PDGFA in Cashmere Goat: A Motivation for the Hair Follicle Stem Cells to Activate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects Recruiting and Tissue Collection
2.2. RNA and DNA Isolation
2.3. Primer Design
2.4. PCR Amplification of Full-Length PDGFA Gene
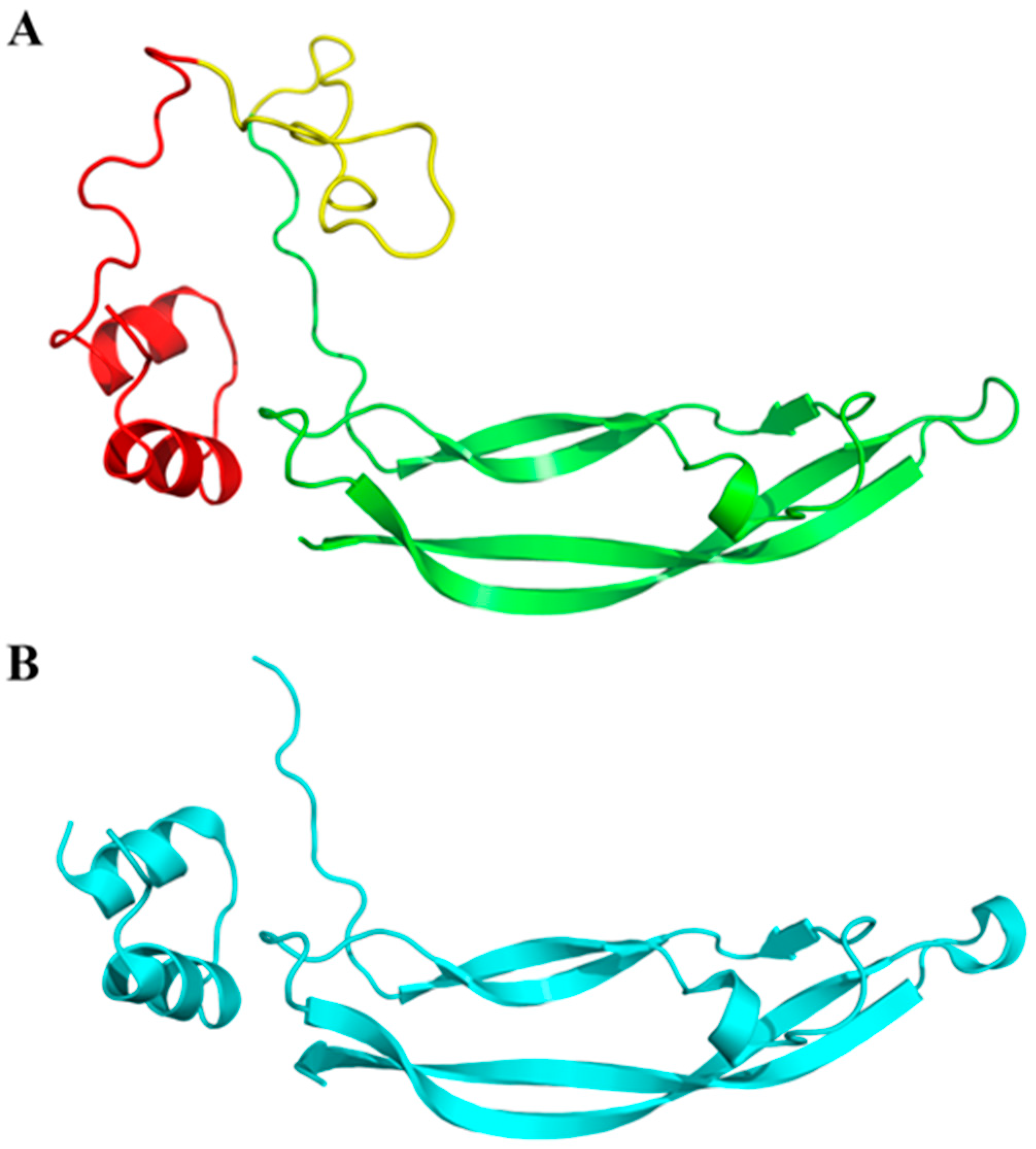
2.5. Homology Modelling of PDGFA Three-Dimensional Structure
2.6. Basic Histology
2.7. Immunohistochemistry
2.8. qPCR on Target Genes
3. Results
3.1. PDGFA Full-Length Transcript
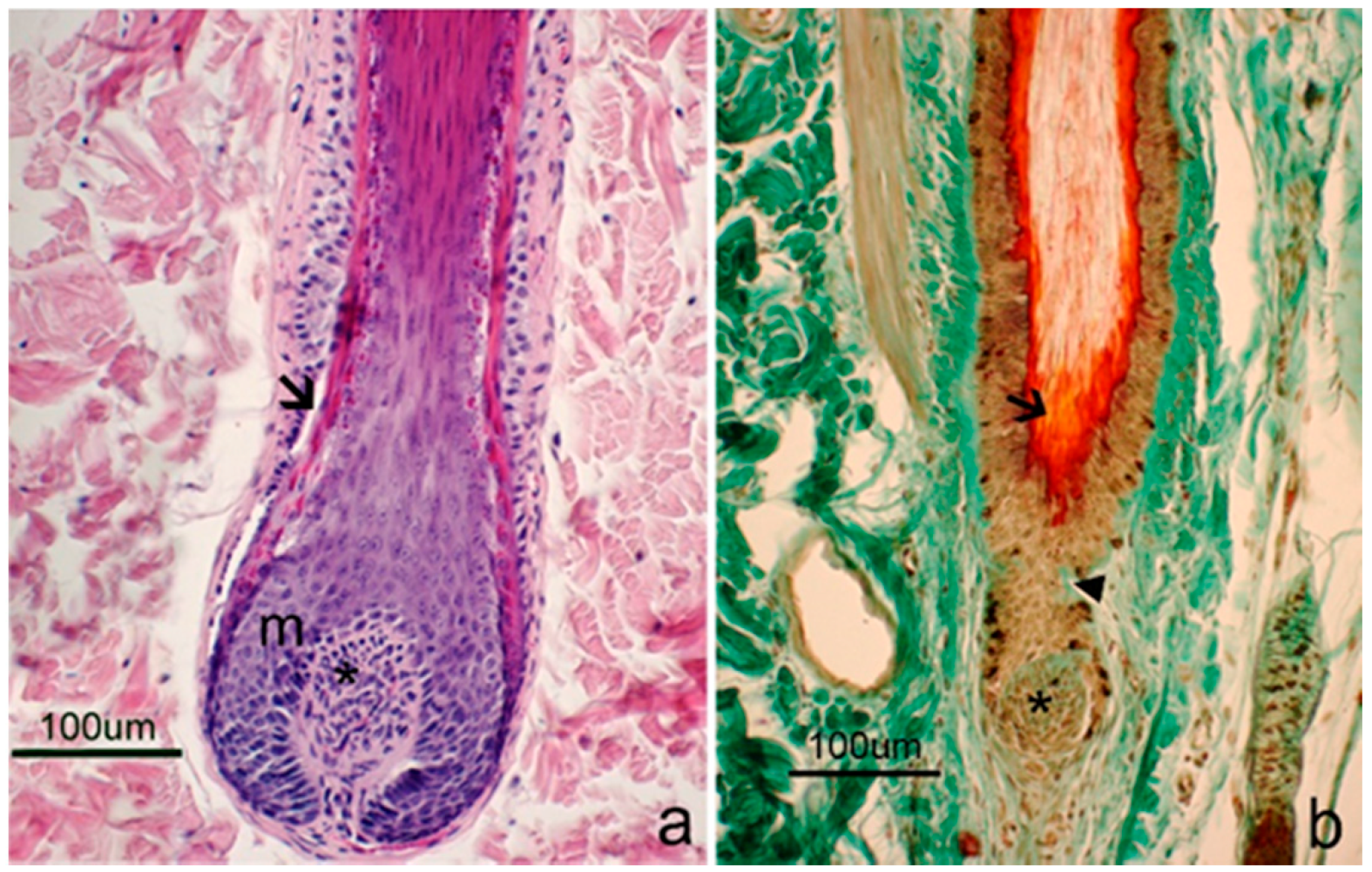
3.2. Morphological Evaluation
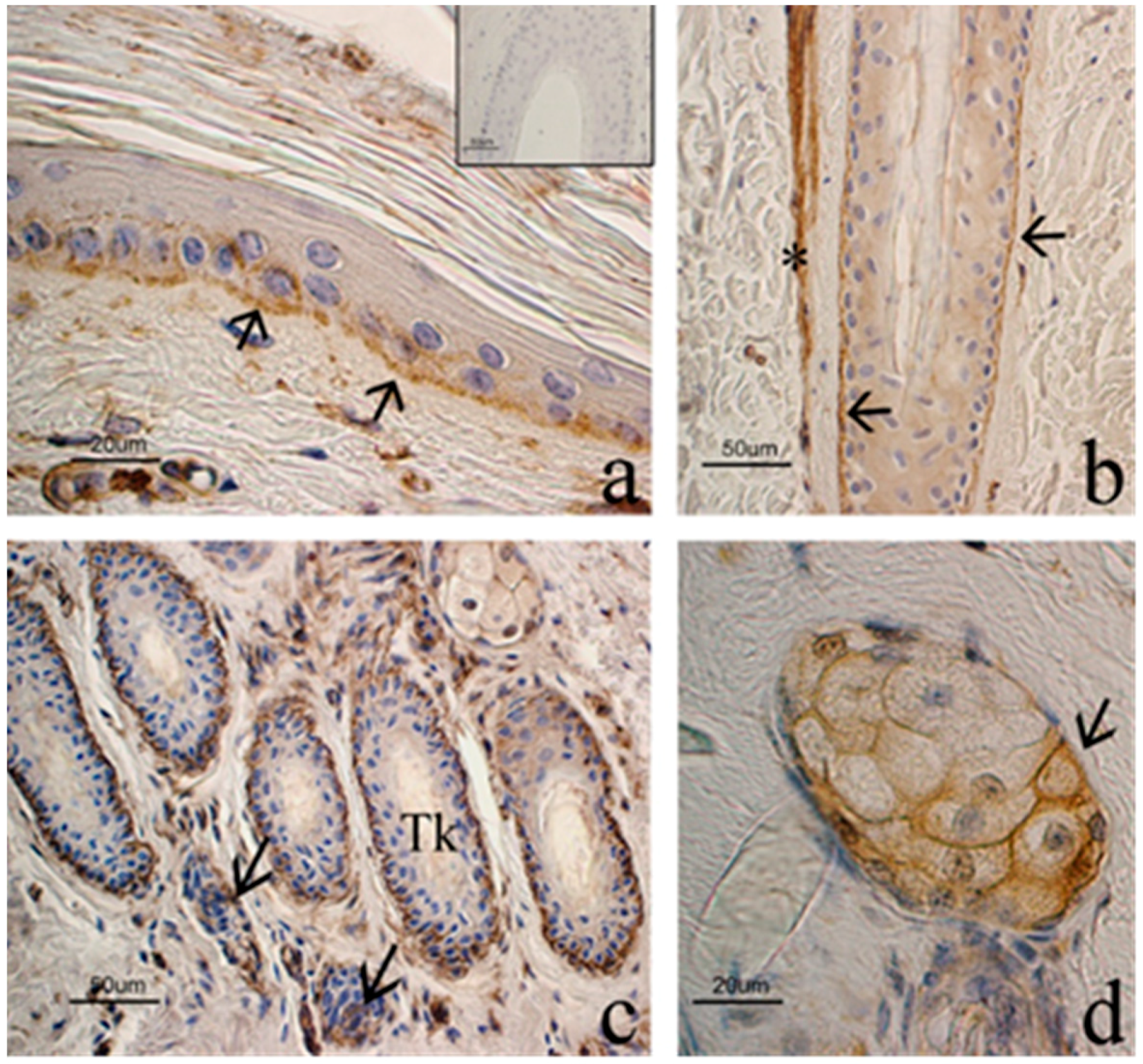
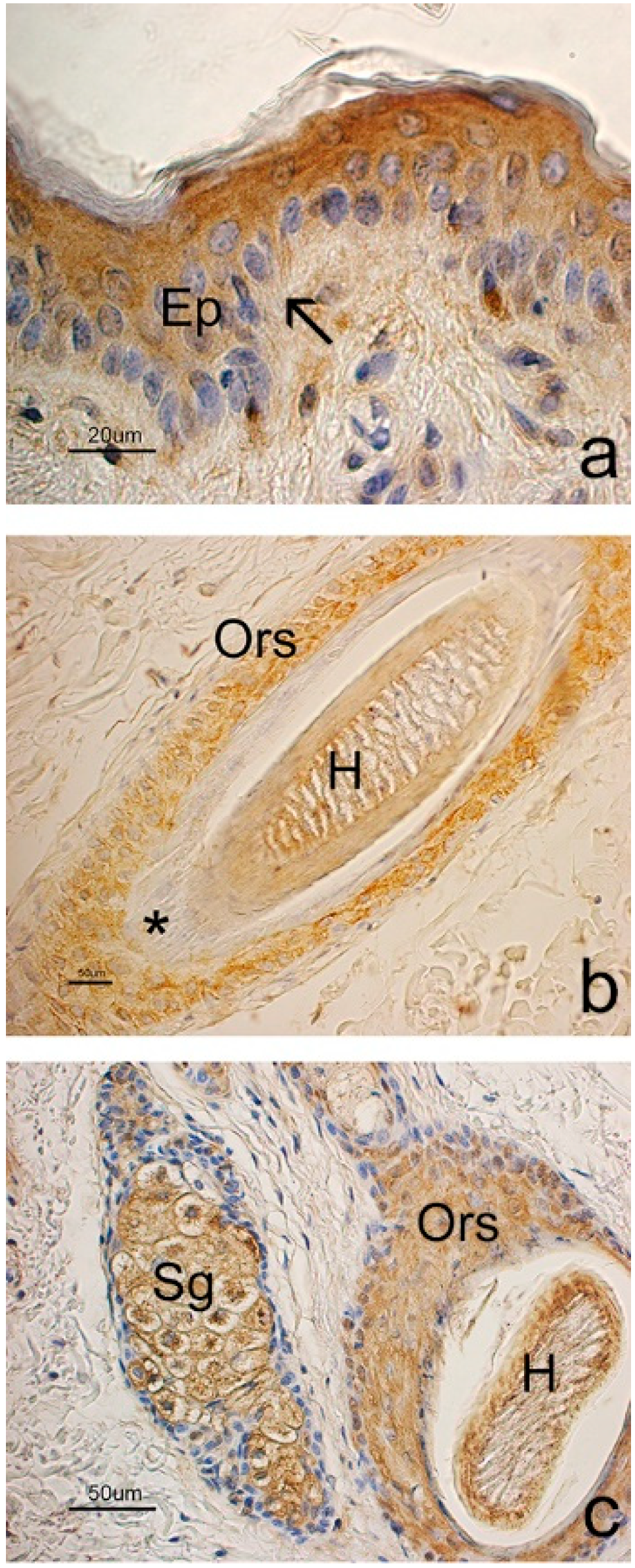
3.3. Immunohistochemical Evaluation
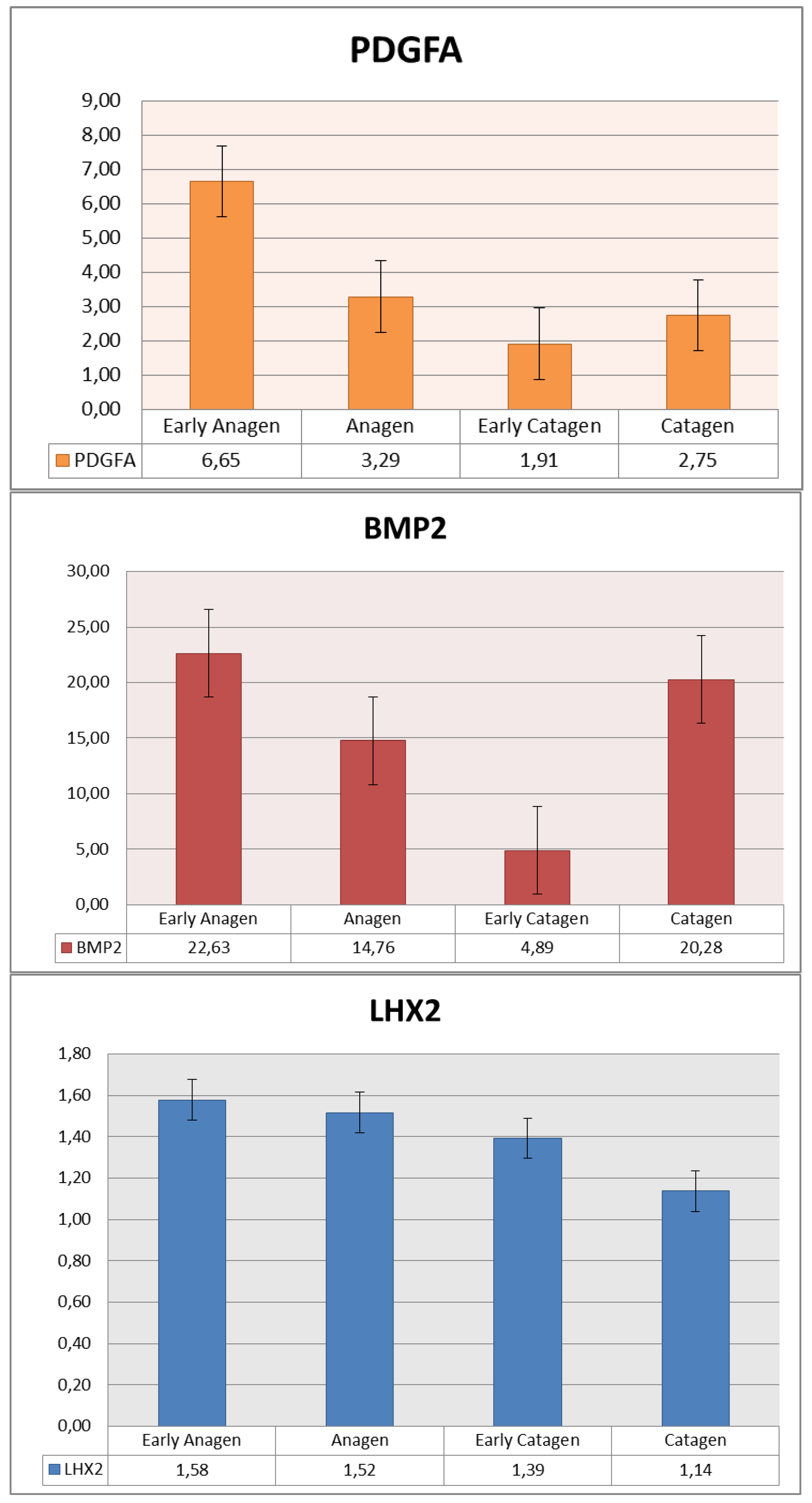
3.4. qPCR Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, Y.L.; Wang, X.L.; Yang, Z.X. The genetic diversity of seven indigenous Chinese goat breeds. Small Rumin. Res. 2008, 74, 231–237. [Google Scholar] [CrossRef]
- Di, R.; Vahidi, S.M.F.; Ma, Y.H.; He, X.H.; Zhao, Q.J.; Han, J.L.; Guan, W.J.; Chu, M.X.; Sun, W.; Pu, Y.P. Microsatellite analysis revealed genetic diversity and population structure among Chinese cashmere goats. Anim. Genet. 2011, 42, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Antonini, M.; Wang, J.; Lou, Y.; Tang, P.; Renieri, C.; Pazzaglia, I.; Valbonesi, A. Effects of year and sampling site on mean fibre diameter of Alashan cashmere goat. Small Rumin. Res. 2016, 137, 71–72. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Gao, F. Isolation and identification of stem cells from adult cashmere goat skin. Int. J. Dermatol. 2008, 47, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Foitzik, K. Biology of the Hair Follicle: The Basics. Semin. Cutan. Med. Surg. 2006, 25, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.R.; Sabine, M. Secondary follicle development in Australian cashmere goats. Small Rumin. Res. 1991, 4, 349–363. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Waters, J.M.; Richardson, G.D.; Jahoda, C.A.B. Hair follicle stem cells. Semin. Cell Dev. Biol. 2007, 18, 245–254. [Google Scholar] [CrossRef]
- Park, B.S.; Kim, W.S.; Choi, J.S.; Kim, H.K.; Won, J.H.; Ohkubo, F.; Fukuoka, H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. BioMed Res. 2010, 31, 27–34. [Google Scholar] [CrossRef]
- Schmidt, B.; Horsley, V. Unravelling hair follicle-adipocyte communication. Exp. Dermatol. 2012, 21, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Akiyama, M.; Shimizu, H. PDGF isoforms induce and maintain anagen phase of murine hair follicles. J. Dermatol. Sci. 2006, 43, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kiso, M.; Hamazaki, T.S.; Itoh, M.; Kikuchi, S.; Nakagawa, H.; Okochi, H. Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J. Dermatol. Sci. 2015, 79, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Rezza, A.; Sennett, R.L.; Tanguy, M.; Clavel, C.; Rendl, M. PDGF signalling in the dermis and in dermal condensates is dispensable for hair follicle induction and formation. Exp. Dermatol. 2015, 24, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.R.; Sales, A.D.; Rodrigues, G.Q.; Lobo, C.H.; Castro, S.V.; Silva, A.W.; Moura, A.A.; Silva, J.R.; Rodrigues, A.P.; Figueiredo, J.R. Differential gene expression and immunolocalization of platelet-derived growth factors and their receptors in caprine ovaries. Domest. Anim. Endocrinol. 2015, 51, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Li, H.; Eriksson, U. The PDGF family: Four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Pi, L.Q.; Hwang, S.T.; Lee, W.S. Effect of IGF-I on hair growth is related to the anti-apoptotic effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann. Dermatol. 2012, 24, 26–31. [Google Scholar] [CrossRef]
- Geng, R.; Wang, L.; Wang, X.; Chen, Y. Cyclic expression of Lhx2 is involved in secondary hair follicle development in cashmere goat. Gene Expr. Patterns 2014, 16, 31–35. [Google Scholar] [CrossRef]
- Su, R.; Zhang, W.G.; Sharm, R.; Chang, Z.L.; Yin, J.; Li, J.Q. Characterization of BMP2 gene expression in embryonic and adult Inner Mongolia Cashmere goat (Capra hircus) hair follicles. Can. J. Anim. Sci. 2009, 89, 457–462. [Google Scholar] [CrossRef]
- Lee, J.; Tumbar, T. Hairy tale of signaling in hair follicle development and cycling. Semin. Cell Dev. Biol. 2012, 23, 906–916. [Google Scholar] [CrossRef]
- Turksen, K. Tissue Specific Stem Cell Niche, 1st ed.; Springer International Publishing: Basel, Switzerland, 2015; pp. 154–196. [Google Scholar]
- Plikus, M.V.; Mayer, J.A.; De la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Mardaryev, A.N.; Meier, N.; Poterlowicz, K.; Sharov, A.A.; Sharova, T.Y.; Ahmed, M.I.; Rapisarda, V.; Lewis, C.; Fessing, M.Y.; Ruenger, T.M.; et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development 2011, 138, 4843–4852. [Google Scholar] [CrossRef]
- Rhee, H.; Polak, L.; Fuchs, E. Lhx2 Maintains Stem Cell Character in Hair Follicles. Science 2006, 312, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhou, G.; Guo, J.; Yan, H.; Niu, Y.; Li, Y.; Yuan, C.; Geng, R.; Lan, X.; et al. Whole-genome sequencing of eight goat populations for the detection of selection signatures underlying production and adaptive traits. Sci. Rep. 2016, 6, 38932. [Google Scholar] [CrossRef] [PubMed]
- Shim, A.H.; Liu, H.; Focia, P.J.; Chen, X.; Lin, P.C.; He, X. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc. Natl. Acad. Sci. USA 2010, 107, 11307–11312. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. In Protein Structure Prediction. Methods in Molecular Biology (Methods and Protocols), 3rd ed.; Kihara, D., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1137, pp. 1–15. [Google Scholar]
- Ryder, M.L.; Stephenson, S.K. Wool Growth; Academic Press: London, UK; New York, NY, USA, 1968. [Google Scholar]
- Melis, M.; Carpino, F.; Di Tondo, U. Tecniche in Anatomia Patologica; Edi-Ermes: Milano, Italy, 1992. [Google Scholar]
- Mercati, F.; Dall’Aglio, C.; Timperi, L.; Scocco, P.; De Felice, E.; Maranesi, M. Epithelial expression of the hormone leptin by bovine skin. Eur. J. Histochem. 2019, 63, 1. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.E.; Young, R.; Orkin, S.H.; Collins, T. Increased platelet-derived growth factor A-chain expression in human uterine smooth muscle cells during the physiologic hypertrophy of pregnancy. Proc. Natl. Acad. Sci. USA 1990, 87, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.B.; Rong, H.Y.; Rong, L.Y.; Wu, Q.J.; Jiao, J.W.; Ze, Y.W.; Yu, B.Z.; Zhi, H.Z.; Rong, J.Y.; Guang, B.L.; et al. Selection and validation of suitable reference genes in skin tissue of Liaoning cashmere goat during hair follicle cycle. Livest. Sci. 2014, 161, 28–35. [Google Scholar]
- Ibraheemi, M.; Galbrait, H.; Scaife, J.; Ewen, S. Growth of secondary hair follicles of the Cashmere goat in vitro and their response to prolactin and melatonin. J. Anat. 1994, 185 Pt 1, 135–142. [Google Scholar]
- Karlsson, L.; Bondjers, C.; Betsholtz, C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 1999, 126, 2611–2621. [Google Scholar]
- Akiyama, M.; Smith, L.T.; Holbrook, K.A. Growth factor and growth factor receptor localization in the hair follicle bulge and associated tissue in human fetus. J. Investig. Dermatol. 1996, 106, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Takakura, N.; Yoshida, H.; Kunisada, T.; Nishikawa, S.; Nishikawa, S.I. Involvement of platelet-derived growth factor receptor-alpha in hair canal formation. J. Investig. Dermatol. 1996, 107, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Kamp, H.; Geilen, C.C.; Sommer, C.; Blume-Peytavi, U. Regulation of PDGF and PDGF receptor in cultured dermal papilla cells and follicular keratinocytes of the human hair follicle. Exp. Dermatol. 2003, 12, 662–672. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence | TM (°C) |
|---|---|---|
| FWDA | CTCGGGACGCGATGAGGAC | 60.3 |
| FWDB | GATGAGGACCTGGGCTTG | 58.2 |
| OLIGODT | GAGAGAGAGAGAGACAGAGAACTAGTCTCGAGTTTTTTTTTTTTTTTTTT | 74.9 |
| Primer | Sequence (Forward and Reverse) | TM (°C) | Efficiency (100%) |
|---|---|---|---|
| PDGFA- | TCCGCTAACTTCCTGATCT CTTTCAACTTCGCCTTCTT | 56 | 95.6 |
| BMP2- | CTACATGCTGGACTTGTAC GTTGTTTTCCCACTCATTTC | 55 | 83.3 |
| LHX2- | GGAAGCATCTACTGCAAGGAAG GAGGTGATAAACCAAGTCCCG | 55 | 93.2 |
| UBC | GCATTGTTGGGTTCCTGTGT TTTGCATTTTGACCTGTGAG | 57 | 116.2 |
| YWHAZ | TGTAGGAGCCCGTAGGTCATCT TTCTCTCTGTATTCTCGAGCCATCT | 57 | 105 |
| SDHA | AGCACTGGAGGAAGCACAC CACAGTCGGTCTCGTTCAA | 57 | 128.2 |
| Gene | p Value |
|---|---|
| PDGFA | 8 × 10−8 |
| BMP2 | 0.00024102 |
| LHX2 | 0.04075542 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazzaglia, I.; Mercati, F.; Antonini, M.; Capomaccio, S.; Cappelli, K.; Dall’Aglio, C.; La Terza, A.; Mozzicafreddo, M.; Nocelli, C.; Pallotti, S.; et al. PDGFA in Cashmere Goat: A Motivation for the Hair Follicle Stem Cells to Activate. Animals 2019, 9, 38. https://doi.org/10.3390/ani9020038
Pazzaglia I, Mercati F, Antonini M, Capomaccio S, Cappelli K, Dall’Aglio C, La Terza A, Mozzicafreddo M, Nocelli C, Pallotti S, et al. PDGFA in Cashmere Goat: A Motivation for the Hair Follicle Stem Cells to Activate. Animals. 2019; 9(2):38. https://doi.org/10.3390/ani9020038
Chicago/Turabian StylePazzaglia, Irene, Francesca Mercati, Marco Antonini, Stefano Capomaccio, Katia Cappelli, Cecilia Dall’Aglio, Antonietta La Terza, Matteo Mozzicafreddo, Cristina Nocelli, Stefano Pallotti, and et al. 2019. "PDGFA in Cashmere Goat: A Motivation for the Hair Follicle Stem Cells to Activate" Animals 9, no. 2: 38. https://doi.org/10.3390/ani9020038
APA StylePazzaglia, I., Mercati, F., Antonini, M., Capomaccio, S., Cappelli, K., Dall’Aglio, C., La Terza, A., Mozzicafreddo, M., Nocelli, C., Pallotti, S., Pediconi, D., & Renieri, C. (2019). PDGFA in Cashmere Goat: A Motivation for the Hair Follicle Stem Cells to Activate. Animals, 9(2), 38. https://doi.org/10.3390/ani9020038







