Conference, Rockhampton, Australia, 15–18 August 2016; North Australia Beef Research Council: Gympie, Australia, 2016. [Google Scholar]
- Gardiner, C.P.; Parker, A. Steer liveweight gains on ProgardesTM/buffel pastures in Qld. In Proceedings of the 2nd Australian and New Zealand Societies of Animal Production Joint Conference, Lincoln University, Christchurch, New Zealand, 2–5 July 2012. [Google Scholar]
- Ngo, T.; Parker, A.; Gardiner, C.P. The effects of diet preference on feed intake, digestibility and nitrogen balance of sheep given Flinders grass (Iseilema spp.) hay and/or Desmanthus leptophyllus cv. JCU 1 ad libitum. In Proceedings of the TropAg2017: International Tropical Agriculture Conference, Brisbane, Australia, 20–22 November 2017. [Google Scholar]
- Rangel, J.; Gardiner, C.P. Stimulation of wool growth by Desmanthus spp. as a supplement to a diet of Mitchell grass hay. Trop. Grassl. 2009, 43, 106–111. [Google Scholar]
- Gardiner, C.; Kempe, N.; Hannah, I. New pasture legumes for clay soils in dry environments. North. Muster 2012, 30, 27–28. [Google Scholar]
- Wetselaar, R. Estimation of nitrogen fixation by four legumes in a dry monsoonal area of north-western Australia. Aust. J. Exp. Agric. 1967, 7, 518–522. [Google Scholar] [CrossRef]
- Jones, R.; Probert, M.; Dalgliesh, N.; McCown, R. Nitrogen inputs from a pasture legume in rotations with cereals in the semi-arid tropics of northern Australia: Experimentation and modelling on a clay loam soil. Aust. J. Exp. Agric. 1996, 36, 985–994. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [PubMed]
- Peck, G.; Buck, S.; Hoffmann, A.; Holloway, C.; Johnson, B.; Lawrence, D.; Paton, C. Review of Productivity Decline in Sown Grass Pastures; Meat & Livestock Australia Limited: North Sydney, Australia, 2011; p. 86. [Google Scholar]
- Sturz, A.; Christie, B.; Matheson, B.; Nowak, J. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol. Fertil. Soils 1997, 25, 13–19. [Google Scholar] [CrossRef]
- Rao, I.M.; Peters, M.; Castro, A.; Schultze-Kraft, R.; White, D.; Fisher, M.; Rudel, T. LivestockPlus: The sustainable intensification of forage-based agricultural systems to improve livelihoods and ecosystem services in the tropics. Trop. Grassl. 2015, 3, 59–82. [Google Scholar] [CrossRef]
- Peters, M.; Herrero, M.; Fisher, M.; Erb, K.-H.; Rao, I.; Subbarao, G.V.; Searchinger, T. Challenges and opportunities for improving eco-efficiency of tropical forage-based systems to mitigate greenhouse gas emissions. Trop. Grassl. 2013, 1, 156–167. [Google Scholar]
- Mero, R.N.; Udén, P. Promising tropical grasses and legumes as feed resources in Central Tanzania V. Effect of supplementing Cenchrus ciliaris hay with leaves from four legumes on intake and digestibility by growing Mpwapwa bulls. Anim. Feed Sci. Techol. 1998, 70, 111–122. [Google Scholar] [CrossRef]
- Bowen, M.; Chudleigh, F.; Buck, S.; Hopkins, K. Productivity and profitability of forage options for beef production in the subtropics of northern Australia. Anim. Prod. Sci. 2016, 58, 332–342. [Google Scholar] [CrossRef]
- Gardiner, C.P.; Swan, S.J. Abandoned pasture legumes offer potential economic and environmental benefits in semiarid clay soil rangelands. In Proceedings of the 15th Biennial Conference Proceedings ‘A Climate of Change in the Rangelands’, Charters Towers, Australia, 28 September–2 October 2008. [Google Scholar]
- CSIRO. Nutrient Requirements of Domesticated Ruminants; CSIRO publishing: Clayton, VIC, Australia, 2007. [Google Scholar]
- Clements, R.J.; Henzell, E.F. Pasture research and development in northern Australia: An ongoing scientific adventure. Trop. Grassl. 2010, 44, 221–230. [Google Scholar]
- Jones, R.; Lowry, J. Australian goats detoxify the goitrogen 3-hydroxy-4 (1H) pyridone (DHP) after rumen infusion from an Indonesian goat. Experientia 1984, 40, 1435–1436. [Google Scholar] [CrossRef]
- Pengelly, B.C.; Liu, C.J. Genetic relationships and variation in the tropical mimosoid legume Desmanthus assessed by random amplified polymorphic DNA. Genet. Resour. Crop Evol. 2001, 48, 93–101. [Google Scholar] [CrossRef]
- Luckow, M. Monograph of Desmanthus (leguminosae-mimosoideae). In Systematic Botany Monographs; Anderson, C., Ed.; The American Society of Plant Taxonomists: Laramie, WY, USA, 1993; Volume 38, pp. 1–166. [Google Scholar]
- Tropical Forages. Desmanthus Leptophyllus. Available online: http://www.tropicalforages.info/key/forages/Media/Html/entities/desmanthus_leptophyllus.htm (accessed on 26 July 2019).
- State of the Environment. Average Rainfall. 2017. Available online: https://www.stateoftheenvironment.des.qld.gov.au/2015/climate/climate-observations/average-rainfall (accessed on 26 July 2019).
- Jones, R.; Brandon, N. Persistence and productivity of eight accessions of Desmanthus virgatus under a range of grazing pressures in subtropical Queensland. Trop. Grassl. 1998, 32, 145–152. [Google Scholar]
- Department of Agriculture Forestry and Fisheries. Pasture Management for the Inland Burnett, 3rd ed.; DAFF: Brisbane, Australia, 2014.
- Gardiner, C.P.; Kempe, N.; Hannah, I.; McDonald, J. PROGARDES TM: A legume for tropical/subtropical semi-arid clay soils. Trop. Grassl. 2013, 1, 78–80. [Google Scholar] [CrossRef]
- Pandrangi, S.; Elwell, M.; Anantheswaran, R.; LaBorde, L. Efficacy of sulfuric acid scarification and disinfectant treatments in eliminating Escherichia coli O157: H7 from alfalfa seeds prior to sprouting. J. Food Sci. 2003, 68, 613–617. [Google Scholar] [CrossRef]
- Clem, B. Desmanthus. 2009. Available online: https://keys.lucidcentral.org/keys/v3/pastures/Html/Desmanthus.htm (accessed on 26 July 2019).
- Gardiner, C.; Wright, C.; Coventry, M. The germination, passage and viability of Desmanthus virgatus (L.) Willenow seed through sheep and its implication for dispersal in tropical rangelands. In Proceedings of the 16th Australian Society of Agronomy Conference, Armidale, NSW, Australia, 14–18 October 2012. [Google Scholar]
- Kanani, J.; Lukefahr, S.; Stanko, R. Evaluation of tropical forage legumes (Medicago sativa, Dolichos lablab, Leucaena leucocephala and Desmanthus bicornutus) for growing goats. Small Rumin. Res. 2006, 65, 1–7. [Google Scholar] [CrossRef]

| Animal Nutrient Needs | Supplement | Critical Season |
|---|---|---|
| Energy | Grains, molasses | Dry |
| Protein | Urea | Dry |
| Roughage | Silage, hay | Dry and wet |
| Minerals | Phosphorus | Wet |
| Desmanthus Species | Experiment | Dosage | Control Dosage | Effects | References |
|---|---|---|---|---|---|
| D. bicornutus, D. leptophyllus or D. virgatus | In vitro (Brahman steers rumen fluid) | 1 g Desmanthus + 125 mL rumen fluid | 1 g Rhodes grass forage + 125 mL rumen fluid | ↓ ME, VFA | [81] |
| D. leptophyllus | In vitro (sheep rumen fluid) | 10 mL of 1:1.3 or 1:1.5 dilution of inoculum:buffer + 0.1 g Desmanthus | 10 mL of 1:1.3 or 1:1.5 dilution of inoculum:buffer + 0.1 g grass | ↓ ME, VFA | [68] |
| Progardes™ | Steers | Paddock with buffel grass and Progardes™ | Paddock with buffel grass | ↑ LW | [83] |
| Progardes™ | Steers | Paddock Progardes™ (7 plants/m2) and buffel grass | Paddock with buffel grass | ↑ LW | [82] |
| D. bicornutus | Goats | 40% Desmanthus in the diet + alfalfa | Alfalfa | ↓ LW | [110] |
| D. virgatus, D. pubescens or D. leptophyllus | Sheep | 30% Desmanthus + Mitchell grass hay | Mitchell grass | ↑ LW, ↑ Intake, ↑ Wool growth | [85] |
| D. leptophyllus | Sheep | Ad libitum flinders grass hay + D. leptophyllusor either D. leptophyllus or flinders grass hay | ↑ LW, ↑ positive N balance with Desmanthus | [84] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suybeng, B.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Methane Emissions and the Use of Desmanthus in Beef Cattle Production in Northern Australia. Animals 2019, 9, 542. https://doi.org/10.3390/ani9080542
Suybeng B, Charmley E, Gardiner CP, Malau-Aduli BS, Malau-Aduli AEO. Methane Emissions and the Use of Desmanthus in Beef Cattle Production in Northern Australia. Animals. 2019; 9(8):542. https://doi.org/10.3390/ani9080542
Chicago/Turabian StyleSuybeng, Bénédicte, Edward Charmley, Christopher P. Gardiner, Bunmi S. Malau-Aduli, and Aduli E. O. Malau-Aduli. 2019. "Methane Emissions and the Use of Desmanthus in Beef Cattle Production in Northern Australia" Animals 9, no. 8: 542. https://doi.org/10.3390/ani9080542
APA StyleSuybeng, B., Charmley, E., Gardiner, C. P., Malau-Aduli, B. S., & Malau-Aduli, A. E. O. (2019). Methane Emissions and the Use of Desmanthus in Beef Cattle Production in Northern Australia. Animals, 9(8), 542. https://doi.org/10.3390/ani9080542





