Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Free Radicals and Reactive Oxygen and Nitrogen Species
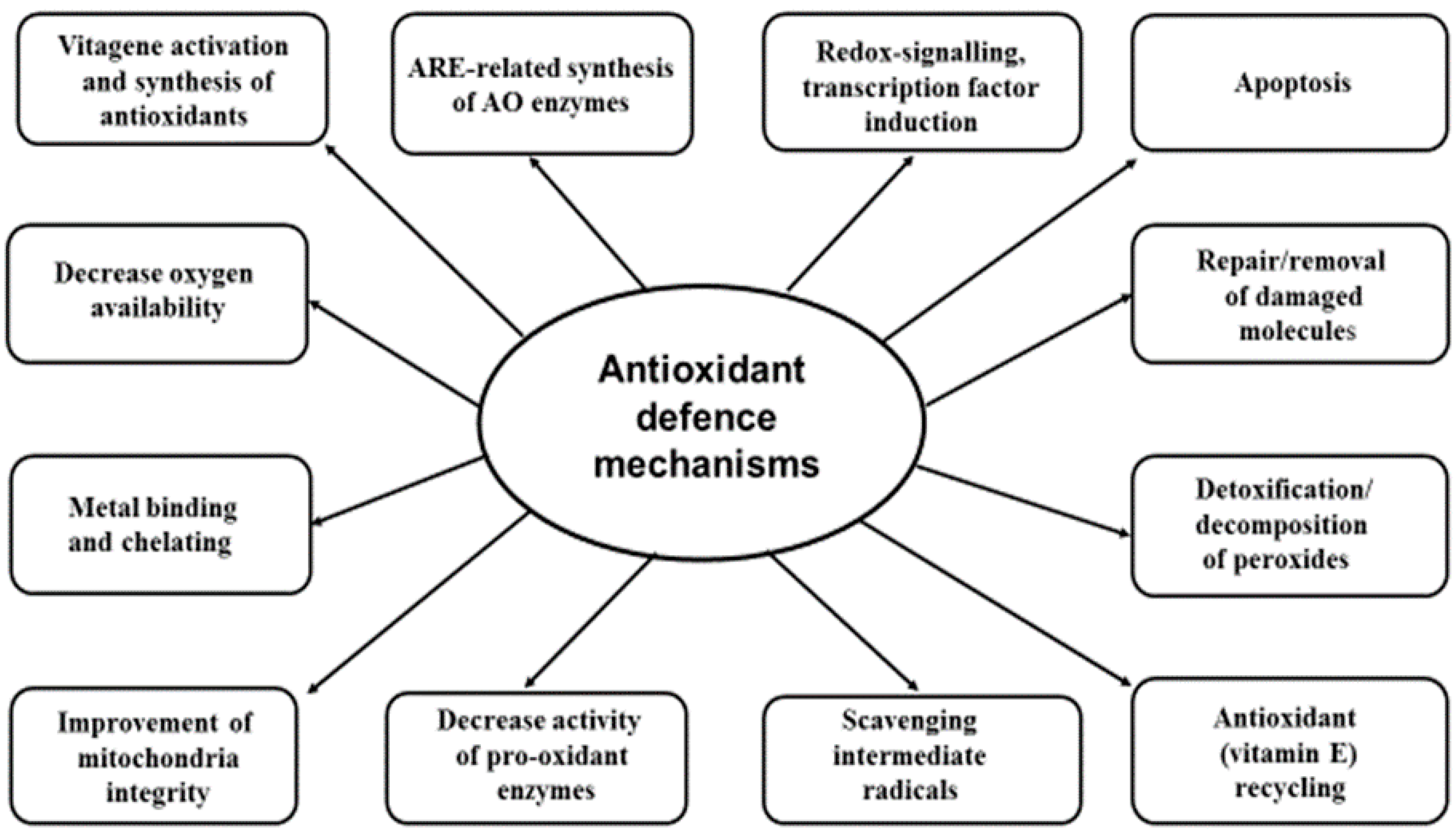
3. Biological Antioxidant Systems
4. Oxidative Stress in Dairy Cattle
5. Nutritional Modulation of the Antioxidant Network to Prevent Oxidative Stress
6. Organic Selenium Concept Development
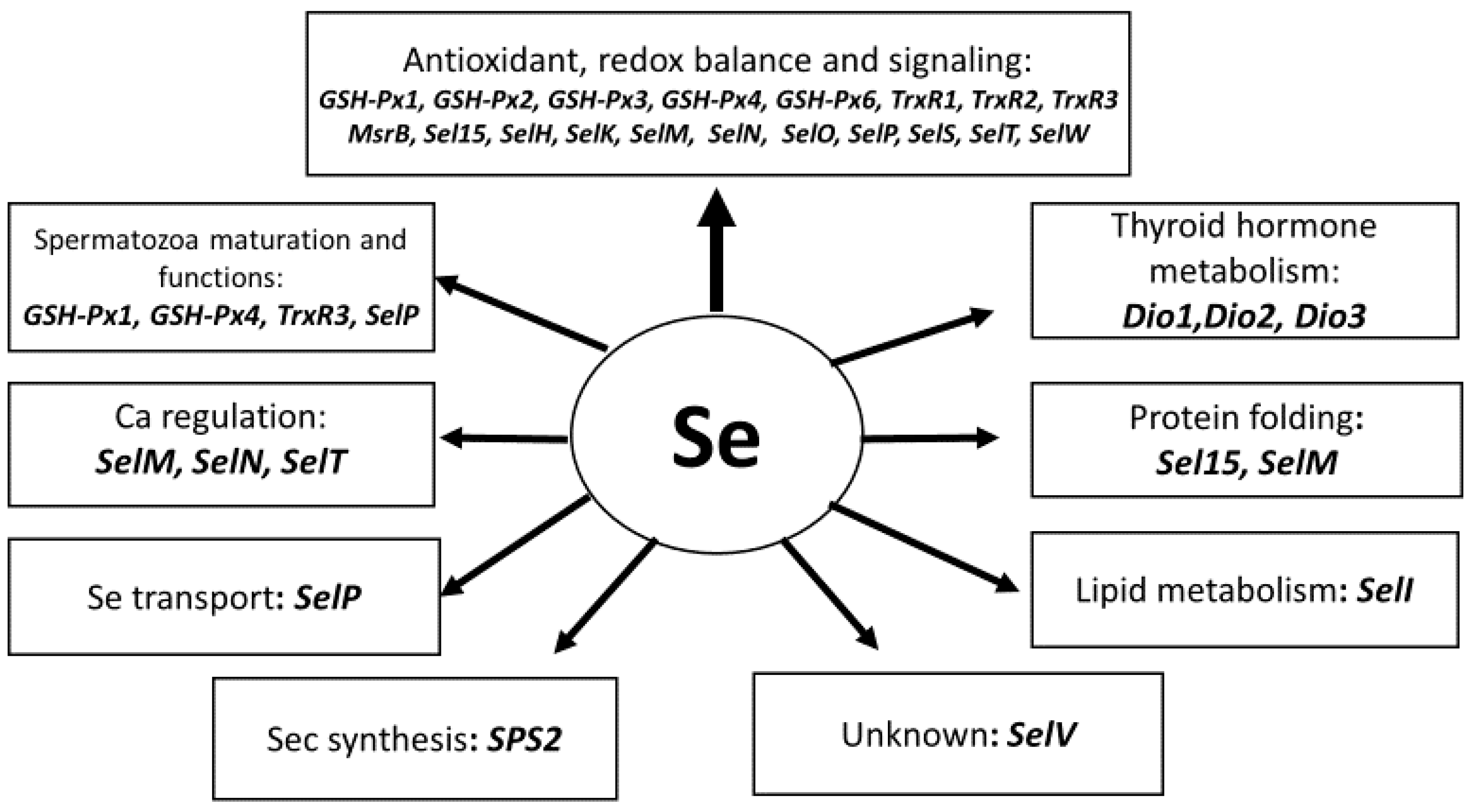
7. Important Features of Selenium Metabolism in Ruminants
8. Beneficial Effects of Organic Selenium in Cows
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AP1 | activator protein 1 (a transcription factor) |
| BD | basic diet |
| DM | dry matter |
| GSH | reduced glutathione |
| GSH-Px | glutathione peroxidase |
| 4-HNE | 4-hydroxyalkenal |
| MAPK | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| Met | methionine |
| NF-κB | nuclear factor-kappa B (a transcription factor) |
| Nrf2 | NF-E2-related factor 2 (a transcription factor) |
| OS | oxidative stress |
| OSi | oxidative stress index |
| PMN | polymorphonuclear neutrophil |
| PPAR | peroxisome proliferator-activated receptor |
| PUFA | polyunsaturated fatty acids |
| PRRs | pathogen recognition receptors |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RP | retained placenta |
| SeCys | selenocysteine |
| SeMet | selenomethionine |
| SepP | selenoprotein P |
| Se-Yeast | selenized yeast |
| SOD | superoxide dismutase |
| SS | sodium selenite |
| TLR | Toll-like receptor |
References
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox Biology in Transition Periods of Dairy Cattle: Role in the Health of Periparturient and Neonatal Animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z. Effect of Heat Stress on Reproduction in Dairy Cows: Insights into the Cellular and Molecular Responses of the Oocyte. Annu. Rev. Anim. Biosci. 2017, 5, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.C.; Zoidis, E.; Chadio, S.E. Maternal Selenium and Developmental Programming. Antioxidants 2019, 8, 145. [Google Scholar] [CrossRef]
- Surai, P.F. Selenium in Poultry Nutrition and Health; Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. In vivo and in vitro testing for selenium and selenium compounds bioavailability assessment in foodstuff. Crit. Rev. Food Sci. Nutr. 2017, 57, 805–833. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I. Selenium in sow nutrition. Anim. Feed Sci. Technol. 2016, 211, 18–30. [Google Scholar] [CrossRef]
- Juniper, D.T.; Rymer, C.; Briens, M. Bioefficacy of hydroxy-selenomethionine as a selenium supplement in pregnant dairy heifers and on the selenium status of their calves. J. Dairy Sci. 2019, 102, 7000–7010. [Google Scholar] [CrossRef]
- Galbraith, M.L.; Vorachek, W.R.; Estill, C.T.; Whanger, P.D.; Bobe, G.; Davis, T.Z.; Hall, J.A. Rumen Microorganisms Decrease Bioavailability of Inorganic Selenium Supplements. Biol. Trace Elem. Res. 2016, 171, 338–343. [Google Scholar] [CrossRef]
- Sun, L.L.; Gao, S.T.; Wang, K.; Xu, J.C.; Sanz-Fernandez, M.V.; Baumgard, L.H.; Bu, D.P. Effects of source on bioavailability of selenium, antioxidant status, and performance in lactating dairy cows during oxidative stress-inducing conditions. J. Dairy Sci. 2019, 102, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Xiao, M. Effect of Organic Selenium Supplementation on Selenium Status, Oxidative Stress, and Antioxidant Status in Selenium-Adequate Dairy Cows During the Periparturient Period. Biol. Trace Elem. Res. 2018, 186, 430–440. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Santos, A.L.; Sinha, S.; Lindner, A.B. The Good, the Bad, and the Ugly of ROS: New Insights on Aging and Aging-Related Diseases from Eukaryotic and Prokaryotic Model Organisms. Oxid. Med. Cell. Longev. 2018, 2018, 1941285. [Google Scholar] [CrossRef]
- Yoshida, Y.; Umeno, A.; Akazawa, Y.; Shichiri, M.; Murotomi, K.; Horie, M. Chemistry of lipid peroxidation products and their use as biomarkers in early detection of diseases. J. Oleo Sci. 2015, 64, 347–356. [Google Scholar] [CrossRef]
- Chandra, G.; Aggarwal, A.; Singh, A.K.; Kumar, M.; Upadhyay, R.C. Effect of vitamin e and zinc supplementation on energy metabolites, lipid peroxidation, and milk production in peripartum sahiwal cows. Asian—Australas. J. Anim. Sci. 2013, 26, 1569–1576. [Google Scholar] [CrossRef]
- Grune, T.; Reinheckel, T.; Davies, K.J. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997, 11, 526–534. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Cui, Y.; Niedernhofer, L.J.; Wang, Y. Occurrence, Biological Consequences, and Human Health Relevance of Oxidative Stress-Induced DNA Damage. Chem. Res. Toxicol. 2016, 29, 2008–2039. [Google Scholar] [CrossRef] [Green Version]
- Kawai, Y.; Nuka, E. Abundance of DNA adducts of 4-oxo-2-alkenals, lipid peroxidation-derived highly reactive genotoxins. J. Clin. Biochem. Nutr. 2018, 62, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Drake, J.W.; Charlesworth, B.; Charlesworth, D.; Crow, J.F. Rates of spontaneous mutation. Genetics 1998, 148, 1667–1686. [Google Scholar]
- Colitti, M.; Stefanon, B.; Gabai, G.; Gelain, M.E.; Bonsembiante, F. Oxidative Stress and Nutraceuticals in the Modulation of the Immune Function: Current Knowledge in Animals of Veterinary Interest. Antioxidants 2019, 8, 28. [Google Scholar] [CrossRef]
- Putman, A.K.; Brown, J.L.; Gandy, J.C.; Wisnieski, L.; Sordillo, L.M. Changes in biomarkers of nutrient metabolism, inflammation, and oxidative stress in dairy cows during the transition into the early dry period. J. Dairy Sci. 2018, 101, 9350–9359. [Google Scholar] [CrossRef] [Green Version]
- Boudjellaba, S.; Ainouz, L.; Tennah, S.; Temim, S.; Iguer-Ouada, M. Reproduction performance and blood biochemical parameters in dairy cows: Relationship with oxidative stress status. Vet. World 2018, 11, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life: A molecular timescale from Earth’s earliest history to the rise of complex life. Bioessays 2018, 40, 3. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Buttemer, W.A.; Hulbert, A.J. Does the oxidative stress theory of aging explain longevity differences in birds? II. Antioxidant systems and oxidative damage. Exp. Gerontol. 2012, 47, 211–222. [Google Scholar] [CrossRef]
- Sun, L.H.; Huang, J.Q.; Deng, J.; Lei, X.G. Avian selenogenome: Response to dietary Se and vitamin E deficiency and supplementation. Poult. Sci. 2018. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I. Antioxidant systems in poultry biology: Nutritional modulation of vitagenes. Eur. J. Poult. Sci. 2017, 81. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y.; Zhang, J.; Hu, Q. Subcellular Redox Signaling. Adv. Exp. Med. Biol. 2017, 967, 385–398. [Google Scholar]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Rattan, S.I. The nature of gerontogenes and vitagenes. Antiaging effects of repeated heat shock on human fibroblasts. Ann. N. Y. Acad. Sci. 1998, 854, 54–60. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Aspects Med. 2011, 32, 279–304. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta 2012, 1822, 753–783. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, V.; Scapagnini, G.; Davinelli, S.; Koverech, G.; Koverech, A.; De Pasquale, C.; Salinaro, A.T.; Scuto, M.; Calabrese, E.J.; Genazzani, A.R. Sex hormonal regulation and hormesis in aging and longevity: Role of vitagenes. J. Cell Commun. Signal. 2014, 8, 369–384. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Crupi, R.; Di Paola, R.; Ruggieri, M.; Bianchini, R.; Ontario, M.L.; Cuzzocrea, S.; Calabrese, E.J. Hormesis, cellular stress response and neuroinflammation in schizophrenia: Early onset versus late onset state. J. Neurosci. Res. 2017, 95, 1182–1193. [Google Scholar] [CrossRef]
- Surai, P.F.; Fisinin, V.I. Vitagenes in poultry production: Part 3. Vitagene concept development. Worlds Poult. Sci. J. 2016, 72, 793–804. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Osorio, F.G.; Soria-Valles, C.; Santiago-Fernández, O.; Freije, J.M.; López-Otín, C. NF-κB signaling as a driver of ageing. Int. Rev. Cell Mol. Biol. 2016, 326, 133–174. [Google Scholar]
- Begalli, F.; Bennett, J.; Capece, D.; Verzella, D.; D’Andrea, D.; Tornatore, L.; Franzoso, G. Unlocking the NF-κB Conundrum: Embracing Complexity to Achieve Specificity. Biomedicines 2017, 5, 50. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, M.M.L.; Peralta, M.B.; Angeli, E.; Stassi, A.F.; Gareis, N.C.; Durante, L.; Cainelli, S.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Immune status during postpartum, peri-implantation and early pregnancy in cattle: An updated view. Anim. Reprod. Sci. 2019, 206, 1–10. [Google Scholar] [CrossRef]
- Roche, J.R.; Burke, C.R.; Crookenden, M.A.; Heiser, A.; Loor, J.L.; Meier, S.; Mitchell, M.D.; Phyn, C.V.C.; Turner, S.A. Fertility and the transition dairy cow. Reprod. Fertil. Dev. 2017, 30, 85–100. [Google Scholar] [CrossRef]
- Zebeli, Q.; Ghareeb, K.; Humer, E.; Metzler-Zebeli, B.U.; Besenfelder, U. Nutrition, rumen health and inflammation in the transition period and their role on overall health and fertility in dairy cows. Res. Vet. Sci. 2015, 103, 126–136. [Google Scholar] [CrossRef]
- Mordak, R.; Stewart, P.A. Periparturient stress and immune suppression as a potential cause of retained placenta in highly productive dairy cows: Examples of prevention. Acta Vet. Scand. 2015, 57, 84. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; Schukken, Y.H.; van Schaik, G. Quantifying calf mortality on dairy farms: Challenges and solutions. J. Dairy Sci. 2019, 102, 6404–6417. [Google Scholar] [CrossRef] [Green Version]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Celi, P.; Merlo, M.; Da Dalt, L.; Stefani, A.; Barbato, O.; Gabai, G. Relationship between late embryonic mortality and the increase in plasma advanced oxidised protein products (AOPP) in dairy cows. Reprod. Fertil. Dev. 2011, 23, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Ling, T.; Hernandez-Jover, M.; Sordillo, L.M.; Abuelo, A. Maternal late-gestation metabolic stress is associated with changes in immune and metabolic responses of dairy calves. J. Dairy Sci. 2018, 101, 6568–6580. [Google Scholar] [CrossRef]
- Laubenthal, L.; Ruda, L.; Sultana, N.; Winkler, J.; Rehage, J.; Meyer, U.; Dänicke, S.; Sauerwein, H.; Häussler, S. Effect of increasing body condition on oxidative stress and mitochondrial biogenesis in subcutaneous adipose tissue depot of nonlactating dairy cows. J. Dairy Sci. 2017, 100, 4976–4986. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Omidi, A.; Fathi, M.H.; Parker, M.O. Alterations of antioxidant status markers in dairy cows during lactation and in the dry period. J. Dairy Res. 2017, 84, 49–53. [Google Scholar] [CrossRef]
- Schubach, K.M.; Cooke, R.F.; Brandão, A.P.; Lippolis, K.D.; Silva, L.G.T.; Marques, R.S.; Bohnert, D.W. Impacts of stocking density on development and puberty attainment of replacement beef heifers. Animal 2017, 11, 2260–2267. [Google Scholar] [CrossRef] [Green Version]
- Alomari, E.; Bruno, S.; Ronda, L.; Paredi, G.; Bettati, S.; Mozzarelli, A. Protein carbonylation detection methods: A comparison. Data Brief 2018, 19, 2215–2220. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 2013, 7, 1374–1378. [Google Scholar] [CrossRef] [Green Version]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Kuhn, M.J.; Mavangira, V.; Gandy, J.C.; Sordillo, L.M. Production of 15-F(2t)-isoprostane as an assessment of oxidative stress in dairy cows at different stages of lactation. J. Dairy Sci. 2018, 101, 9287–9295. [Google Scholar] [CrossRef]
- Hanschke, N.; Kankofer, M.; Ruda, L.; Höltershinken, M.; Meyer, U.; Frank, J.; Dänicke, S.; Rehage, J. The effect of conjugated linoleic acid supplements on oxidative and antioxidative status of dairy cows. J. Dairy Sci. 2016, 99, 8090–8102. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Pedernera, M.; Celi, P.; García, S.C.; Salvin, H.E.; Barchia, I.; Fulkerson, W.J. Effect of diet, energy balance and milk production on oxidative stress in early-lactating dairy cows grazing pasture. Vet. J. 2010, 186, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kargar, S.; Ghorbani, G.R.; Fievez, V.; Schingoethe, D.J. Performance, bioenergetic status, and indicators of oxidative stress of environmentally heat-loaded Holstein cows in response to diets inducing milk fat depression. J. Dairy Sci. 2015, 98, 4772–4784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.L.; Wang, Y.M.; Zhou, H.L.; Liu, J.X. Effects of dietary adsorbent on milk aflatoxin M(1) content and the health of lactating dairy cows exposed to long-term aflatoxin B(1) challenge. J. Dairy Sci. 2018, 101, 8944–8953. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Kra, G.; Livshitz, L.; Portnick, Y.; Yakoby, S.; Friedlander, G.; Levin, Y. Seasonal heat stress affects adipose tissue proteome toward enrichment of the Nrf2-mediated oxidative stress response in late-pregnant dairy cows. J. Proteom. 2017, 158, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Skibiel, A.L.; Zachut, M.; do Amaral, B.C.; Levin, Y.; Dahl, G.E. Liver proteomic analysis of postpartum Holstein cows exposed to heat stress or cooling conditions during the dry period. J. Dairy Sci. 2018, 101, 705–716. [Google Scholar] [CrossRef]
- da Silva, A.D.; da Silva, A.S.; Baldissera, M.D.; Schwertz, C.I.; Bottari, N.B.; Carmo, G.M.; Machado, G.; Lucca, N.J.; Henker, L.C.; Piva, M.M.; et al. Oxidative stress in dairy cows naturally infected with the lungworm Dictyocaulus viviparus (Nematoda: Trichostrongyloidea). J. Helminthol. 2017, 91, 462–469. [Google Scholar] [CrossRef]
- Glombowsky, P.; Bottari, N.B.; Klauck, V.; Fávero, J.F.; Soldá, N.M.; Baldissera, M.D.; Perin, G.; Morsch, V.M.; Schetinger, M.R.C.; Stefani, L.M.; et al. Oxidative stress in dairy cows seropositives for Neospora caninum. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 34–37. [Google Scholar] [CrossRef]
- Fidan, A.F.; Cingi, C.C.; Karafakioglu, Y.S.; Utuk, A.E.; Pekkaya, S.; Piskin, F.C. The levels of antioxidant activity, malondialdehyde and nitric oxide in cows naturally infected with Neospora caninum. J. Anim. Vet. Adv. 2010, 9, 1707–1711. [Google Scholar] [CrossRef]
- Li, Y.; Ding, H.Y.; Wang, X.C.; Feng, S.B.; Li, X.B.; Wang, Z.; Liu, G.W.; Li, X.W. An association between the level of oxidative stress and the concentrations of NEFA and BHBA in the plasma of ketotic dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Gabai, G.; De Luca, E.; Miotto, G.; Zin, G.; Stefani, A.; Da Dalt, L.; Barberio, A.; Celi, P. Relationship between Protein Oxidation Biomarkers and Uterine Health in Dairy Cows during the Postpartum Period. Antioxidants 2019, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Urh, C.; Denißen, J.; Gerster, E.; Kraus, N.; Stamer, E.; Heitkönig, B.; Spiekers, H.; Sauerwein, H. Short communication: Pro- and antioxidative indicators in serum of dairy cows during late pregnancy and early lactation: Testing the effects of parity, different dietary energy levels, and farm. J. Dairy Sci. 2019, 102, 6672–6678. [Google Scholar] [CrossRef] [PubMed]
- Celi, P. The role of oxidative stress in small ruminants’ health and production. Rev. Bras. de Zootec. 2010, 39, 348–363. [Google Scholar] [CrossRef]
- Sordillo, L.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Jozwik, A.; Krzyzewski, J.; Strzalkowska, N.; Polawska, E.; Bagnicka, E.; Wierzbicka, A.; Niemczuk, K.; Lipinska, P.; Horbanczuk, J.O. Relations between the oxidative status, mastitis, milk quality and disorders of reproductive functions in dairy cows—A review. Anim. Sci. Pap. Rep. 2012, 30, 297–307. [Google Scholar]
- Agarwal, A.; Gupta, S.; Sekhon, L.; Shah, R. Redox considerations in female reproductive function and assisted reproduction: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 1375–1403. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Talukder, S.; Kerrisk, K.L.; Gabai, G.; Celi, P. Role of oxidant–antioxidant balance in reproduction of domestic animals. Anim. Prod. Sci. 2017, 57, 1588–1597. [Google Scholar] [CrossRef]
- Gilbert, R.O. Symposium review: Mechanisms of disruption of fertility by infectious diseases of the reproductive tract. J. Dairy Sci. 2019, 102, 3754–3765. [Google Scholar] [CrossRef] [Green Version]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Gandy, J.C.; Sordillo, L.M. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev. Vet. Med. 2019, 163, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Antončić-Svetina, M.; Turk, R.; Svetina, A.; Gereš, D.; Rekić, B.; Juretić, D. Lipid status, paraoxonase-1 activity and metabolic parameters in serum of heifers and lactating cows related to oxidative stress. Res. Vet. Sci. 2011, 90, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Han, L.Q.; Zhou, Z.; Ma, Y.; Batistel, F.; Osorio, J.S.; Loor, J.J. Phosphorylation of nuclear factor erythroid 2-like 2 (NFE2L2) in mammary tissue of Holstein cows during the periparturient period is associated with mRNA abundance of antioxidant gene networks. J. Dairy Sci. 2018, 101, 6511–6522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res. Vet. Sci. 2016, 108, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, R.; Kumar, H.; Singh, G.; Krishnan, B.B.; Dey, S. Depressed polymorphonuclear cell functions in periparturient cows that develop postpartum reproductive diseases. Vet. Res. Commun. 2017, 41, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kehrli, M.E., Jr.; Nonnecke, B.J.; Roth, J.A. Alterations in bovine lymphocyte function during the periparturient period. Am. J. Vet. Res. 1989, 50, 215–220. [Google Scholar]
- Kehrli, M.E., Jr.; Nonnecke, B.J.; Roth, J.A. Alterations in bovine neutrophil function during the periparturient period. Am. J. Vet. Res. 1989, 50, 207–214. [Google Scholar]
- Ateya, A.I.; Hussein, M.S.; Ghanem, H.M.; Saleh, R.M.; El-Domany, W.B.; Elseady, Y.Y. Expression profiles of immunity and reproductive genes during transition period in Holstein cattle. Reprod. Domest. Anim. 2018, 53, 352–358. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. (Berl). 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006. [Google Scholar]
- Bassel, L.L.; Caswell, J.L. Bovine neutrophils in health and disease. Cell Tissue Res. 2018, 371, 617–637. [Google Scholar] [CrossRef]
- Earley, B.; Buckham Sporer, K.; Gupta, S. Invited review: Relationship between cattle transport, immunity and respiratory disease. Animal 2017, 11, 486–492. [Google Scholar] [CrossRef]
- Mariotti, M.; Ridge, P.G.; Zhang, Y.; Lobanov, A.V.; Pringle, T.H.; Guigo, R.; Hatfield, D.L.; Gladyshev, V.N. Composition and evolution of the vertebrate and mammalian selenoproteomes. PLoS ONE 2012, 7, e33066. [Google Scholar] [CrossRef]
- Sordillo, L.M. Selenium-dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet. Med. Int. 2013, 2013, 154045. [Google Scholar] [CrossRef]
- Gladyshev, V.N. Eukaryotic proteomes. In Selenium. Its Molecular Biology and Role in Human Health, 4th ed.; Hatfield, D.L., Schweizer, U., Tsui, P.A., Gladyshev, V.N., Eds.; Springer: New York, NY, USA, 2016; pp. 127–139. [Google Scholar]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Duntas, L.H.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Velichko, O.A. Selenium in poultry nutrition: From sodium selenite to organic Se sources. J. Poult. Sci. 2018, 55, 79–93. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Zhao, B.; Xin, X.; Deng, X.; Zhang, H. Influence of long-term fertilization on selenium accumulation in soil and uptake by crops. Pedosphere 2016, 26, 120–129. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Irshad, S.; Abbas, Q.; Ahmad, R. A comprehensive review on environmental transformation of selenium: Recent advances and research perspectives. Environ. Geochem. Health 2019, 41, 1003–1035. [Google Scholar] [CrossRef]
- Van Metre, D.C.; Callan, R.J. Selenium and vitamin E. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 373–402. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Müller, A.; Bertram, A.; Freude, B. Differences in the selenium supply of cattle across Europe. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2014, 42, 131–144. [Google Scholar]
- Peter, D.W.; Whanger, P.D.; Lindsay, J.P.; Buscall, D.J. Excretion of selenium, zinc and copper by sheep receiving continuous intraruminal infusions of selenite or selenomethionine. Proc. Nutr. Soc. Austr. 1982, 7, 178–181. [Google Scholar]
- Cousins, F.R.; Cairney, I.M. Some aspects of selenium metabolism in sheep. Aust. J. Agric. Res. 1961, 12, 927–943. [Google Scholar] [CrossRef]
- Peterson, P.J.; Spedding, D.J. The excretion by sheep of 75-selenium incorporated into red clover: The chemical nature of the excreted selenium and its uptake by three plant species. N. Z. J. Agric. Res. 1963, 6, 13–23. [Google Scholar] [CrossRef]
- Spears, J.W. Trace mineral bioavailability in ruminants. J. Nutr. 2003, 133, 1506S–1509S. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Ha, P.; Gu, L. Determination of the selenomethionine content in grain and human blood. Wei Sheng Yan Jiu 1997, 26, 113–116. [Google Scholar]
- Cubadda, F.; Aureli, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Acharya, R.; Ramana, V.; Tejo Prakash, R.; Tejo Prakash, N. Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J. Agric. Food Chem. 2010, 58, 2295–2301. [Google Scholar] [CrossRef]
- Kretz-Remy, C.; Arrigo, A.P. Modulation of the chymotrypsin-like activity of the 20S proteasome by intracellular redox status: Effects of glutathione peroxidase-1 overexpression and antioxidant drugs. Biol. Chem. 2003, 384, 589–595. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Wright, P.L.; Bell, M.C. Comparative metabolism of selenium and tellurium in sheep and swine. Am. J. Physiol. 1966, 211, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid-base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef]
- Costa, N.D.; Glled, P.T.; Sansom, B.F.; Symonds, H.; Allen, W.M. Monensin and narasin increase selenium and zinc absorption in steers. In Trace Elements in Man and Animals; Mills, C.F., Bremner, I., Chesters, J.K., Eds.; Commonwealth Agricultural Bureaux: Slough, UK, 1985; pp. 472–474. [Google Scholar]
- Koenig, K.M.; Beauchemin, K.A. Supplementing selenium yeast to diets with adequate concentrations of selenium: Selenium status, thyroid hormone concentrations and passive transfer of immunoglobulins in dairy cows and calves. Can. J. Anim. Sci. 2009, 89, 111–122. [Google Scholar] [CrossRef]
- Koenig, K.M.; Buckley, W.T.; Shelford, J.A. True absorption of selenium in dairy cows: Stable isotope tracer methodology and effect of dietary copper. Can. J. Anim. Sci. 1991, 71, 175–183. [Google Scholar] [CrossRef]
- Kamada, H.; Terada, F.; Nishida, T.; Yoshida, H.; Hodate, K.; Shibata, M. Selenium balance in the late pregnancy and lactation of dairy cattle, and blood selenium concentration of dam and its calf. Anim. Sci. Technol. 1998, 69, 1044–1049. [Google Scholar]
- Podoll, K.L.; Bernard, J.B.; Ullrey, D.E.; Debar, S.R.; Ku, P.K.; Magee, W. Dietary selenate versus selenite for cattle, sheep, and horses. J. Anim. Sci. 1992, 70, 1965–1970. [Google Scholar] [CrossRef] [Green Version]
- Ortman, K.; Pehrson, B. Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J. Anim. Sci. 1999, 77, 3365–3370. [Google Scholar] [CrossRef]
- Mainville, A.M.; Odongo, N.E.; Bettger, W.J.; McBride, B.W.; Osborne, V.R. Selenium uptake by ruminal microorganisms from organic and inorganic sources in dairy cows. Can. J. Anim. Sci. 2009, 89, 105–110. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Jenkins, K.J.; Knipfel, J.E. Metabolism of selenomethionine in the rumen. Can. J. Anim. Sci. 1974, 54, 325–330. [Google Scholar]
- Serra, A.B.; Serra, S.D.; Shinchi, K.; Fujihara, T. Bioavailability of rumen bacterial selenium in mice using tissue uptake technique. Biol. Trace Elem. Res. 1997, 58, 255–261. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Yang, W.Z.; Dong, Q.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest. Sci. 2009, 126, 239–244. [Google Scholar] [CrossRef]
- Wei, J.Y.; Wang, J.; Liu, W.; Zhang, K.Z.; Sun, P. Short communication: Effects of different selenium supplements on rumen fermentation and apparent nutrient and selenium digestibility of mid-lactation dairy cows. J. Dairy Sci. 2019, 102, 3131–3135. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th rev. ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Andrieu, S. Is there a role for organic trace element supplements in transition cow health? Vet. J. 2008, 176, 77–83. [Google Scholar] [CrossRef]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef]
- Malbe, M.; Klaassen, M.; Fang, W.; Myllys, V.; Vikerpuur, M.; Nyholm, K.; Sankari, S.; Suoranta, K.; Sandholm, M. Comparisons of selenite and selenium yeast feed supplements on Se-incorporation, mastitis and leucocyte function in Se-deficient dairy cows. Zentralbl. Veterinarmed. A 1995, 42, 111–121. [Google Scholar] [CrossRef]
- Calamari, L.; Petrera, F.; Bertin, G. Effects of either sodium selenite or Se yeast (Sc CNCM I-3060) supplementation on selenium status and milk characteristics in dairy cows. Livest. Sci. 2010, 128, 154–165. [Google Scholar] [CrossRef]
- Gunter, S.A.; Beck, P.A.; Phillips, J.K. Effects of supplementary selenium source on the performance and blood measurements in beef cows and their calves. J. Anim. Sci. 2003, 81, 856–864. [Google Scholar] [CrossRef]
- Liao, S.F.; Brown, K.R.; Stromberg, A.J.; Burris, W.R.; Boling, J.A.; Matthews, J.C. Dietary supplementation of selenium in inorganic and organic forms differentially and commonly alters blood and liver selenium concentrations and liver gene expression profiles of growing beef heifers. Biol. Trace Elem. Res. 2011, 140, 151–169. [Google Scholar] [CrossRef]
- Cerny, K.L.; Garbacik, S.; Skees, C.; Burris, W.R.; Matthews, J.C.; Bridges, P.J. Gestational form of Selenium in Free-Choice Mineral Mixes Affects Transcriptome Profiles of the Neonatal Calf Testis, Including those of Steroidogenic and Spermatogenic Pathways. Biol. Trace Elem. Res. 2016, 169, 56–68. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Ramos-Morales, E.; Bertin, G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in beef cattle. J. Anim. Sci. 2008, 86, 3100–3109. [Google Scholar] [CrossRef]
- Gong, J.; Ni, L.; Wang, D.; Shi, B.; Yan, S. Effect of dietary organic selenium on milk selenium concentration and antioxidant and immune status in midlactation dairy cows. Livest. Sci. 2014, 170, 84–90. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Liu, W.; Bu, D.P.; Liu, S.J.; Zhang, K.Z. Hydroxy-selenomethionine: A novel organic selenium source that improves antioxidant status and selenium concentrations in milk and plasma of mid-lactation dairy cows. J. Dairy Sci. 2017, 100, 9602–9610. [Google Scholar] [CrossRef] [Green Version]
- Phipps, R.H.; Grandison, A.S.; Jones, A.K.; Juniper, D.T.; Ramos-Morales, E.; Bertin, G. Selenium supplementation of lactating dairy cows: Effects on milk production and total selenium content and speciation in blood, milk and cheese. Animal 2008, 2, 1610–1618. [Google Scholar] [CrossRef]
- Guyot, H.; Spring, P.; Andrieu, S.; Rollin, F. Comparative responses to sodium selenite and organic selenium supplements in Belgian Blue cows and calves. Livest. Sci. 2007, 111, 259–263. [Google Scholar] [CrossRef]
- Heard, J.W.; Stockdale, C.R.; Walker, G.P.; Leddin, C.M.; Dunshea, F.R.; McIntosh, G.H.; Shields, P.M.; McKenna, A.; Young, G.P.; Doyle, P.T. Increasing selenium concentration in milk: Effects of amount of selenium from yeast and cereal grain supplements. J. Dairy Sci. 2007, 90, 4117–4127. [Google Scholar] [CrossRef]
- Slavik, P.; Illek, J.; Brix, M.; Hlavicova, J.; Rajmon, R.; Jilek, F. Influence of organic versus inorganic dietary selenium supplementation on the concentration of selenium in colostrum, milk and blood of beef cows. Acta Vet. Scand. 2008, 50, 43. [Google Scholar] [CrossRef]
- Gunter, S.A.; Beck, P.A.; Hallford, D.M. Effects of supplementary selenium source on the blood parameters in beef cows and their nursing calves. Biol. Trace Elem. Res. 2013, 152, 204–211. [Google Scholar] [CrossRef]
- Oltramari, C.E.; Pinheiro, M.D.G.; De Miranda, M.S.; Arcaro, J.R.; Castelani, L.; Toledo, L.M.; Ambrósio, L.A.; Leme, P.R.; Manella, M.Q.; Júnior, I.A. Selenium sources in the diet of dairy cows and their effects on milk production and quality, on udder health and on physiological indicators of heat stress. Ital. J. Anim. Sci. 2014, 13, 2921. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Chernov, N.N.; Novichkova, M.D. Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes. Biochemistry (Mosc) 2014, 79, 1562–1583. [Google Scholar] [CrossRef]
- Surai, P.F. Antioxidant systems in poultry biology: Superoxide dismutase. J. Anim. Res. Nutr. 2016, 1, 1. [Google Scholar] [CrossRef]
- Saran Netto, A.; Salles, M.S.V.; Roma Júnior, L.C.; Cozzolino, S.M.F.; Gonçalves, M.T.M.; Freitas Júnior, J.E.; Zanetti, M.A. Increasing Selenium and Vitamin E in Dairy Cow Milk Improves the Quality of the Milk as Food for Children. Nutrients 2019, 11, 1218. [Google Scholar] [CrossRef]
- Ceballos, A.; Sánchez, J.; Stryhn, H.; Montgomery, J.B.; Barkema, H.W.; Wichtel, J.J. Meta-analysis of the effect of oral selenium supplementation on milk selenium concentration in cattle. J. Dairy Sci. 2009, 92, 324–342. [Google Scholar] [CrossRef] [Green Version]
- Givens, D.I.; Allison, R.; Cottrill, B.; Blake, J.S. Enhancing the selenium content of bovine milk through alteration of the form and concentration of selenium in the diet of the dairy cow. J. Sci. Food Agric. 2004, 84, 811–817. [Google Scholar] [CrossRef]
- Juniper, D.T.; Bertin, G. Distribution of total selenium and selenium species within the tissues and products of food producing animals offered diets containing either selenoyeasts or sodium selenite. In Proceedings of the TEMA14, Enshi, China, 19–24 September 2011. [Google Scholar]
- Walker, G.P.; Dunshea, F.R.; Heard, J.W.; Stockdale, C.R.; Doyle, P.T. Output of selenium in milk, urine, and feces is proportional to selenium intake in dairy cows fed a total mixed ration supplemented with selenium yeast. J. Dairy Sci. 2010, 93, 4644–4650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehrson, B.; Ortman, K.; Madjid, N.; Trafikowska, U. The influence of dietary selenium as selenium yeast or sodium selenite on the concentration of selenium in the milk of suckler cows and on the selenium status of their calves. J. Anim. Sci. 1999, 77, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Olson, G.E.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Kurokawa, S. Maternal-fetal transfer of selenium in the mouse. FASEB J. 2013, 27, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C.; Peters, J.C. Long-term effects of dietary organic and inorganic selenium sources and levels on reproducing sows and their progeny. J. Anim. Sci. 2004, 82, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Reed, J.J.; Neville, T.L.; Thorson, J.F.; Maddock-Carlin, K.R.; Taylor, J.B.; Reynolds, L.P.; Redmer, D.A.; Luther, J.S.; Hammer, C.J.; et al. Nutritional plane and selenium supply during gestation affect yield and nutrient composition of colostrum and milk in primiparous ewes. J. Anim. Sci. 2011, 89, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Dinse, D.; Khol-Parisini, A.; Schafft, H.; Lahrssen-Wiederholt, M.; Schreiner, M.; Scharek-Tedin, L.; Zentek, J. Colostrum and milk selenium, antioxidative capacity and immune status of dairy cows fed sodium selenite or selenium yeast. Arch. Anim. Nutr. 2013, 67, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.P.; Hogan, J.S. Effect of selenium source on selenium status, neutrophil function, and response to intramammary endotoxin challenge of dairy cows. J. Dairy Sci. 2005, 88, 4366–4374. [Google Scholar] [CrossRef]
- Apperson, K.D.; Vorachek, W.R.; Dolan, B.P.; Bobe, G.; Pirelli, G.J.; Hall, J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on passive transfer of ovalbumin in their newborn calves. J. Trace Elem. Med. Biol. 2018, 50, 640–645. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of supranutritional maternal and colostral selenium supplementation on passive absorption of immunoglobulin G in selenium-replete dairy calves. J. Dairy Sci. 2014, 97, 4379–4391. [Google Scholar] [CrossRef]
- Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Burk, R.F. Selenoprotein P is the major selenium transport protein in mouse milk. PLoS ONE 2014, 9, e103486. [Google Scholar] [CrossRef]
- Dalgaard, T.S.; Briens, M.; Engberg, R.M.; Lauridsen, C. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Anim. Feed Sci. Technol. 2018, 238, 73–83. [Google Scholar] [CrossRef]
- Matthews, J.C.; Zhang, Z.; Patterson, J.D.; Bridges, P.J.; Stromberg, A.J.; Boling, J.A. Hepatic transcriptome profiles differ among maturing beef heifers supplemented with inorganic, organic, or mixed (50% inorganic:50% organic) forms of dietary selenium. Biol. Trace Elem. Res. 2014, 160, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, Y.; Burris, W.R.; Bridges, P.J.; Matthews, J.C. Forms of selenium in vitamin-mineral mixes differentially affect the expression of genes responsible for prolactin, ACTH, and α-MSH synthesis and mitochondrial dysfunction in pituitaries of steers grazing endophyte-infected tall fescue. J. Anim. Sci. 2019, 97, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, L.H.; Huang, J.Q.; Briens, M.; Qi, D.S.; Xu, S.W.; Lei, X.G. A novel organic selenium compound exerts unique regulation of selenium speciation, selenogenome, and selenoproteins in broiler chicks. J. Nutr. 2017, 147, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Muegge, C.R.; Brennan, K.M.; Schoonmaker, J.P. Supplementation of organic and inorganic selenium to late gestation and early lactation beef cows effect on progeny feedlot performance and carcass characteristics. J. Anim. Sci. 2017, 95, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Mohrekesh, M.; Shahraki, A.F.; Ghalamkari, G.R.; Guyot, H. Effects of three methods of oral selenium-enriched yeast supplementation on blood components and growth in Holstein dairy calves. Anim. Prod. Sci. 2019, 59, 260–265. [Google Scholar] [CrossRef]
- Cerri, R.L.; Rutigliano, H.M.; Lima, F.S.; Araújo, D.B.; Santos, J.E. Effect of source of supplemental selenium on uterine health and embryo quality in high-producing dairy cows. Theriogenology 2009, 71, 1127–1137. [Google Scholar] [CrossRef]
- Muegge, C.R.; Brennan, K.M.; Schoonmaker, J.P. Supplementation of organic and inorganic selenium to late gestation and early lactation beef cows effect on cow and preweaning calf performance. J. Anim. Sci. 2016, 94, 3399–3408. [Google Scholar] [CrossRef] [PubMed]
- Kegley, E.B.; Ball, J.J.; Beck, P.A. Impact of mineral and vitamin status on beef cattle immune function and health. J. Anim. Sci. 2016, 94, 5401–5413. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Blalock, H.M.; Miller, L.C.; Loveday, H.D. Selenium in tissues of calves supplemented with selenium yeast. J. Anim. Sci. 2004, 82, 269. [Google Scholar]
- Khatti, A.; Mehrotra, S.; Patel, P.K.; Singh, G.; Maurya, V.P.; Mahla, A.S.; Chaudhari, R.K.; Das, G.K.; Singh, M.; Sarkar, M.; et al. Supplementation of vitamin E, selenium and increased energy allowance mitigates the transition stress and improves postpartum reproductive performance in the crossbred cow. Theriogenology 2017, 104, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fisinin, V.I. Selenium in poultry breeder nutrition: An update. Anim. Feed Sci. Technol. 2014, 191, 1–15. [Google Scholar] [CrossRef]
- Juniper, D.T.; Phipps, R.H.; Bertin, G. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in commercial-line turkeys. Animal 2011, 5, 1751–1760. [Google Scholar] [CrossRef]
- Juniper, D.T.; Bertin, G. Effects of dietary selenium supplementation on tissue selenium distribution and glutathione peroxidase activity in Chinese Ring necked Pheasants. Animal 2013, 7, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.G.; Bobe, G.; Vorachek, W.R.; Dolan, B.P.; Estill, C.T.; Pirelli, G.J.; Hall, J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn calves. J. Anim. Sci. 2017, 95, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Prevedello, P.; Stefani, A.L.; Piron, A.; Contiero, B.; Lante, A.; Gottardo, F.; Chevaux, E. Effect of dietary supplementation with different sources of selenium on growth response, selenium blood levels and meat quality of intensively finished Charolais young bulls. Animal 2011, 5, 1531–1538. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Van Saun, R.J.; Bobe, G.; Stewart, W.C.; Vorachek, W.R.; Mosher, W.D.; Nichols, T.; Forsberg, N.E.; Pirelli, G.J. Organic and inorganic selenium: I. Oral bioavailability in ewes. J. Anim. Sci. 2012, 90, 568–576. [Google Scholar] [CrossRef]
- Stewart, W.C.; Bobe, G.; Vorachek, W.R.; Pirelli, G.J.; Mosher, W.D.; Nichols, T.; Van Saun, R.J.; Forsberg, N.E.; Hall, J.A. Organic and inorganic selenium: II. Transfer efficiency from ewes to lambs. J. Anim. Sci. 2012, 90, 577–584. [Google Scholar] [CrossRef]
- Vignola, G.; Lambertini, L.; Mazzone, G.; Giammarco, M.; Tassinari, M.; Martelli, G.; Bertin, G. Effects of selenium source and level of supplementation on the performance and meat quality of lambs. Meat Sci. 2009, 81, 678–685. [Google Scholar] [CrossRef]
- Sevcikova, L.; Pechova, A.; Pavlata, L.; Antos, D.; Mala, E.; Palenik, T.; Panev, A.; Dvorak, R. The effect of various forms of selenium supplied to pregnant goats on the levels of selenium in the body of their kids at the time of weaning. Biol. Trace Elem. Res. 2011, 143, 882–892. [Google Scholar] [CrossRef]
- Reczyńska, D.; Witek, B.; Jarczak, J.; Czopowicz, M.; Mickiewicz, M.; Kaba, J.; Zwierzchowski, L.; Bagnicka, E. The impact of organic vs. inorganic selenium on dairy goat productivity and expression of selected genes in milk somatic cells. J. Dairy Res. 2019, 86, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Calamari, L.; Abeni, F.; Bertin, G. Metabolic and hematological profiles in mature horses supplemented with different selenium sources and doses. J. Anim. Sci. 2010, 88, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Calamari, L.; Ferrari, A.; Bertin, G. Effect of selenium source and dose on selenium status of mature horses. J. Anim. Sci. 2009, 87, 167–178. [Google Scholar] [CrossRef] [PubMed]
| Free Radicals | Non-Radicals |
|---|---|
| Reactive Oxygen Species (ROS) | |
| Superoxide, O2*− | Hydrogen peroxide, H2O2 |
| Hydroxyl, OH* | Organic peroxides, ROOH |
| Hydroperoxyl, HO2* | Peroxinitrite, ONOO− |
| Peroxyl, RO2* | Hypochlorous acid, HOCl |
| Alkoxyl, RO* | Hypobromous acid, HOBr |
| Reactive Nitrogen Species (RNS) | |
| Nitric oxide, NO* | Nitrous acid, HNO2 |
| Nitrogen dioxide, NO2* | Dinitrogen trioxide, N2O3 |
| Nitrate radical, NO3* | Dinitrogen tetroxide, N2O4 |
| Biomarker | Advantages | Disadvantages |
|---|---|---|
| MDA | Sensitive and reproducible | Non-specific product of lipid peroxidation |
| TBARS | Rapid, popular, easy, and economical | Non-specific, non-reproducible, no quantitative relationship with lipid peroxidation |
| F2-Isoprostane | Specific, reproducible, sensitive | Expensive, auto-oxidation of samples, sample derivatisation is required |
| ORAC | Sensitive and covers a wide variety of antioxidants | Requires spectrofluorometer; AAPH, a free radical source is sensitive to temperature, low reactivity of fluorescein toward ROO• radicals |
| FRAP | inexpensive, reagents are simple to prepare, results are highly reproducible, and the procedure is straightforward and speedy | The reaction is non-specific, and the result of the test depends on the reaction time. |
| TEAC | Extremely fast and simple | Results vary with sample dilution; antioxidant used may interact with solvent molecules; specificity varies |
| TRAP | Gives an idea of the rate of free radical formation | Antioxidant employed may not trap all types of free radicals |
| ROMs | Extremely fast, simple; can be performed directly in whole blood, inflammatory fluids, cell extracts and respiratory condensate | Inhibited by sodium azide, lack of reference values |
| RONS | Fast, commercial Kits are available | lack of reference values |
| BAP | fast, simple and covers a wide variety of antioxidants | Can be performed only in plasma and serum samples; hyperlipemic samples can underestimate results |
| AOPPs | Novel markers of protein oxidation, quickly developing, mediators of pro-inflammatory response | lack of reference values |
| Protein carbonylation | Easy to perform | lack of reference values |
| AO enzymes (SOD, GSH-Px, Catalase, etc.) | Common, widely used tests, commercial kits are available | Difficulties with results interpretation, since some enzymes are stress-inducible |
| Plasma total thiols | Important part of the Redox system, commercial kits are available | Very sensitive to oxidation during sample preparation and storage |
| Non-enzymatic antioxidants: glutathione, α-tocopherol, β-carotene, uric acid, etc. | Common, widely used tests. | Individually reflect only a small proportion of the antioxidant defence potential |
| HSP | Important elements of antistress protection | Difficult to perform, difficulties with results interpretation, since HSP are stress-inducible |
| Conditions | Markers | References |
|---|---|---|
| Biological/Metabolic Stresses | ||
| Periparturient dairy cow | Plasma ROS + RNS↑, AOA↓, OSi↑, 15-F2t-isoprostane↑, TBARS↑, hydroperoxides↑ | [62,63] |
| Dairy cow at the end of the first week (Day 7) after parturition | GSH↓, GSH-Px↓, CAT↓, vitamin E↓, T-AOC↓, ROS↑, H2O2↑, MDA↑ | [12,64] |
| Nutritional Stresses | ||
| Dairy cows with body weight and body condition increase due to a ration of increasing energy density for 15 wk | dROM↑, TBARS↑ | [53] |
| Dairy cows in severe negative energy balance during early lactation | BAP↓ | [65] |
| Fish oil-fed dairy cows | Plasma MDA↑, AST↑, ALP↑ | [66] |
| Dairy cows fed AFB1-contaminated diets | MDA↑, SOD↓, GSH-Px↓, T-AOA↓ | [67] |
| Environmental Stresses | ||
| Heat stress in late-pregnant dairy cows | MDA↑, cortisol↑, Nrf2-mediated oxidative stress response↑ | [68] |
| Heat stress in postpartum Holstein cows | Oxidative phosphorylation↑, mitochondria disfunction↑, Nrf2-mediated oxidative stress response↑ | [69] |
| Pathogen/Disease Stresses | ||
| Dairy cows naturally infected with the lungworm Dictyocaulus viviparus (Nematoda: Trichostrongyloidea). | TBARS↑, ROS↑, SOD↑, CAT↓ | [70] |
| Dairy cows seropositive and symptomatic for Neospora caninum | serum ROS↑, TBARS↑, NO↑, GST↓, T-AOA↓ | [71,72] |
| Ketotic dairy cows | plasma SOD↓, CAT↓, vitamin C↓, vitamin E↓, hydroxyl radical capacity↓, H2O2↑, MDA↑ | [73] |
| Dairy cows with Grade 2 Endometritis | AOOP↑ | [74] |
| Parameter | Effect of Organic vs. Inorganic Selenium | References |
|---|---|---|
| Se in cow plasma | Increased | [138] |
| Se in cow serum | Increased | [11] |
| Se in cow whole blood | Increased | [139,140,141,142,143] |
| Se in cow milk | Increased | [138,143,144,145,146,147] |
| Se in cow whole blood, red blood cells and liver | Increased | [140] |
| Se in cow cheese | Increased | [145] |
| SeMet in cow milk | Increased | [145] |
| Se in cow colostrum | Increased | [9,148] |
| Se in heart, kidney and muscle of beef cattle | Increased | [142] |
| Se in whole blood of calves at birth | Increased | [149] |
| Se in whole blood of calves | Increased | [149] |
| Se in plasma of calves | Increased | [148] |
| GSH-Px in serum of cows | Increased | [143] |
| GSH-Px in whole blood of cows | Increased | [142,148] |
| GSH-Px in erythrocytes of calves at birth | Increased | [149] |
| SelP in serum of cows | Increased | [143] |
| TrxR in serum of cows | Increased | [143] |
| Total AOA in serum of cows | Increased | [143] |
| Catalase in serum of cows | Increased | [143] |
| IL1 in serum of cows | Increased | [143] |
| IgA in serum of cows | Increased | [143] |
| Somatic cell counts | Decreased | [150] |
| Fat in milk | Increased | [150] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals 2019, 9, 462. https://doi.org/10.3390/ani9070462
Surai PF, Kochish II, Fisinin VI, Juniper DT. Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals. 2019; 9(7):462. https://doi.org/10.3390/ani9070462
Chicago/Turabian StyleSurai, Peter F., Ivan I. Kochish, Vladimir I. Fisinin, and Darren T. Juniper. 2019. "Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition" Animals 9, no. 7: 462. https://doi.org/10.3390/ani9070462
APA StyleSurai, P. F., Kochish, I. I., Fisinin, V. I., & Juniper, D. T. (2019). Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals, 9(7), 462. https://doi.org/10.3390/ani9070462