Diet-Induced Rabbit Models for the Study of Metabolic Syndrome
Abstract
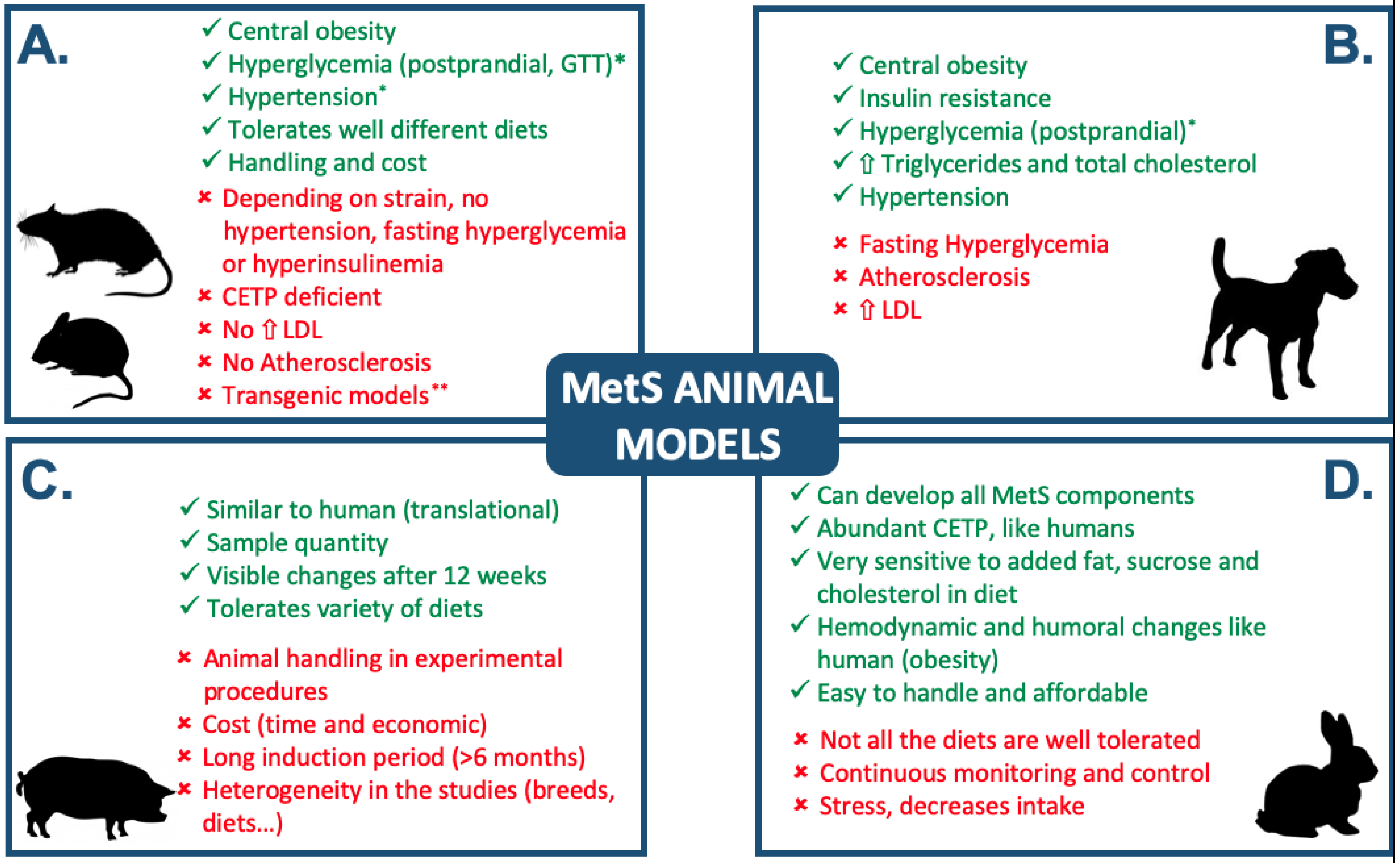
Simple Summary
Abstract
1. Introduction
2. Diet Regimes
2.1. High-Fat Enriched Diet
2.2. Diet Supplemented with Cholesterol
2.3. Sucrose and Fat-Enriched Diet
2.4. Fructose and Fat-Enriched Diet
3. Other Experimental Models
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.; James, W.P.T.; Loria, C.M.; Sidney, C.S., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.M.; Girman, C.; Rhodes, T.; Nijpels, G.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation 2005, 112, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Balkau, B. Epidemiology of the metabolic syndrome and the RISC study. Eur. Heart J. Suppl. 2005, 7, 6–9. [Google Scholar]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef]
- Schmerbach, K.; Patzak, A. The metabolic syndrome: Is it the mother’s fault? Acta Physiol. 2014, 210, 702–704. [Google Scholar] [CrossRef]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Aromolaran, A.S.; Boutjdir, M. Cardiac ion channel regulation in obesity and the metabolic syndrome: Relevance to long QT syndrome and atrial fibrillation. Front. Physiol. 2017, 8, 431. [Google Scholar] [CrossRef]
- Rozendaal, Y.J.W.; Wang, Y.; Paalvast, Y.; Tambyrajah, L.L.; Li, Z.; Van Dijk, K.W.; Rensen, P.C.N.; Kuivenhoven, J.A.; Groen, A.K.; Hilbers, P.A.J.; et al. In vivo and in silico dynamics of the development of Metabolic Syndrome. PLoS Comput. Biol. 2018, 14, e1006145. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Asrafuzzaman, M.; Cao, Y.; Afroz, R.; Kamato, D.; Gray, S.; Little, P.J. Animal models for assessing the impact of natural products on the aetiology and metabolic pathophysiology of Type 2 diabetes. Biomed. Pharmacother. 2017, 89, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Arias-Mutis, O.J.; Marrachelli, V.G.; Ruiz-Sauri, A.; Alberola, A.; Morales, J.M.; Such-Miquel, L.; Monleón, D.; Chorro, F.J.; Such, L.; Zarzoso, M. Development and characterization of an experimental model of diet-induced metabolic syndrome in rabbit. PLoS ONE 2017, 12, e0178315. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Arias-Mutis, O.J.; Genovés, P.; Calvo, C.J.; Diaz, A.; Parra, G.; Such-Miquel, L.; Such, L.; Alberola, A.; Chorro, F.J.; Zarzoso, M. An Experimental Model of Diet-Induced Metabolic Syndrome in Rabbit: Methodological Considerations, Development, and Assessment. J. Vis. Exp. 2018, 134, e57117. [Google Scholar] [CrossRef] [PubMed]
- Bertram, C.E.; Hanson, M.A. Animal models and programming of the metabolic syndrome. Br. Med. Bull. 2001, 60, 103–121. [Google Scholar] [CrossRef]
- Zhang, X.; Lerman, L.O. Investigating the Metabolic Syndrome. Toxicol. Pathol. 2016, 44, 358–366. [Google Scholar] [CrossRef]
- Carroll, J.F.; Dwyer, T.M.; Grady, A.W.; Reinhart, G.A.; Montani, J.P.; Cockrell, K.; Meydrech, E.F.; Mizelle, H.L. Hypertension, cardiac hypertrophy, and neurohumoral activity in a new animal model of obesity. Am. J. Physiol. 1996, 271, H373–H378. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [PubMed]
- Go, J.L.; Prem, K.; Al-Hijji, M.A.; Qin, Q.; Noble, C.; Young, M.D.; Lerman, L.O.; Lerman, A. Experimental Metabolic Syndrome Model Associated with Mechanical and Structural Degenerative Changes of the Aortic Valve. Sci. Rep. 2018, 8, 17835. [Google Scholar] [PubMed]
- Waqar, A.B.; Koike, T.; Yu, Y.; Inoue, T.; Aoki, T.; Liu, E.; Fan, J. High-fat diet without excess calories induces metabolic disorders and enhances atherosclerosis in rabbits. Atherosclerosis 2010, 213, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Watanabe, T. Cholesterol-fed Atherosclerosis and Transgenic Rabbit Models for the Study of Atherosclerosis. J. Atheroscler. Thromb. 2000, 7, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J. Animal lipoproteins: Chemistry, structure, and comparative aspects. J. Lipid Res. 1980, 21, 789–853. [Google Scholar] [PubMed]
- Taylor, J.M. Transgenic rabbit models for the study of atherosclerosis. Ann. N. Y. Acad. Sci. 1997, 811, 146–154. [Google Scholar] [CrossRef]
- Fan, J.; Wang, J.; Bensadoun, A.; Lauer, S.J.; Dang, Q.; Mahley, R.W.; Taylor, J.M. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8724–8728. [Google Scholar] [CrossRef]
- Shore, V.G.; Shore, B.; Hart, R.G. Changes in Apolipoproteins and Properties of Rabbit Very Low Density Lipoproteins on Induction of Cholesteremia. Biochemistry 1974, 13, 1579–1585. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Al-Sagheer, A.A.; Naiel, M.A.; Saadeldin, I.M.; Swelum, A.A. Dietary cold pressed watercress and coconut oil mixture enhances growth performance, intestinal microbiota, antioxidant status, and immunity of growing rabbits. Animals 2018, 8, 212. [Google Scholar]
- Filippi, S.; Vignozzi, L.; Morelli, A.; Chavalmane, A.K.; Sarchielli, E.; Fibbi, B.; Saad, F.; Sandner, P.; Ruggiano, P.; Vannelli, G.B.; et al. Testosterone Partially Ameliorates Metabolic Profile and Erectile Responsiveness to PDE5 Inhibitors in an Animal Model of Male Metabolic Syndrome. J. Sex. Med. 2009, 6, 3274–3288. [Google Scholar] [CrossRef]
- Helfenstein, T.; Fonseca, F.A.; Ihara, S.S.; Bottós, J.M.; Moreira, F.T.; Pott, H.; Farah, M.E.; Martins, M.C.; Izar, M.C.; Pott, H., Jr. Impaired glucose tolerance plus hyperlipidaemia induced by diet promotes retina microaneurysms in New Zealand rabbits. Int. J. Exp. Pathol. 2011, 92, 40–49. [Google Scholar] [CrossRef]
- Ning, B.; Wang, X.; Yu, Y.; Waqar, A.B.; Yu, Q.; Koike, T.; Shiomi, M.; Liu, E.; Wang, Y.; Fan, J. High-fructose and high-fat diet-induced insulin resistance enhances atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Nutr. Metab. 2015, 12, 30. [Google Scholar] [CrossRef]
- Yin, W.; Yuan, Z.; Wang, Z.; Yang, B.; Yang, Y. A diet high in saturated fat and sucrose alters glucoregulation and induces aortic fatty streaks in New Zealand White rabbits. Int. J. Exp. Diabetes Res. 2002, 3, 179–184. [Google Scholar] [CrossRef]
- Liu, Y.R.; Li, B.; Li, M.H.; Yu, Y.H.; Wang, Z.M.; Chen, S.L. Improvement of cardiac dysfunction by bilateral surgical renal denervation in animals with diabetes induced by high fructose and high fat diet. Diabetes Res. Clin. Pract. 2016, 115, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.; Henn, C.; Drewniak, E.; Lesieur-Brooks, A.; Machan, J.; Crisco, J.; Ehrlich, M. High dietary fat and the development of osteoarthritis in a rabbit model. Osteoarthr. Cartil. 2012, 20, 584–592. [Google Scholar] [CrossRef]
- Peiretti, P.G. Effects of Dietary Fatty Acids on Lipid Traits in the Muscle and Perirenal Fat of Growing Rabbits Fed Mixed Diets. Animals 2012, 2, 55–67. [Google Scholar] [CrossRef]
- Drimba, L.; Hegedűs, C.; Yin, D.; Sári, R.; Németh, J.; Szilvássy, Z.; Peitl, B. Beneficial Cardiac Effects of Cicletanine in Conscious Rabbits With Metabolic Syndrome. J. Cardiovasc. Pharmacol. 2012, 60, 208–218. [Google Scholar] [CrossRef]
- Niimi, M.; Yang, D.; Kitajima, S.; Ning, B.; Wang, C.; Li, S.; Liu, E.; Zhang, J.; Chen, Y.E.; Fan, J. ApoE knockout rabbits: A novel model for the study of human hyperlipidemia. Atherosclerosis 2016, 245, 187–193. [Google Scholar] [CrossRef]
- Nielsen, S.; Karpe, F. Determinants of VLDL-triglycerides production. Curr. Opin. Lipidol. 2012, 23, 321–326. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Klein, S.; Carraro, F.; Weber, J.M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. J. Physiol. Metab. 1990, 258, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, G.; Roco, J.; Medina, M.; Medina, A.; Peral, M.; Jerez, S. High fat diet-induced metabolically obese and normal weight rabbit model shows early vascular dysfunction: Mechanisms involved. Int. J. Obes. 2018, 42, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Cervera, C.; Blas, E.; Fernández-Carmona, J. Growth of Rabbits under Different Environmental Temperatures Using High Fat Diets. World Rabbit Sci. 1997, 5, 71–75. [Google Scholar] [CrossRef]
- Halade, G.; Rahman, M.; Williams, P.; Fernandes, G. High Fat Diet-Induced Animal Model of a Age-associated Obesity and Osteoporosis. J. Nutr. Biochem. 2010, 21, 1162–1169. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, S.; Vignozzi, L.; Filippi, S.; Gurrieri, B.; Comeglio, P.; Morelli, A.; Danza, G.; Bartolucci, G.; Maggi, M.; Baldi, E.; et al. Metabolic syndrome-associated sperm alterations in an experimental rabbit model: Relation with metabolic profile, testis and epididymis gene expression and effect of tamoxifen treatment. Mol. Cell. Endocrinol. 2015, 401, 12–24. [Google Scholar] [CrossRef]
- Morelli, A.; Comeglio, P.; Filippi, S.; Sarchielli, E.; Vignozzi, L.; Maneschi, E.; Cellai, I.; Gacci, M.; Lenzi, A.; Vannelli, G.B.; et al. Mechanism of action of phosphodiesterase type 5 inhibition in metabolic syndrome-associated prostate alterations: An experimental study in the rabbit. Prostate 2013, 73, 428–441. [Google Scholar] [CrossRef]
- Maneschi, E.; Cellai, I.; Aversa, A.; Mello, T.; Filippi, S.; Comeglio, P.; Bani, D.; Guasti, D.; Sarchielli, E.; Salvatore, G.; et al. Tadalafil reduces visceral adipose tissue accumulation by promoting preadipocytes differentiation towards a metabolically healthy phenotype: Studies in rabbits. Mol. Cell. Endocrinol. 2016, 424, 50–70. [Google Scholar] [CrossRef]
- Murray, R.K.; Mayes, P.A.; Granner, D.K.; Rodwell, V.W. Harper’s Illustrated Biochemistry, 29th ed.; Section 2: Bioenergetics and the Metabolism of Carbohydrates and Lipids; Mc Graw-Hill Education: Columbus, OH, USA, 2012. [Google Scholar]
- Zhao, S.; Chu, Y.; Zhang, C.; Lin, Y.; Xu, K.; Yang, P.; Fan, J.; Liu, E. Diet-induced central obesity and insulin resistance in rabbits. J. Anim. Physiol. Anim. Nutr. 2008, 92, 105–111. [Google Scholar] [CrossRef]
- Bray, G.A. How bad is fructose? Am. J. Clin. Nutr. 2007, 86, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Albright, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition Recommendations and Interventions for Diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31, S61–S78. [Google Scholar]
- Jürgens, H.; Haass, W.; Castaneda, T.R.; Schürmann, A.; Koebnick, C.; Dombrowski, F.; Otto, B.; Nawrocki, A.R.; Scherer, P.E.; Spranger, J.; et al. Consuming Fructose-sweetened Beverages Increases Body Adiposity in Mice. Obes. Res. 2005, 13, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W. Health implications of fructose consumption: A review of recent data. Nutr. Metab. 2010, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar]
- Warren, R.J.; Ebert, D.L.; Mitchell, A.; Barter, P.J. Rabbit hepatic lipase cDNA sequence: Low activity is associated with low messenger RNA levels. J. Lipid Res. 1991, 32, 1333–1339. [Google Scholar]
- Rothblat, G.H.; Mahlberg, F.H.; Johnson, W.J.; Phillips, M.C. Apolipoproteins, membrane cholesterol domains, and the regulation of cholesterol efflux. J. Lipid Res. 1992, 33, 1091–1097. [Google Scholar]
- Koike, T.; Liang, J.; Wang, X.; Ichikawa, T.; Shiomi, M.; Liu, G.; Sun, H.; Kitajima, S.; Morimoto, M.; Watanabe, T. Overexpression of Lipoprotein Lipase in Transgenic Watanabe Heritable Hyperlipidemic Rabbits Improves Hyperlipidemia and Obesity. J. Biol. Chem. 2004, 279, 7521–7529. [Google Scholar] [CrossRef]
- Liu, E.; Kitajima, S.; Higaki, Y.; Morimoto, M.; Sun, H.; Watanabe, T.; Yamada, N.; Fan, J. High lipoprotein lipase activity increases insulin sensitivity in transgenic rabbits. Metabolism 2005, 54, 132–138. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Schulze, M.B.; Manson, J.; Ludwig, D. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. ACC Curr. J. Rev. 2004, 13, 34–35. [Google Scholar] [CrossRef]
| Author | Diet | Duration | Rabbit Breed | MetS Components | ||||
|---|---|---|---|---|---|---|---|---|
| Ob | HG | HT | DL | FFA | ||||
| Waqar et al. [23] | 3% coconut oil | 22 weeks | Japanese White male | 🗴 | ✓ | ✓ | ✓ | ✓ |
| 10% coconut oil | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Brunner et al. [36] | 17% fat (added palmitic, oleic and linoleic acid) | 28 weeks | New Zealand White | - | - | - | - | - |
| Carroll et al. [19] | 15% fat, 10% corn oil and 5% lard | 12 weeks | New Zealand White female | ✓ | ✓ | ✓ | ✓ | ✓ |
| Alarcon et al. [43] | 18% fat, 10% corn oil and 8% lard | 6 weeks | Hybrid Flanders | 🗴 | ✓ | ✓ | ✓ | ✓ |
| Cervera et al. [44] | 8.5% animal fat | 12 weeks | Crossbred | ✓ | - | - | - | - |
| 2.5% soya full fat | ✓ | - | - | - | - | |||
| Author | Diet | Duration | Rabbit Breed | MetS Components | ||||
|---|---|---|---|---|---|---|---|---|
| Ob | HG | HT | DP | FFA | ||||
| Drimba et al. [38] | 1.5% cholesterol and 2.6% fat | 8 weeks | New Zealand White male | - | - | ✓ | ✓ | ✓ |
| Filippi et al. [31] | 0.5% cholesterol and 4% peanut oil | 12 weeks | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Maneschi et al. [49] | 0.5% cholesterol and 4% peanut oil | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Marchiani et al. [47] | 0.5 cholesterol and 4% peanut oil | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Morelli et al. [48] | 0.5% cholesterol and 4% peanut oil | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Author | Diet | Duration | Rabbit Breed | MetS Components | ||||
|---|---|---|---|---|---|---|---|---|
| Ob | HG | HT | DP | FFA | ||||
| Arias-Mutis et al. [14] | 10% hydrogenated coconut oil, 5% pork fat, 15% sucrose dissolved in water | 28 weeks | New Zealand White male | ✓ | ✓ | ✓ | ✓ | ✓ |
| Helfenstein et al. [32] | 10% lard, 40% sucrose and cholesterol (0.5% for the first 12 weeks and 0.1% for up to 24 weeks) | 24 weeks | ✓ | ✓ | - | ✓ | ✓ | |
| Liu et al. [35] | 30% sucrose and 10% fat | 48 weeks | ✓ | ✓ | - | ✓ | ✓ | |
| Yin et al. [34] | 10% pork fat and 37% sucrose | 6.5 months (28 weeks) | ✓ | ✓ | - | ✓ | ✓ | |
| Zhao et al. [51] | 10% pork fat and 30% sucrose (11% protein, 11.2% fat, 10.1% fiber, 6.8% ash) | 36 weeks | Japanese White male | ✓ | ✓ | ✓ | ✓ | ✓ |
| Author | Diet | Duration | Rabbit Breed | MetS Components | ||||
|---|---|---|---|---|---|---|---|---|
| Ob | HG | HT | DP | FFA | ||||
| Ning et al. (33) | 30% fructose and 10% coconut oil (91% saturated fatty acids) | 16 weeks | WHHL | ✓ | ✓ | 🗴 | ✓ | ✓ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, W.M.; Arias-Mutis, O.J.; Calvo, C.J.; Chorro, F.J.; Zarzoso, M. Diet-Induced Rabbit Models for the Study of Metabolic Syndrome. Animals 2019, 9, 463. https://doi.org/10.3390/ani9070463
Lozano WM, Arias-Mutis OJ, Calvo CJ, Chorro FJ, Zarzoso M. Diet-Induced Rabbit Models for the Study of Metabolic Syndrome. Animals. 2019; 9(7):463. https://doi.org/10.3390/ani9070463
Chicago/Turabian StyleLozano, Wilson M., Oscar J. Arias-Mutis, Conrado J. Calvo, Francisco J. Chorro, and Manuel Zarzoso. 2019. "Diet-Induced Rabbit Models for the Study of Metabolic Syndrome" Animals 9, no. 7: 463. https://doi.org/10.3390/ani9070463
APA StyleLozano, W. M., Arias-Mutis, O. J., Calvo, C. J., Chorro, F. J., & Zarzoso, M. (2019). Diet-Induced Rabbit Models for the Study of Metabolic Syndrome. Animals, 9(7), 463. https://doi.org/10.3390/ani9070463





