Mirrors Improve Rabbit Natural Behavior in a Free-Range Breeding System
Abstract
Simple Summary
Abstract
1. Introduction
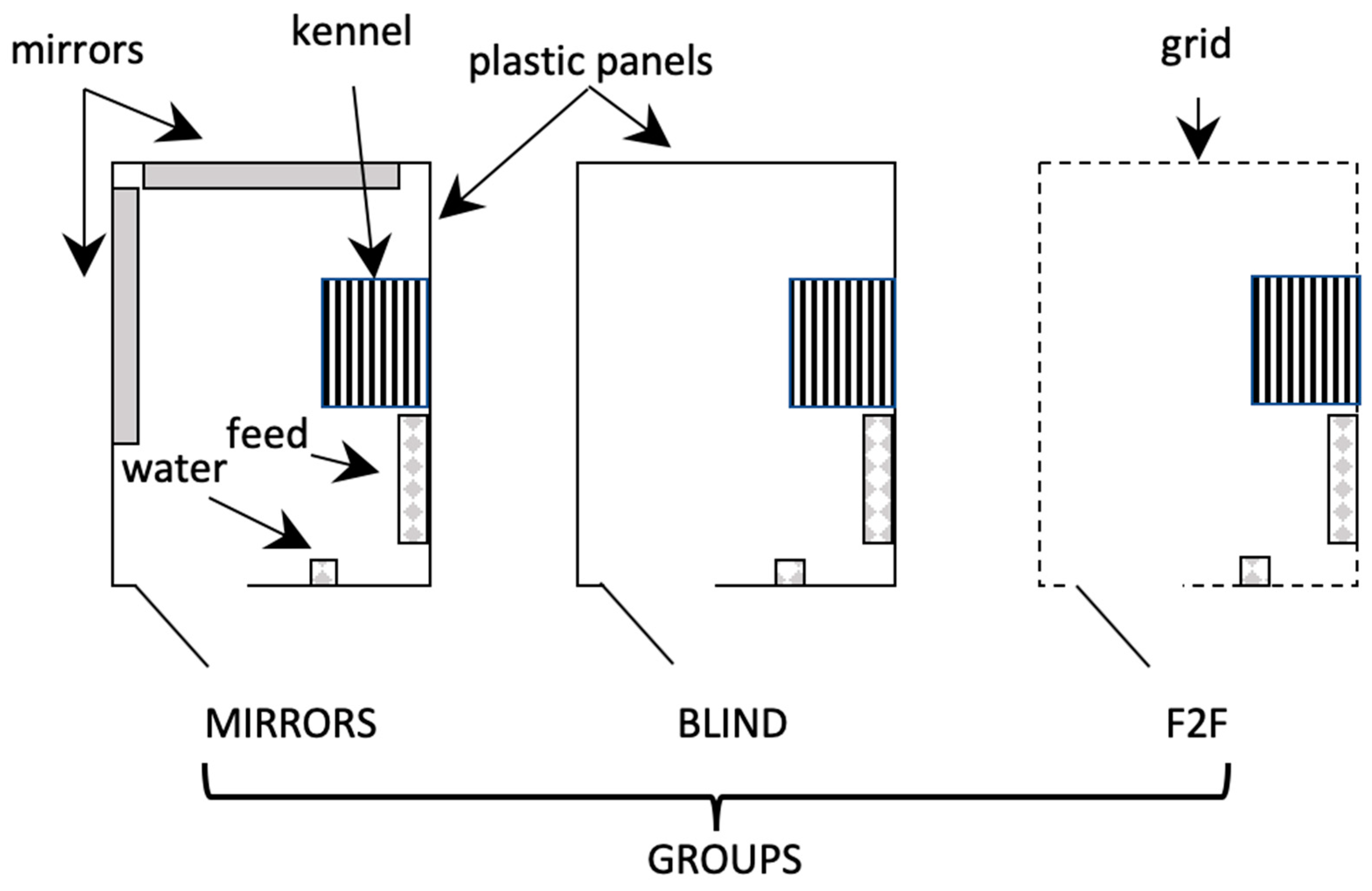
2. Materials and Methods
2.1. Animals
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Keskin, S.; Kor, A.; Karaca, S. Evaluation of sensory characteristics of sheep and goat meat by Procrustes Analysis. Czech. J. Anim. Sci. 2012, 57, 516–521. [Google Scholar] [CrossRef]
- Trocino, A.; Filiou, E.; Tazzoli, M.; Bertotto, D.; Negrato, E.; Xiccato, G. Behaviour and welfare of growing rabbits housed in cages and pens. Livest. Sci. 2014, 167, 305–314. [Google Scholar] [CrossRef]
- Five Freedoms. Farm Animal Welfare Council (FAWC), the National Archives. 2012. Available online: http://webarchive.nationalarchives.gov.uk/20121007104210/http:/www.fawc.org.uk/freedoms.htm (accessed on 11 July 2019).
- Lawrence, A.; Terlouw, C. A review of behavioral factors involved in the development and continued performance of stereotypic behavior in pigs. J. Anim. Sci. 1993, 71, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Trocino, A.; Xiccato, G. Animal welfare in reared rabbits: a review with emphasis on housing systems. World Rabbit Sci. 2006, 14, 77–93. [Google Scholar] [CrossRef]
- Maertens, L.; van Herck, A. Performance of weaned rabbits raised in pens or in classical cages: First results. World Rabbit Sci. 2000, 8, 435–440. [Google Scholar]
- Dalle Zotte, A.; Princz, Z.; Matics, Z.; Gerencsér, Z.; Metzger, S.; Szendrő, Z. Rabbit preference for cages and pens with or without mirrors. Appl. Anim. Behav. Sci. 2009, 116, 273–278. [Google Scholar] [CrossRef]
- Princz, Z.; Dalle Zotte, A.; Radnai, I.; Bíró-Németh, E.; Matics, Z.; Gerencsér, Z.; Nagy, I.; Szendrő, Z. Behaviour of growing rabbits under various housing conditions. Appl. Anim. Behav. Sci. 2008, 111, 342–356. [Google Scholar] [CrossRef]
- Matics, Z.; Farkas, T.P.; Dal Bosco, A.; Szendrő, Z.; Filiou, E.; Nagy, I.; Odermatt, M.; Paci, G.; Gerencsér, Z. Comparison of pens without and with multilevel platforms for growing rabbits. Ital. J. Anim. Sci. 2018, 17, 469–476. [Google Scholar] [CrossRef]
- Jones, S.E.; Phillips, C.J.C. The effects of mirrors on the welfare of caged rabbits. Anim. Welfare 2005, 14, 195–202. [Google Scholar]
- Edgar, J.L.; Seaman, S.C. The effect of mirrors on the behaviour of singly housed male and female laboratory rabbits. Anim. Welfare 2010, 19, 461–471. [Google Scholar]
- Hoy, S.; Verga, M. Welfare indicators. In Recent Advances in Rabbit Sciences; Maertens, L., Coudert, P., Eds.; Ilvo-Institute for Agriculture and Fisheries Research: Melle, Belgium, 2006; pp. 71–74. [Google Scholar]
- Jenkins, J.R. Rabbit behavior. Vet. Clin. North Am. Exot. Anim. Pract. 2001, 4, 669–679. [Google Scholar] [CrossRef]
- Daszkiewicz, T.; Gugołek, A.; Janiszewski, P.; Kubiak, D.; Czoik, M. The effect of intensive and extensive production systems on carcass quality in New Zealand White rabbits. World Rabbit Sci. 2012, 20, 25–33. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Mugnai, C. Rearing rabbits on a wire net floor or straw litter: Behaviour, growth and meat qualitative traits. Livest. Prod. Sci. 2002, 75, 149–156. [Google Scholar] [CrossRef]
- Lambertini, L.; Vignola, G.; Zaghini, G. Alternative pen housing system for fattening rabbits: Effects of group density and litter. World Rabbit Sci. 2001, 9, 141–147. [Google Scholar] [CrossRef][Green Version]
- Princz, Z.; Szendrő, Z.; Dalle Zotte, A.; Radnai, I.; Biró Németh, E.; Metzger, S.Z.; Gyovai, M.; Orova, Z. Effect of different housing on productive traits and on some behaviour patterns of growing rabbits. In Proceedings of the 17th Hungarian Conference on Rabbit Production, Kaposvár, Hungary, 25 May 2005; pp. 95–102. [Google Scholar]
- Morton, D.B. Refinement in rabbit husbandry. Lab. Anim. 1993, 27, 301–329, The BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Available online: https://journals.sagepub.com/doi/pdf/10.1258/002367793780745633 (accessed on 5 August 2019). [CrossRef]
- Mykytowycz, R.; Rowley, I. Continuous observations of the activity of the wild rabbit, Oryctolagus cuniculus (L.), during 24 hour periods. CSIRO Wildlife Research 1958, 3, 26–31. [Google Scholar] [CrossRef]
- Lehmann, M. Interference of a restricted environment as found in battery cages with normal behaviour of young fattening rabbits. In Rabbit Production Systems Including Welfare; Auxilia, T., Ed.; CEC Agriculture, ECSC-EEC-EAEC: Brussels, Belgium, 1987; pp. 257–268. [Google Scholar]
- Xu, H.T. The behaviour of the rabbit. In Proceedings of the 6th World Rabbit Congress, Toulouse, France, 9–12 July 1996; pp. 437–440. [Google Scholar]
- Gibb, J.A. Sociality, time and space in a sparse population of rabbits (Oryctolagus cuniculus). J. Zool. 1993, 229, 581–607. [Google Scholar] [CrossRef]
- Batchelor, G.R. Group housing on floor pens and environmental enrichment in sandy lop rabbits I. Anim. Technol. 1991, 42, 109–120. [Google Scholar]
- Lidfors, L. Behavioural effects of environmental enrichment for individually caged rabbits. Appl. Anim. Behav. Sci. 1997, 52, 157–169. [Google Scholar] [CrossRef]
- Terlouw, E.M.C.; Arnould, C.; Auperin, B.; Berri, C.; Le Bihan-Duval, E.; Deiss, V.; Lefèvre, F.; Lensink, B.J.; Mounier, L. Pre-slaughter conditions, animal stress and welfare: Current status and possible future research. Animal 2008, 2, 1501–1517. [Google Scholar] [CrossRef]
- Szeto, A.; Gonzales, J.A.; Spitzer, S.B.; Levine, J.E.; Zaias, J.; Saab, P.G.; Schneiderman, N.; McCabe, P.M. Circulating levels of glucocorticoid hormones in WHHL and NZW rabbits: Circadian cycle and response to repeated social encounter. Psychoneuroendocrinology 2004, 29, 861–866. [Google Scholar] [CrossRef]
- Bayatiz, V. Evaluation of cortisol and stress in captive animals. Aust. J. Basic Appl. Sci. 2009, 3, 1022–1031. [Google Scholar]
- Mazzone, G.; Vignola, G.; Giammarco, M.; Manetta, A.C.; Lambertini, L. Effects of loading methods on rabbit welfare and meat quality. Meat Sci. 2010, 85, 33–39. [Google Scholar] [CrossRef]
- Choe, J.H.; Kim, B.C. Association of blood glucose, blood lactate, serum cortisol levels, muscle metabolites, muscle fiber type composition, and pork quality traits. Meat Sci. 2014, 97, 137–142. [Google Scholar] [CrossRef]
- Fazio, F.; Casella, S.; Giudice, E.; Giannetto, C.; Piccione, G. Evaluation of secondary stress biomarkers during road transport in rabbit. Livest. Sci. 2015, 173, 106–110. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Jones, S.D.M.; Stanley, R.W. The use of electrolyte solutions for reducing transport stress. J. Anim. Sci. 1997, 75, 258–265. [Google Scholar] [CrossRef]
- Negretti, P.; Bianconi, G.; Finzi, A. Mutual visual relationships of rabbits raised in individual cages. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008; pp. 1213–1216. [Google Scholar]
- Seaman, C.S.; Waran, K.N.; Mason, G.; d’Eath, B.R. Animal economics: Assessing the motivation of female laboratory rabbits to reach a platform, social contact and food. Anim. Behav. 2009, 75, 31–42. [Google Scholar] [CrossRef]
- Negretti, P.; Bianconi, G.; Finzi, A. Mutual olfactory relationships in rabbits raised in individual cages. World Rabbit Sci. 2010, 18, 33–36. [Google Scholar] [CrossRef]
- Finzi, A.; Negretti, P.; Bianconi, G.; González-Redondo, P. Sex effect in mutual olfactory relationships of individually caged rabbits. Cogent Food Agric. 2015, 1, 992154. [Google Scholar] [CrossRef]
- Verga, M.; Zingarelli, I.; Heinzl, E.; Ferrante, V.; Martino, P.A.; Luzi, F. Effect of housing and environmental enrichment on performance and behaviour in fattening rabbits. In Proceedings of the 8th World Rabbit Congress, Puebla, Mexico, 7–10 September 2004; pp. 1283–1288. [Google Scholar]
| Behavioral Repertoire | Group | Time Slot | RMSE | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F2F | Blind | Mirrors | 1 | 2 | 3 | Group | Time | G × T | ||
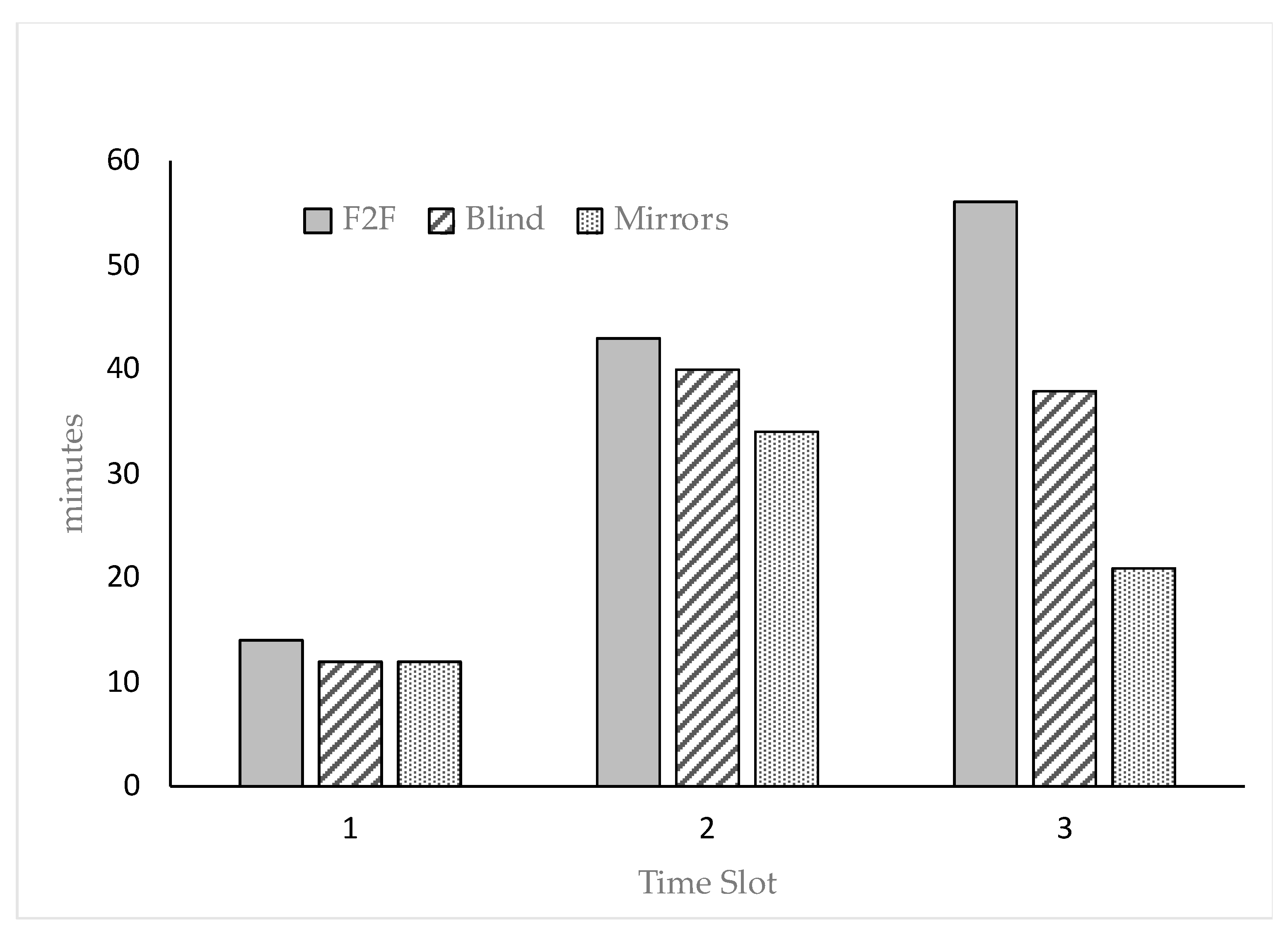
| Drinking, min | 43.9 | 45.1 | 40.0 | 31.8 B | 64.0 A | 33.3 B | 10.71 | 0.8127 | 0.0006 | 0.2264 |
| Feeding, min | 135.6 | 137.2 | 137.1 | 89.5 B | 157.9 A | 156.4 A | 20.68 | 0.9339 | 0.0003 | 0.1944 |
| OI, min | 30.6 a | 22.2 b | 22.5 b | 13.8 B | 37.9 A | 23.6 A,B | 6.08 | 0.0325 | 0.0039 | 0.7484 |
| Gnawing, min | 47.7 A | 14.8 B | 13.1 B | 19.9 b | 36.6 a | 19.0 b | 10.72 | 0.0004 | 0.0126 | 0.9666 |
| N2N, s | 5.50 | 3.25 | 4.08 | 2.42 b | 7.17 a | 3.25 b | 1.64 | 0.6190 | 0.0173 | 0.4829 |
| Scratching, min | 4.08 A | 2.58 B | 6.00 A | 3.00 B | 7.33 A | 2.33 B | 2.81 | 0.0028 | <0.0001 | 0.1252 |
| Alarm, s | 20.5 A | 5.08 B | 6.75 B | 1.25 B | 18.3 A | 12.8 A | 6.61 | 0.0008 | 0.0006 | 0.1283 |
| Allo-grooming, min | 44.7 a,b | 43.0 b | 52.3 a | 29.3 B | 46.6 A,B | 64.2 A | 4.74 | 0.0407 | 0.0022 | 0.9688 |
| Kennel stay, n | 14.7 B | 27.6 A | 2.42 C | 15.6 | 10.8 | 18.3 | 8.73 | 0.0023 | 0.5126 | 0.1660 |
| Stretching, s | 14.0 a | 10.6 b | 11.3 b | 6.75 B | 15.3 A | 13.8 A | 0.65 | 0.0350 | 0.0071 | 0.6846 |
| Locomotion, min | 37.9 a | 25.1 b | 28.5 a,b | 13.42 B | 39.67 A | 38.42 A | 11.38 | 0.0280 | <0.0001 | 0.0426 |
| Inactive, min | 135.6 B | 196.2 A | 173.0 A | 277.5 A | 87.0 C | 140.3 B | 9.36 | 0.0034 | <0.0001 | 0.4872 |
| Cecotrophy, n/day | 6.75 | 10.25 | 10.00 | - | - | - | 1.84 | 0.0625 | - | - |
| Mirrors, min | - | - | 22. | 5.75 b | 8.75 a | 8.00 a | 0.65 | - | 0.0381 | - |
| Blood Parameters | Group | RMSE | p-Value | ||
|---|---|---|---|---|---|
| F2F | Blind | Mirrors | |||
| Cortisol, μg/dL | 280.2 | 310.6 | 223.5 | 128.5 | 0.4040 |
| PCV, % | 37.7 | 41.0 | 40.8 | 4.95 | 0.3416 |
| Glucose, mg/dL | 112.5 B | 132.5 A | 135.4 A | 11.5 | 0.0007 |
| Total Protein, g/dL | 6.63 A,B | 7.10 A | 6.23 B | 0.40 | 0.0013 |
| Lactate, mg/dL | 81.9 a | 65.3 b | 69.2 b | 8.24 | 0.0480 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastellone, V.; Bovera, F.; Musco, N.; Panettieri, V.; Piccolo, G.; Scandurra, A.; Di Meo, C.; Attia, Y.A.; Lombardi, P. Mirrors Improve Rabbit Natural Behavior in a Free-Range Breeding System. Animals 2019, 9, 533. https://doi.org/10.3390/ani9080533
Mastellone V, Bovera F, Musco N, Panettieri V, Piccolo G, Scandurra A, Di Meo C, Attia YA, Lombardi P. Mirrors Improve Rabbit Natural Behavior in a Free-Range Breeding System. Animals. 2019; 9(8):533. https://doi.org/10.3390/ani9080533
Chicago/Turabian StyleMastellone, Vincenzo, Fulvia Bovera, Nadia Musco, Valentina Panettieri, Giovanni Piccolo, Anna Scandurra, Carmelo Di Meo, Youssef A. Attia, and Pietro Lombardi. 2019. "Mirrors Improve Rabbit Natural Behavior in a Free-Range Breeding System" Animals 9, no. 8: 533. https://doi.org/10.3390/ani9080533
APA StyleMastellone, V., Bovera, F., Musco, N., Panettieri, V., Piccolo, G., Scandurra, A., Di Meo, C., Attia, Y. A., & Lombardi, P. (2019). Mirrors Improve Rabbit Natural Behavior in a Free-Range Breeding System. Animals, 9(8), 533. https://doi.org/10.3390/ani9080533






