Potential of Insect Meals as Protein Sources for Meat-Type Ducks Based on In Vitro Digestibility
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Composition of Substrates
3.2. Pearson Correlation between Chemical Composition and In Vitro Digestibility of Insect Meal
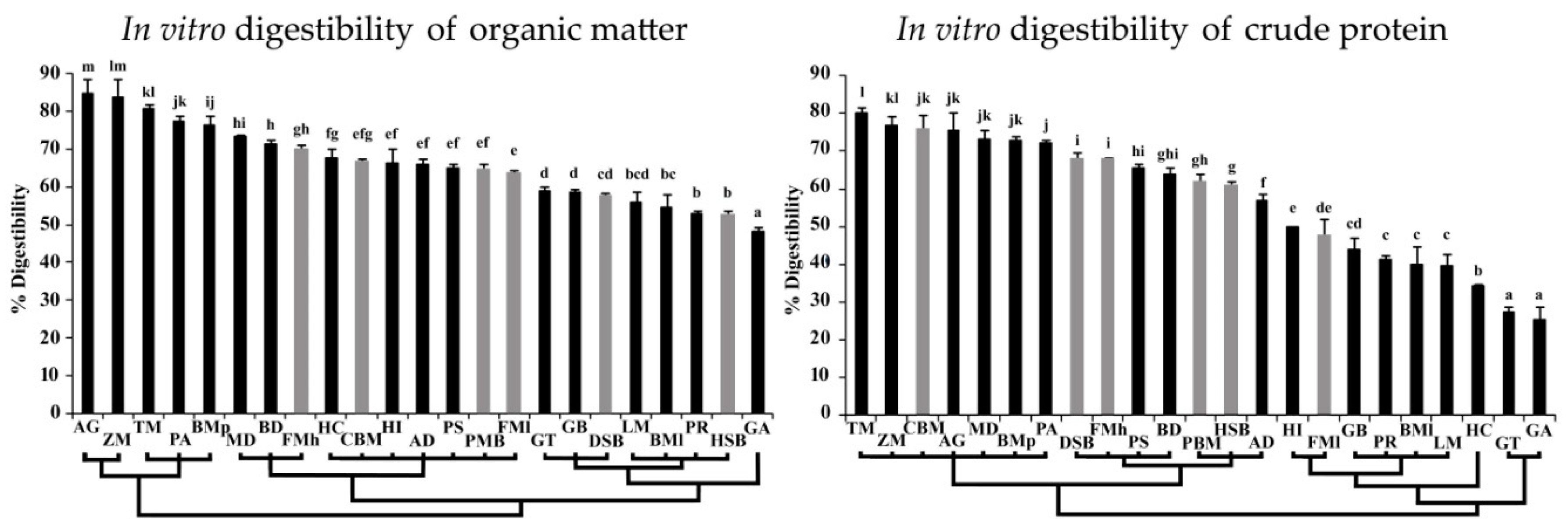
3.3. In Vitro Digestibility of Organic Matter and Crude Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 15 February 2019).
- Adzitey, F.; Adzitey, S.P. Duck production: Has a potential to reduce poverty among rural households in Asian communities—A review. J. World Poult. Res. 2011, 1, 7–10. Available online: https://pdfs.semanticscholar.org/0ed1/d3ae26df7aa3db2e83bae6d2c8094facb6ca.pdf (accessed on 15 February 2019).
- Thongwittaya, N. Substitution of plant protein for fish meal in the diet of laying ducks. Animal Sci. J. 2007, 78, 351–355. [Google Scholar] [CrossRef]
- Fazhi, X.; Lvmu, L.; Jiaping, X.; Kun, Q.; Zhide, Z.; Zhangyi, L. Effects of fermented rapeseed meal on growth performance and serum parameters in ducks. Asian Aust. J. Anim. Sci. 2011, 24, 678–684. Available online: http://www.koreascience.or.kr/article/JAKO201118861579041.page (accessed on 15 February 2019). [CrossRef]
- Gutleb, A.C.; Caloni, F.; Giraud, F.; Cortinovis, C.; Pizzo, F.; Hoffmann, L.; Bohn, T.; Pasquali, M. Detection of multiple mycotoxin occurrences in soy animal feed by traditional mycological identification combined with molecular species identification. Toxicol. Rep. 2015, 2, 275–279. Available online: https://www.sciencedirect.com/science/article/pii/S2214750015000086 (accessed on 15 February 2019). [CrossRef] [PubMed]
- Han, X.Y.; Huang, Q.C.; Li, W.F.; Jiang, J.F.; Xu, Z.R. Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livest Sci. 2008, 119, 216–220. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1871141308001169 (accessed on 15 February 2019). [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Bianchi, C.; et al. Effects of yellow mealworm larvae (Tenebrio molitor) inclusion in diets for female broiler chickens: implications for animal health and gut histology. Anim. Feed Sci. Technol. 2017, 234, 253–263. Available online: https://academic.oup.com/ps/article/97/2/540/4601755 (accessed on 15 February 2019). [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellonea, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow mealworm larvae (Tenebrio molitor, L.) as possible alternative to soybean meal in broiler diets. Br. Poult Sci 2015, 5, 569–575. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L.; et al. Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: Effects on growth performance, gut morphology, and histological findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2018, 49, 1–10. [Google Scholar] [CrossRef]
- Hanboonsong, Y.; Jamjanya, T.; Durst, B.P. Six-Legged Livestock: Edible Insect Farming, Collection and Marketing in Thailand, 1st ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Makkar, P.S.H.; Tran, G.; Heuzé, V.; Ankersa, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. Available online: https://www.sciencedirect.com/science/article/pii/S0377840114002326 (accessed on 15 February 2019). [CrossRef]
- Sánchez-Muros, M.; Fernando, G.B.; Manzano-Agugliaro, F. Insect meal as renewable source of food for animal feeding: A review. J. Clean Prod. 2014, 65, 16–27. Available online: https://www.sciencedirect.com/science/article/pii/S095965261300841X (accessed on 15 February 2019).
- Gunawan, A.; Erlina, S.; Samudera, R.; Syarif, D.M.; Noor, M.Y.; Lantu, A.X. Effect of supplement maggot black soldier fly live on the percentage of carcass and weight of carcass of male Alabio ducks. In Proceedings of the 1st International Conference on Food and Agriculture, Nusa Dua, Bali, Indonesia, 20–21 October 2018. [Google Scholar]
- Gariglio, M.; Dabbou, S.; Biasato, I.; Capucchio, M.T.; Colombino, E.; Hernandez, F.; Madrid Sanchez, J.; Martinez, S.; Gai, F.; Caimi, C.; et al. Nutritional effects of the dietary inclusion of partially defatted Hermetia illucens larva meal in Muscovy duck. J. Anim. Sci. Biotechnol. in press.
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of Tenebrio molitor and Hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 338–343. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Mi, B.M.; Zhang, H.F.; Hou, S.S.; Zhang, Z.Y. Using a computer-controlled simulated digestion system to predict the energetic value of corn for ducks. Poult. Sci. 2014, 93, 1410–1420. Available online: https://academic.oup.com/ps/article/93/6/1410/1564622 (accessed on 15 February 2019). [CrossRef]
- Kovitvadhi, A.; Luapan, J.; Amarapitak, P.; Sriyaphai, P.; Buahom, R.; Cham-iam, T.; Chandang, P.; Leelehapongsathon, K.; Tirawattanawanich, C.; Thongprajukaew, K. Screening three cricket species (Gryllus bimaculatus, Acheta domestica and Modicogryllus confirmata) for broiler diets by in vitro digestibility techniques. In Proceedings of the 6th Mediterranean Poultry Summit, Turin, Italy, 18–21 June 2018. [Google Scholar]
- Kluth, H.; Rodehutscord, M. Comparison of amino acid digestibility in broiler chickens, turkeys, and Pekin ducks. Poult. Sci. 2006, 85, 1953–1960. Available online: https://academic.oup.com/ps/article/85/11/1953/2962600 (accessed on 15 February 2019).
- Association of Official Analytical Chemists, Official Method of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2006.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fibre, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3591. Available online: https://www.sciencedirect.com/science/article/pii/S0022030291785512 (accessed on 15 February 2019). [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Barroso, F.G.; Haro, C.; Sánchez-Muros, M.J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquacul 2014, 422, 193–201. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0044848613006790 (accessed on 15 February 2019). [CrossRef]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional potential of selected insect species reared on the Island of Sumatra. Int. J. Environ. Res. Public Health 2017, 14, 521. Available online: https://www.mdpi.com/1660-4601/14/5/521 (accessed on 15 February 2019). [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784 . [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
| Substrates | Chemical Composition (%DM) | |||||
|---|---|---|---|---|---|---|
| DM 1 | Ash | CP | EE | CF | ADF | |
| General protein sources (animal-based proteins) | ||||||
| Fishmeal: high protein (FMh) | 91.1 | 20.7 | 58.6 | 9.60 | 0.57 | - |
| Fishmeal: low protein (FMl) | 92.2 | 24.6 | 38.9 | 14.8 | 0.59 | - |
| Chicken by-product meal (CBM) | 95.5 | 16.3 | 60.9 | 10.2 | 2.46 | - |
| Pork by-product meal (PBM) | 95.3 | 33.4 | 46.2 | 10.4 | 0.33 | - |
| General protein sources (plant-based proteins) | ||||||
| Dehulled-soybean meal (DSB) | 87.7 | 6.18 | 45.9 | 0 | 4.31 | - |
| Hulled-soybean meal (HSB) | 88.4 | 6.96 | 41.0 | 0 | 7.55 | - |
| Insect meal | ||||||
| Order: Blattodea | ||||||
| Periplaneta americana (PA:nymph) | 94.6 | 3.98 | 64.4 | 23.6 | 4.36 | 5.53 |
| Order: Coleoptera | ||||||
| Hydrous cavistanum (HC:adult) | 86.3 | 1.88 | 41.9 | 38.3 | 14.7 | 20.6 |
| Tenebrio molitor (TM:larvae) | 97.1 | 5.95 | 53.0 | 31.0 | 8.47 | 8.19 |
| Zophobas morio (ZM:larvae) | 96.8 | 5.53 | 42.0 | 41.7 | 6.28 | 6.93 |
| Order: Diptera | ||||||
| Bactrocera dorsalis (BD:larvae) | 95.1 | 9.41 | 45.2 | 31.3 | 5.94 | 13.6 |
| Hermetia illucens (HI:prepupae) | 91.8 | 9.54 | 37.9 | 30.1 | 12.3 | 11.2 |
| Musca domestica (MD:larvae) | 93.8 | 6.78 | 54.8 | 21.7 | 9.65 | 14.9 |
| Order: Lepidoptera | ||||||
| Achroia grisella (AG:larvae) | 97.2 | 6.02 | 37.6 | 48.6 | 3.02 | 12.7 |
| Bombyx mori (BMl:larvae) | 96.7 | 10.1 | 61.2 | 17.6 | 5.39 | 13.6 |
| Bombyx mori (BMp:pupae) | 95.2 | 4.51 | 50.4 | 35.0 | 4.61 | 7.63 |
| Philosamia ricini (PR:pupae) | 92.6 | 7.15 | 64.5 | 11.5 | 8.53 | 10.1 |
| Order: Orthoptera | ||||||
| Acheta domesticus (AD:adult) | 95.8 | 4.66 | 52.8 | 24.5 | 10.0 | 12.3 |
| Gryllotalpa africana (GA:adult) | 94.9 | 4.12 | 54.3 | 17.7 | 17.8 | 24.6 |
| Gryllus bimaculatus (GB:adult) | 92.2 | 5.05 | 53.3 | 22.6 | 8.98 | 13.5 |
| Gryllus testaceus (GT:adult) | 95.7 | 4.54 | 40.2 | 24.7 | 8.35 | 13.2 |
| Locusta migratoria (LM:adult) | 91.9 | 4.56 | 58.5 | 3.59 | 12.7 | 15.8 |
| Patanga succincta (PS:adult) | 92.0 | 4.29 | 63.3 | 12.9 | 15.1 | 12.8 |
| Parameters | CP | EE | CF | ADF | OMd | CPd |
|---|---|---|---|---|---|---|
| Ash | −0.05 | −0.03 | −0.35 | −0.21 | −0.05 | 0.11 |
| CP | −0.77 ** | 0.11 | −0.10 | −0.39 | −0.03 | |
| EE | −0.45 | −0.23 | 0.77 ** | 0.47 | ||
| CF | 0.71 ** | −0.56 * | −0.54 * | |||
| ADF | −0.59 * | −0.68 ** | ||||
| OMd | 0.89 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovitvadhi, A.; Chundang, P.; Thongprajukaew, K.; Tirawattanawanich, C.; Srikachar, S.; Chotimanothum, B. Potential of Insect Meals as Protein Sources for Meat-Type Ducks Based on In Vitro Digestibility. Animals 2019, 9, 155. https://doi.org/10.3390/ani9040155
Kovitvadhi A, Chundang P, Thongprajukaew K, Tirawattanawanich C, Srikachar S, Chotimanothum B. Potential of Insect Meals as Protein Sources for Meat-Type Ducks Based on In Vitro Digestibility. Animals. 2019; 9(4):155. https://doi.org/10.3390/ani9040155
Chicago/Turabian StyleKovitvadhi, Attawit, Pipatpong Chundang, Karun Thongprajukaew, Chanin Tirawattanawanich, Sunyanee Srikachar, and Banthari Chotimanothum. 2019. "Potential of Insect Meals as Protein Sources for Meat-Type Ducks Based on In Vitro Digestibility" Animals 9, no. 4: 155. https://doi.org/10.3390/ani9040155
APA StyleKovitvadhi, A., Chundang, P., Thongprajukaew, K., Tirawattanawanich, C., Srikachar, S., & Chotimanothum, B. (2019). Potential of Insect Meals as Protein Sources for Meat-Type Ducks Based on In Vitro Digestibility. Animals, 9(4), 155. https://doi.org/10.3390/ani9040155







