Housing Rabbit Does in a Combi System with Removable Walls: Effect on Behaviour and Reproductive Performance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
- -
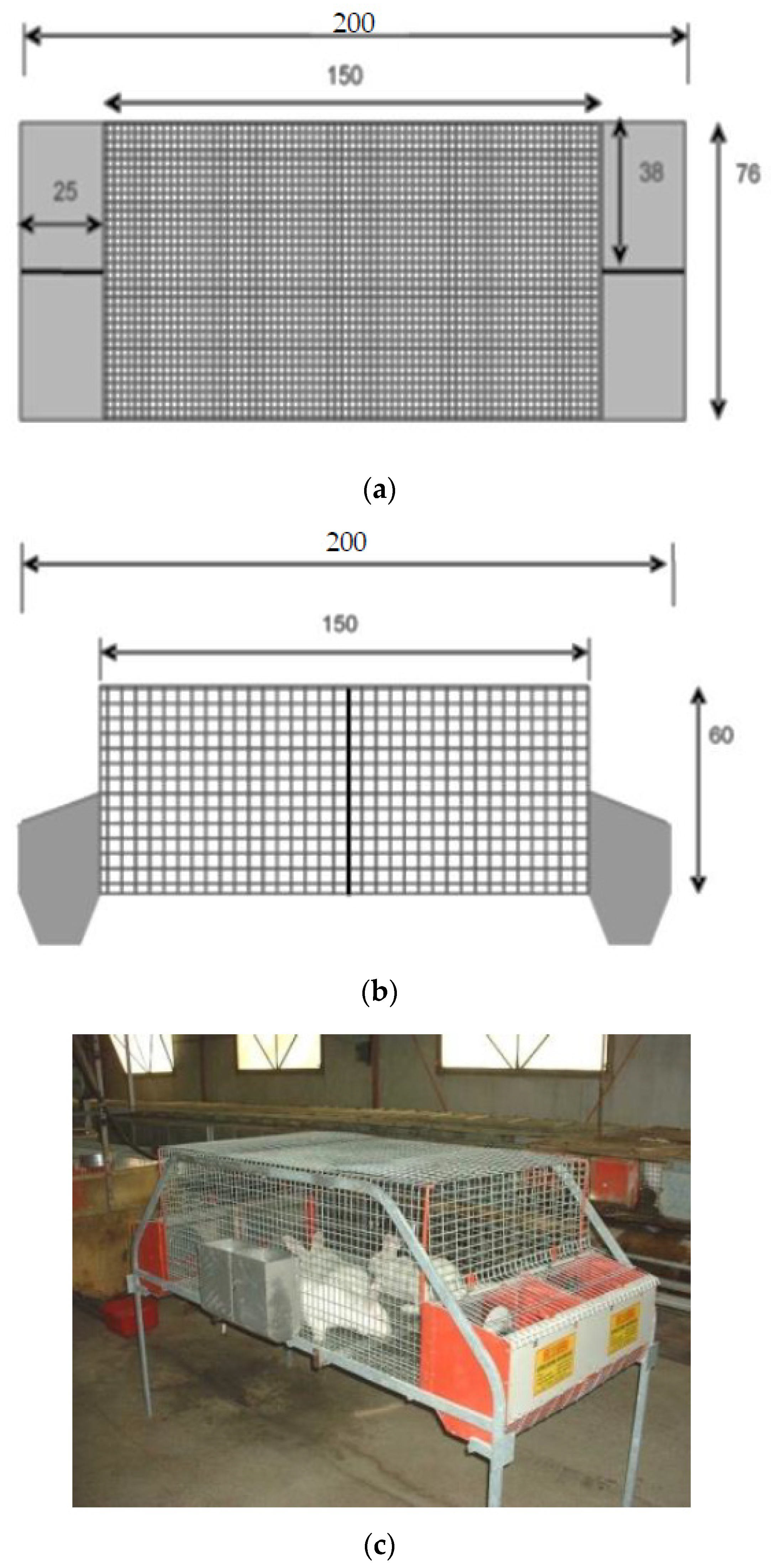
- Simple colony cage (C1; Figure 1), with dimensions of 76W × 150L × 60H cm, and four external shut-out nest boxes (38 × 25 × 35 cm) at each side of the cage.
- -
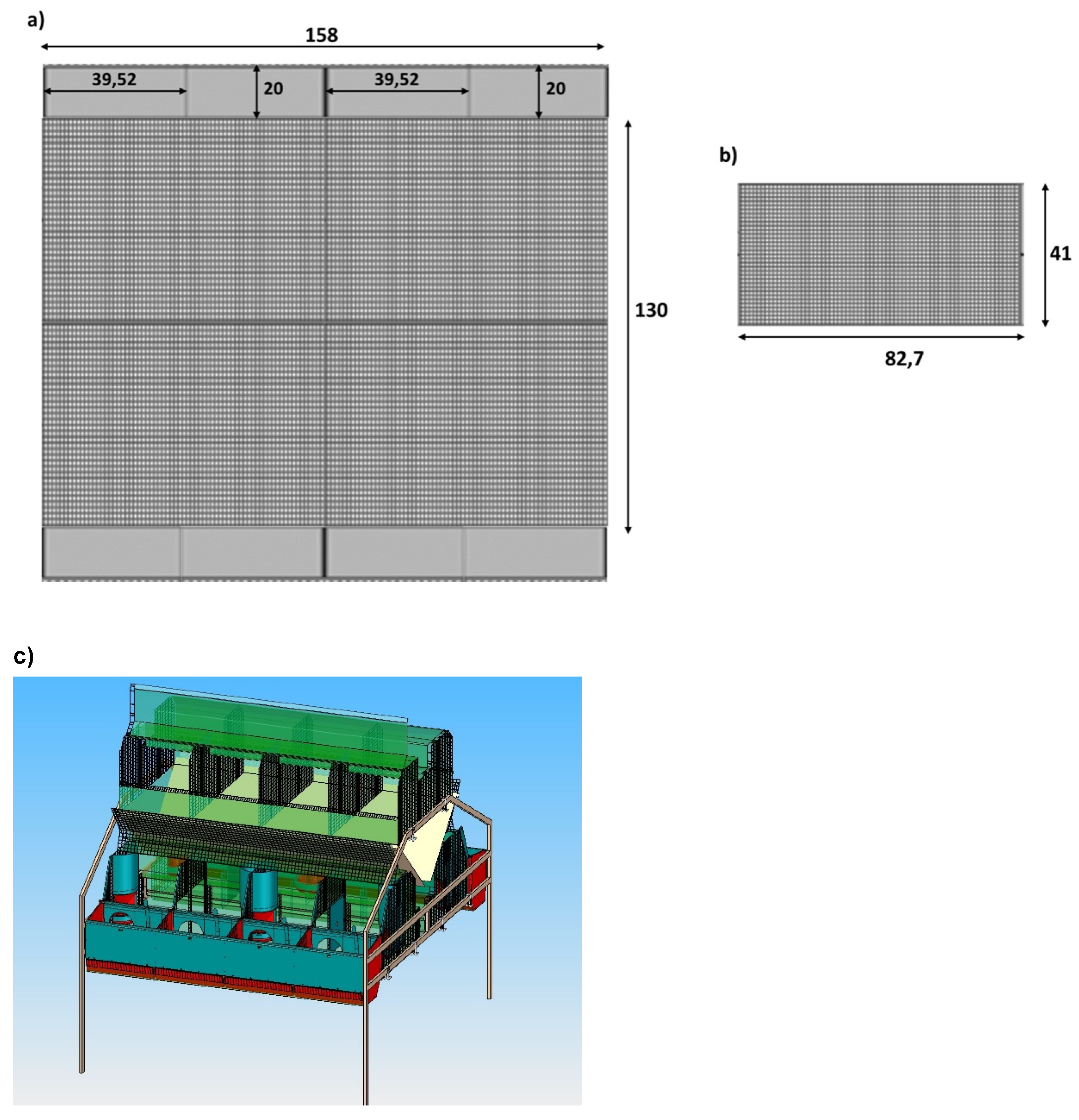
- Combination colony cage (C2; Figure 2) with two levels: in the lower level, the maternity cage was located with eight units and nests, divided into two sides (four rabbit does per side), furnished with removable walls to manage group and individual housing, depending on the different production phases. The dimensions of cage were of 130W × 158L × 60H cm, with eight 39.52 × 20 × 35 cm external shut-out nest boxes at each side of the cage. In the upper level, there was a multi-purpose cage with removable walls, available for hosting the non-pregnant and severely injured does and for potential sopra-numerous pregnant does (possibility to add nests). This cage was considered an autonomous and independent production unit.
- -
- Conventional cage (C), with dimensions of 38W × 60L × 34H cm, provided with an external nest box (38 × 25 × 35 cm).
2.2. Experimental Design
- -
- C1 group (n = 16, four does × four production units–open system);
- -
- C2 group (n = 16, four does per block × four production units–closed system);
- -
- C group (n = 16, one doe × 16 production units).
2.3. Diets
2.4. Behaviour Observation and Ethogram
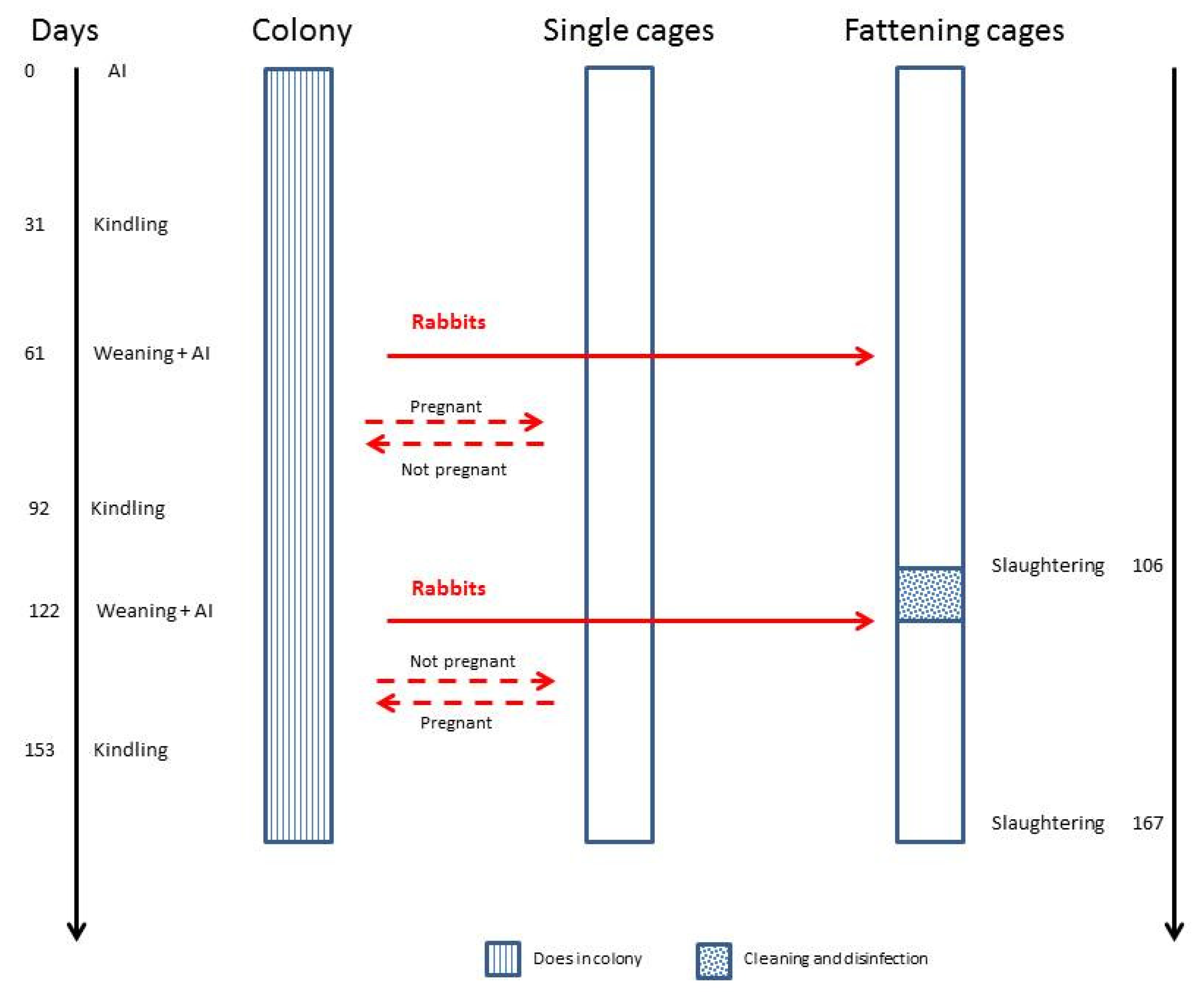
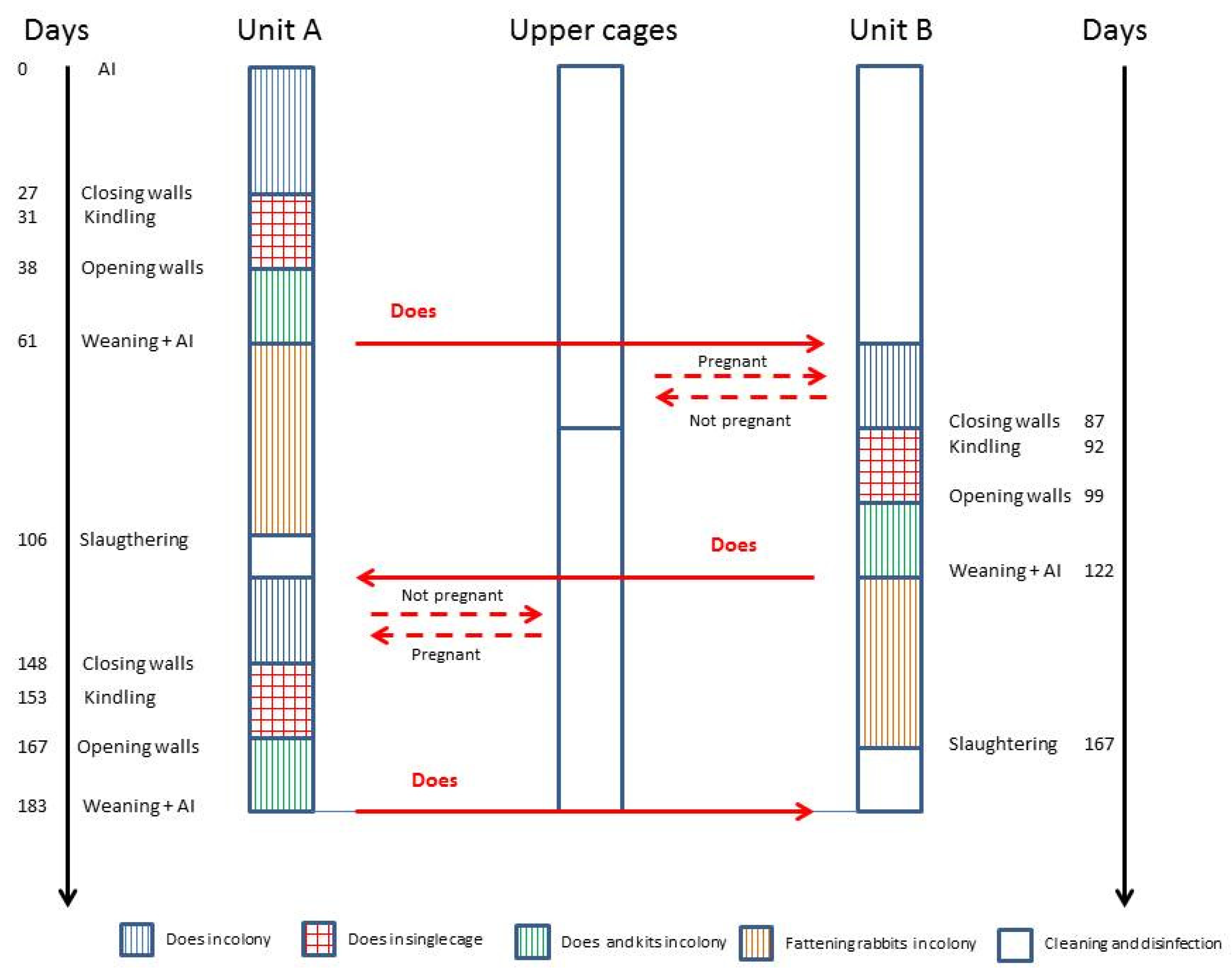
- from days 1 to 7, when does were in the colony (C1 and C2);
- from days 30 to 36, around the kindling period, when in group C2, the internal walls were present and animals were housed individually. The behavioural observations were interrupted on the day of kindling to provide a peaceful and quiet environment for does. In C1, the does were always in the colony;
- from days 38 to 44, when in group C2, the internal walls were removed and the does were grouped again.
2.5. Body Injuries
2.6. Reproductive Performance
2.7. Statistical Analysis
3. Result and Discussion
3.1. Behaviour and Welfare of Does
3.2. Reproductive Performance of Does
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Surridge, A.K.; Bell, D.J.; Hewitt, G.M. From population structure to individual behaviour: Genetic analysis of social structure in the European wild rabbit (Oryctolagus cuniculus). Biol. J. Linnean Soc. 1999, 68, 57–71. [Google Scholar] [CrossRef]
- Lebas, F.; Coudert, P.; Rochambeau, H.; de Thébault, R.G. The Rabbit—Husbandry, Health and Production; FAO Animal Production and Health Series; Food and Agriculture organization of the United Nations: Roma, Italy, 1997; p. 21. [Google Scholar]
- Mirabito, L.; Dumont, F.; Galliot, P.; Souchet, C. Logement collectif des lapines reproductrices: Conséquences sur le comportement. Rech. Cunicole 2005, 29, 57–60. [Google Scholar]
- Mugnai, C.; Dal Bosco, A.; Castellini, C. Effect of different rearing systems and pre-kindling handling on behaviour and performance of rabbit does. Appl. Anim. Behav. Sci. 2009, 118, 91–100. [Google Scholar] [CrossRef]
- Szendrő, Z.; McNitt, J.I. Housing of rabbit does: Group and individual systems: A review. Livest. Sci. 2012, 150, 1–10. [Google Scholar] [CrossRef]
- Rommers, J.M.; Reuvekamp, B.J.F.; Gunnink, H.; de Jong, J.C. Effect of hiding places, straw and territory on aggression in group-housed rabbit does. Appl. Anim. Behav.Sci. 2014, 157, 117–126. [Google Scholar] [CrossRef]
- Buijs, S.; Maertens, L.; Hermans, K.; Vangeyte, J.; Tuyttens, F.S.M. Behaviour, wounds, weight loss and adrenal weight of rabbit does as affected by semi-group-housing. Appl. Anim. Behav. Sci. 2015, 172, 44–51. [Google Scholar] [CrossRef]
- Rommers, J.; de Greef, K. Are combi parks just as useful as regular parks for fatteners for part-time group-housing of rabbit does? World Rabbit Sci. 2018, 26, 299–305. [Google Scholar] [CrossRef]
- Hoy, S.; Matics, Z. Alternative housing systems for rabbit does. In Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15–18 June 2016; pp. 637–651. [Google Scholar]
- Szendrő, Z.; McNitt, J.I.; Matics, Z.; Mikó, A.; Gerencsér, Z. Alternative and enriched housing systems for breeding does: A review. World Rabbit Sci. 2016, 24, 1–14. [Google Scholar] [CrossRef]
- International Rabbit Reproduction Group. Recommendations and guidelines for applied reproduction trials with rabbit does. World Rabbit Sci. 2005, 13, 147–164. [Google Scholar] [CrossRef]
- McBride, A. Rabbits and Hares; Whittet Books: London, UK, 1988. [Google Scholar]
- Castellini, C.; Dal Bosco, A.; Mugnai, C. Comparison of different reproductive protocols for rabbit doe: Effect of litter size and mating interval. Livest. Prod. Sci. 2003, 83, 131–139. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Kalle, G. Aus der Schweizer Praxis: Kaninchen in Gruppenhaltung. DGS Mag. 1994, 25, 16–20. [Google Scholar]
- Castellini, C.; Dal Bosco, A.; Cardinali, R. Long term effect of post-weaning rhythm on the body fat and performance of rabbit does. Repr. Nutr. Dev. 2006, 46 (Suppl. 2), 195–204. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Castellini, C.; Mugnai, C. Evaluation of body condition score in pregnant rabbit does by ultrasound scanner. Ital. Anim. Sci. 2003, 2 (Suppl. 1), 480–482. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 9.0; StataCorp: College Station, TX, USA, 2005. [Google Scholar]
- Jildge, B.; Hudson, R. Diversity and development of circadian rhythm in the European rabbit. Chronobiol. Int. 2001, 18, 1–26. [Google Scholar] [CrossRef]
- Lawrence, A.B.; Rushen, J. Introduction in: Stereotypic Animal Behaviour, Fundamentals and Applications to Welfare; CAB International: Trowbridge, UK, 1993. [Google Scholar]
- Verga, M.; Luzi, F.; Carenzi, C. Effects of husbandry and management systems on physiology and behaviour of farmed and laboratory rabbits. Horm. Behav. 2007, 52, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mugnai, C.; Castellini, C.; Laudazi, S. A prototype of colony cage for improving the welfare of rabbit does: Preliminary results. In Proceedings of the 8th Congress of the World Rabbit Science Association, Puebla, Mexico, 7–10 September 2004; pp. 1229–1234. [Google Scholar]
- Graf, S.; Bigler, L.M.; Failing, K.; Würbel, H.; Buchwalder, T. Regrouping rabbit does in a familiar or novel pen: Effects on agonistic behaviour, injuries and core body temperature. Appl. Anim. Behav. Sci. 2011, 135, 121–127. [Google Scholar] [CrossRef]
- Szendrő, Z.; Mikó, A.; Odermatt, M.; Gerencsér, Z.; Radnai, I.; Dezséry, B.; Garai, E.; Nagy, I.; Szendrő, K.; Matics, Z. Comparison of performance and welfare of single-caged and group-housed rabbit does. Animal 2013, 7, 463–468. [Google Scholar] [CrossRef]
- Andrist, C.A.; Bigler, L.M.; Würbel, H.; Roth, B.A. Effects of group stability on aggression, stress and injuries in breeding rabbits. Appl. Anim. Behav. Sci. 2012, 142, 182–188. [Google Scholar] [CrossRef]
- Andrist, C.A.; van den Borne, B.H.P.; Bigler, L.M.; Buchwalder, T.; Roth, B.A. Epidemiologic survey in Swiss group-housed breeding rabbits: Extent of lesions and potential risk factors. Prev. Vet. Med. 2013, 108, 218–224. [Google Scholar] [CrossRef]
- Andrist, C.A.; Bigler, L.M.; Würbel, H.; Roth, B.A. Masking odour when regrouping rabbit does: Effect on aggression, stress and lesions. Livest. Sci. 2014, 170, 150–157. [Google Scholar] [CrossRef]
- Rommers, J.M.; Boiti, C.; de Jong, I.; Brecchia, G. Performance and behaviour of rabbit does in a group-housing system with natural mating or artificial insemination. Reprod. Nutr. Dev. 2006, 46, 677–687. [Google Scholar] [CrossRef]
- Rommers, J.M.; Gunnink, H.; Klop, A.; de Jong, I.C. Dynamics in aggressive behaviour of rabbit does in a group-housing system: A descriptive study. In Proceedings of the 17th International Symposium on Housing and Diseases of Rabbits, Fur Providing Animals and Pet Animals, Celle, Germany, 11–12 May 2011; pp. 75–85. [Google Scholar]
- Rommers, J.M.; Gunnink, H.; de Jong, I.C. Effect of different types of places on aggression among does in a group-housing system: A pilot study. In Proceedings of the 18th International Symposium on Housing and Diseases of Rabbits, Fur Providing Animals and Pet Animals, Celle, Germany, 22–23 May 2013; pp. 59–68. [Google Scholar]
- Zomeño, C.; Birolo, M.; Zuffellato, A.; Xiccato, G.; Trocino, A. Aggressiveness in group-housed rabbit does: Influence of group size and pen characteristics. Appl. Anim. Behav. Sci. 2017, 194, 79–85. [Google Scholar] [CrossRef]
- Kutsukake, N. Complexity, dynamics and diversity of sociality in group-living mammals. Ecol. Res. 2009, 24, 521–531. [Google Scholar] [CrossRef]
- Von Holst, D.; Hutzelmeyer, H.; Kaetzke, P.; Kaschei, M.; Schönheiter, R. Social rank, stress, fitness, and life expectancy in wild rabbits. Naturwissenschaften 1999, 86, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Von Holst, D.; Hutzelmeyer, H.; Kaetzke, P.; Khaschei, M.; Rödel, H.G.; Schrutka, H. Social rank, fecundity and lifetime reproductive success in wild European rabbits (Oryctolagus cuniculus). Behav. Ecol. Sociobiol. 2002, 51, 245–254. [Google Scholar] [CrossRef]
- Mykytowycz, R. Social behaviour of an experimental colony of wild rabbits. Oryctolagus cuniculus (L.) I. Establishment of the colony. CSIRO Wildl. Res. 1958, 3, 7–25. [Google Scholar] [CrossRef]
- Southern, H.N. Sexual and aggressive behaviour of the wild rabbit. Behaviour 1948, 1, 173–194. [Google Scholar] [CrossRef]
- Albonetti, M.E.; Farabollini, F. Social stress by repeated defeat: Effects on social behaviour and emotionality. Behav. Brain Res. 1994, 62, 187–193. [Google Scholar] [CrossRef]
- Maertens, L.; Buijs, S. Production performances of rabbit does in a part-time group-housing system. In Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15–18 June 2016; pp. 711–714. [Google Scholar]
- Xiccato, G.; Parigi-Bini, R.; DalleZotte, A.; Carazzolo, A.; Cossu, M.E. Effect of dietary energy level, addition of fat and physiological state on performance and energy balance of lactating and pregnant rabbit does. Anim. Sci. 1995, 61, 387–398. [Google Scholar] [CrossRef]
- Szendrő, Z.; Matics, Z.; Szabó, R.T.; Mikó, A.; Odermatt, M.; Gerencsér, Z. Aggressivity and its effect on lifespan of group housed rabbit does. In Proceedings of the 11th World Rabbit Congress, Qingdao, China, 15–18 June 2016; pp. 719–722. [Google Scholar]
- Buhl, M.; Damme, K.; Hoy, S. Erste Ergebnissezueinem Gruppen haltungs system für HäsinnenmitJungen. In Proceedings of the 19th International Symposium on Housing and Diseases of Rabbits, Furproviding Animals and Pet Animals, Celle, Germany, 27–28 May 2015; pp. 229–236. [Google Scholar]
- Hoy, S.; Dal Bosco, A.; Matics, Z.; Villagra, A. Hauptergebnisse des internationalen Anihwa-Kaninchen-Projektes RABHO. In Proceedings of the 20 International Symposium on Housing and Diseases of Rabbits, Furproviding Animals and Pet Animals, Celle, Germany, 17–18 May 2017; pp. 14–26. [Google Scholar]
- Maertens, L. Farmers experiences with park housing of rabbits. In Proceedings of the 20 International Symposium on Housing and Diseases of Rabbits, Fur providing Animals and Pet Animals, Celle, Germany, 17–18 May 2017; pp. 37–41. [Google Scholar]
- Maertens, L.; Rommers, J.; Jacque, M. Le logement des lapinsenparcs, une alternative pour les cages classiques dans un système “duo”? In Proceedings of the 14èmes Journées de la RechercheCunicole, Le Mans, France, 22–23 November 2011; pp. 85–88. [Google Scholar]
- Maertens, L.; Buijs, S. Performances de femelles loges temporairement en groupe dans des parcs polyvalentset ens ystème tout plein tout vide. In Proceedings of the 15èmes Journées de la Recherche Cunicole, Le Mans, France, 19–20 November 2013; pp. 35–38. [Google Scholar]
- Maertens, L.; Buijs, S. Production performances of semi-group housed rabbit does. In Proceedings of the 19th International Symposium on Housing and Diseases of Rabbits, Furproviding Animals and Pet Animals, Celle, Germany, 27–28 May 2015; pp. 22–31. [Google Scholar]
- Maertens, L.; De Bie, Y. Logement de lapines “part-time” en groupe: Résultats dans un élevage équipé avec des parcs polyvalents. In Proceedings of the 17èmes Journées de la Recherche Cunicole, Le Mans, France, 21–22 November 2017; pp. 55–58. [Google Scholar]
- Manteca, X. Neurophysiology and assessment of welfare. Meat Sci. 1998, 49, 205–218. [Google Scholar] [CrossRef]
- Kermabon, A.Y.; Prunier, A.; Djiane, J.; Salesse, R. Gonadotropins in lactating sows exposed to long or short days during pregnancy and lactation: Serum concentrations and ovarian receptors. Biol. Reprod. 1995, 53, 1095–1102. [Google Scholar] [CrossRef]
- Bench, C.J.; Gonyou, H.W. Effect of environmental enrichment and breed line on the incidence of belly nosing in piglets weaned at 7 and 14 days-of-age. Appl. Anim. Behav. Sci. 2007, 105, 26–41. [Google Scholar] [CrossRef]
- Theau-Clement, M. Advances in biostimulation methods applied to rabbit reproduction. World Rabbit Sci. 2000, 1, 61–79. [Google Scholar]
- González-Mariscal, G. Neuroendocrinology of maternal behavior in the rabbit. Horm. Behav. 2001, 40, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.; Distel, H. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol. Behav. 1986, 37, 123–129. [Google Scholar] [CrossRef]
- Bohus, B.; Koolhaas, J.M.; De Ruite, A.J.H.; Heijnen, C.J. Stress and differential alterations in immune system functions: Conclusions from social stress studies in animals. Neth. J. Med. 1991, 3, 306–315. [Google Scholar]
| Categories | Behavior Patterns | Behavior Description |
|---|---|---|
| Move | Moving | Any movement in any direction where all four limbs are involved |
| Jumping | Voluntary movements of jumping | |
| Eat | Eating | Head above the feeder. Eating or chewing pellets |
| Drinking | Head in close proximity to water nipple. Nosing or drinking from water nipple | |
| Self | Comfort | Licking, scratching, or nibbling of the body |
| Stereotypies | Biting bars | Licking or gnawing cage bars and scratching cage floor insistently |
| Smelling bars | Smelling bars and cage floor insistently | |
| Static | Lying down | Resting with chest or stomach on the floor. Fore limbs stretched in front of the body |
| Crouching | Resting with chest or stomach on the floor. Hind and fore limbs crouched under body | |
| Sitting-up | Sat in upright position on hind limbs and fore limbs straight, but without bust touching the floor | |
| Staying | Standing still on four straight limbs | |
| Standing alert | Standing up on the hind legs | Sitting in upright position with ears erect |
| Standing up on hind legs with erect ears | Sitting in upright position on hind limbs and fore limbs straight, with ears erect | |
| Maternal | Nesting | Nest-building consists of digging a burrow, collecting straw, and shaping it into a nest inside the burrow, as well as plucking body hair and lining the straw nest with it |
| Change of nest | A doe that enter in another doe nest | |
| Others | Defecation, urination caecotrophy | - |
| Social relationship | Smelling other | Smelling another doe |
| Allo-grooming | Licking, scratching, or nibbling another doe’s body | |
| Attack | Offensive moves, in which the doe attempts to bite its opponent | |
| Dominance feature | A doe that mounts, bites, or scratches another doe, or that sits with a tense body posture with erected ears and tail near to another doe | |
| Submissive feature | A doe in a crouched posture that avoids visual contact, rolls over onto the back, ears back, and tail tucked (submissive features) near to another doe |
| Period | 1–7 Days | 30–36 Days | 38–44 Days | p-Value | MSE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavior Patterns | Categories | C | C1 | C2 | C | C1 | C2 | C | C1 | C2 | T | S | T × S | |
| Moving | Move | 7.33 a,b | 9.80 b | 9.60 b | 4.01 a | 10.70 b | 2.69 a | 4.08 a | 9.62 b | 9.70 b | * | * | * | 2.95 |
| Jumping | 0.57 a | 1.80 b | 1.88 b | 0.92 a | 1.80 b | 1.77 b | 0.11 a | 1.69 b | 1.29 b | n.s. | * | n.s | 0.18 | |
| Eating | Eat | 8.29 | 9.50 | 9.88 | 10.74 | 10.66 | 10.70 | 11.00 | 8.67 | 9.91 | n.s. | n.s. | * | 1.09 |
| Drinking | 3.55 | 2.70 | 3.20 | 3.00 | 3.89 | 2.75 | 11.18 | 2.61 | 3.49 | n.s. | n.s. | n.s | 0.88 | |
| Comfort | Self | 8.65 | 9.70 | 9.90 | 10.93 | 8.23 | 8.34 | 3.24 | 8.68 | 9.15 | n.s. | n.s. | ns. | 1.75 |
| Biting bars | Stereotypies | 8.71 b | 2.04 a | 2.64 a | 6.43 b | 2.77 a | 2.53 a | 20.04 b | 2.76 a | 2.25 a | n.s. | *** | ** | 1.48 |
| Smelling bars | 7.44 b | 3.91 a | 3.83 a | 7.66 b | 3.65 a | 5.62 a,b | 8.44 b | 3.53 a | 3.14 a | * | ** | * | 1.20 | |
| Lying down | Static | 7.7 a | 15.89 c | 15.70 c | 9.50 a,b | 13.00 b | 16.17 c | 3.90 a | 15.76 c | 16.30 c | * | ** | * | 1.55 |
| Crouching | 28.15 b | 10.32 a | 10.61 a | 32.15 b | 10.20 a | 17.32 a | 28.91 b | 10.43 a | 10.33 a | * | ** | ** | 2.07 | |
| Sitting-up | 3.56 a,b | 6.30 b | 5.74 b | 1.17 a | 5.65 b | 5.44 b | 1.01 a | 6.40 b | 6.62 b | * | ** | * | 0.74 | |
| Staying | 7.78 b | 4.84 a,b | 4.26 a,b | 4.94 a,b | 3.76 a,b | 5.50 a,b | 1.06 a | 4.74 a,b | 4.24 a,b | n.s. | * | * | 1.11 | |
| Standingup on the hind legs | Standing alert | 5.00 b | 5.56 b | 5.58 b | 0.53 a | 5.50 b | 6.41 b | 0.00 a | 5.86 b | 5.47 b | * | ** | * | 1.05 |
| Standingup on hind legs with erect ears | 1.68 a | 8.50 b | 7.57 b | 1.48 a | 8.3 b | 8.65 b | 1.00 a | 8.8 b | 8.4 b | n.s. | *** | n.s | 1.58 | |
| Nesting | Maternal | - | - | - | 5.74 | 3.11 | 4.60 | - | - | - | - | - | - | 0.82 |
| Change of nest | - | - | - | - | 8.50 | - | - | - | - | - | - | - | 0.15 | |
| Defecation, urination caecotrophy | Others | 1.59 | 1.12 | 1.34 | 0.80 | 1.43 | 1.56 | 0.70 | 1.25 | 1.30 | n.s. | n.s. | n.s. | 0.25 |
| Smelling other | Social relationship | - | 2.80 | 2.80 | - | 1.12 | - | - | 3.37 | 2.22 | - | - | - | 0.61 |
| Allo-grooming | - | 1.46 a,b | 1.66 a,b | - | 1.01 a | - | - | 0.83 a | 1.98 b | - | - | - | 0.47 | |
| Attack | - | 2.60 b | 2.87 b | - | 3.12 c | - | - | 2.00 a | 2.70 b | - | - | - | 0.52 | |
| Dominance feature | - | 0.68 a | 0.60 a | - | 1.78 b | - | - | 1.50 b | 1.95 c | - | - | - | 0.41 | |
| Submissive feature | - | 0.48 | 0.34 | - | 0.39 | - | - | 0.54 | 0.67 | - | - | - | 0.15 | |
| Period | Body Part | 1–7 Days | 30–36 Days | 38–44 Days | p-Value | X2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Housing system | C1 | C2 | C1 | C2 | C1 | C2 | T | S | T × S | ||
| Injured animals % | 19 a,b | 20 b | 24 b | - | 17 a | 22 b | * | ** | n.s. | 2 | |
| Part of body | Head and ears | 24 a | 23 a | 25 a | - | 26 a | 36 b | * | ** | * | 4 |
| Body | 10 b | 10 a | 7 a | - | 7 a | 10 b | * | * | * | 2 | |
| Genitals | - | - | - | - | - | - | - | - | - | - | |
| Tail | 66 b | 67 b | 68 b | - | 67 b | 54 a | * | ** | * | 5 | |
| Housing System | Unit | C | C1 | C2 | p-Value | MSE |
|---|---|---|---|---|---|---|
| Sexual receptivity | % | 85.2 c | 72.6 a | 79.2 b | * | 4.52 † |
| Fertility rate | % | 82.8 b | 69.3 a | 76.2 b | * | 3.41 † |
| Kindling rate | % | 78.3 c | 60.2 a | 69.0 b | * | 4.08 † |
| Embryo mortality | % | 4.5 a | 9.1 b | 6.2 a,b | * | 0.50 |
| Doe weight at kindling | g | 3750 | 3455 | 3540 | n.s. | 565 |
| Doe weight at weaning | g | 4220 | 3860 | 3985 | n.s. | 385 |
| Estimated depot fat at AI | g | 53 b | 32 a | 40 a | ** | 1.58 |
| Born alive | n | 8.90 b | 7.50 a | 7.95 a | ** | 0.32 |
| Weaned pups | n | 7.85 c | 6.91 a | 7.20 b | * | 0.21 |
| Weight at weaning | g/pup | 585 | 570 | 565 | n.s. | 39.2 |
| Pre-weaning mortality | % | 5.5 a | 8.0 c | 7.2 b | * | 0.75 † |
| Housing System | Unit | C | C1 | C2 | p-Value | MSE |
|---|---|---|---|---|---|---|
| Rabbits sold/year/doe | 35.5 c | 20.4 a | 25.6 b | * | 2.68 | |
| Live weight sold/year/doe | kg | 81.6 c | 46.6 a | 60.7 b | *** | 4.29 |
| Production losses | kg | 42.4 a | 73.5 b | 48.5 a | ** | 3.58 |
| Kindling interval | day | 75.2 a | 94.3 b | 82.1 a,b | * | 5.82 |
| Kindling/year/doe | n. | 4.80 | 3.78 | 4.35 | n.s. | 0.58 |
| Annual doe replacement | % | 75.0 a | 112.0 b | 87.5 a | ** | 6.57 † |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Bosco, A.; Mugnai, C.; Martino, M.; Szendrő, Z.; Mattioli, S.; Cambiotti, V.; Cartoni Mancinelli, A.; Moscati, L.; Castellini, C. Housing Rabbit Does in a Combi System with Removable Walls: Effect on Behaviour and Reproductive Performance. Animals 2019, 9, 528. https://doi.org/10.3390/ani9080528
Dal Bosco A, Mugnai C, Martino M, Szendrő Z, Mattioli S, Cambiotti V, Cartoni Mancinelli A, Moscati L, Castellini C. Housing Rabbit Does in a Combi System with Removable Walls: Effect on Behaviour and Reproductive Performance. Animals. 2019; 9(8):528. https://doi.org/10.3390/ani9080528
Chicago/Turabian StyleDal Bosco, Alessandro, Cecilia Mugnai, Melania Martino, Zsolt Szendrő, Simona Mattioli, Valentina Cambiotti, Alice Cartoni Mancinelli, Livia Moscati, and Cesare Castellini. 2019. "Housing Rabbit Does in a Combi System with Removable Walls: Effect on Behaviour and Reproductive Performance" Animals 9, no. 8: 528. https://doi.org/10.3390/ani9080528
APA StyleDal Bosco, A., Mugnai, C., Martino, M., Szendrő, Z., Mattioli, S., Cambiotti, V., Cartoni Mancinelli, A., Moscati, L., & Castellini, C. (2019). Housing Rabbit Does in a Combi System with Removable Walls: Effect on Behaviour and Reproductive Performance. Animals, 9(8), 528. https://doi.org/10.3390/ani9080528








