Sexual Dimorphism for Coping Styles Complements Traditional Methods for Sex Determination in a Multivariety Endangered Hen Breed
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Animal Care and Use Committee Statement
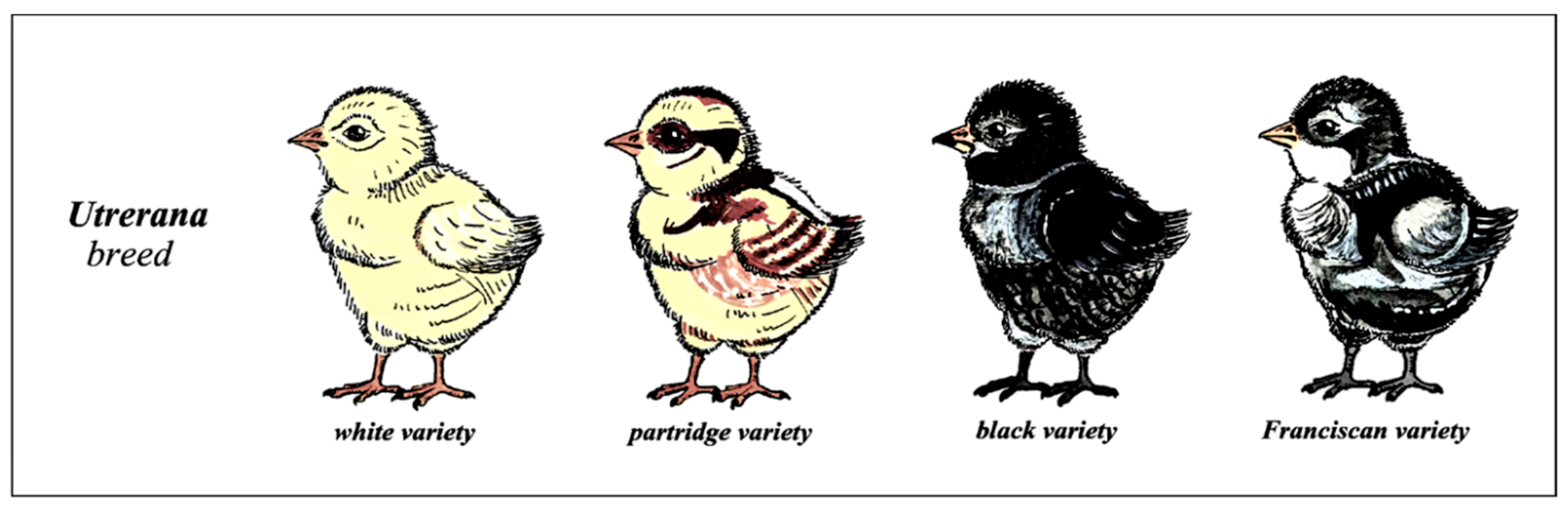
2.2. Sample Size and Background
2.3. Chicken Handling for Examinations
2.4. Sex Assignation Methods
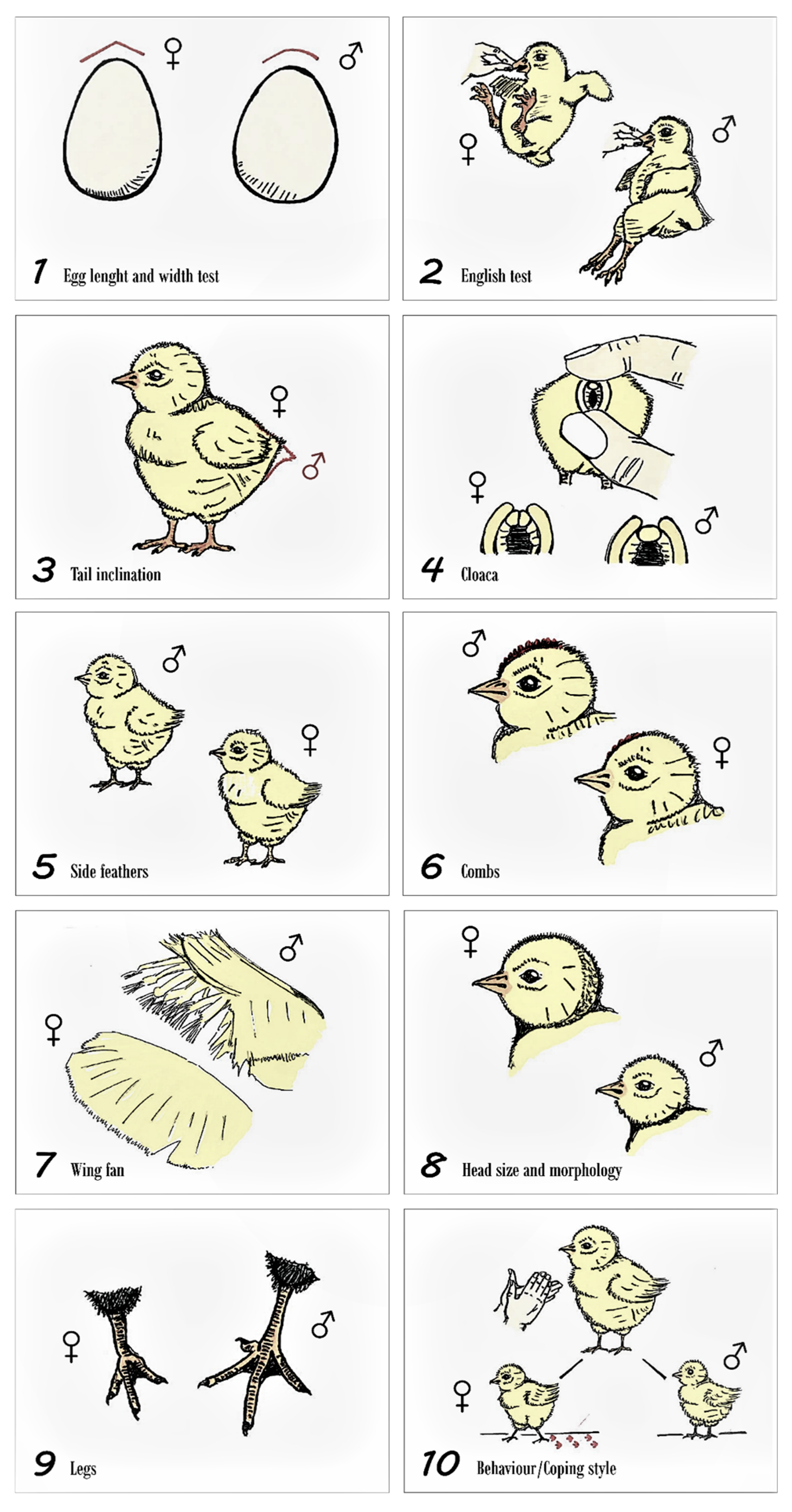
2.5. Sex Assignation Criteria and Method Definition
- (a)
- Method 1. Egg dimorphism: studied as egg length (1.1) and egg width (1.2). Long, pointed eggs for females and, flat and wide eggs for males. In addition, the date of laying, the identification number of the egg, the possible sex predicted, and the hatching date were recorded. To state the limits to consider an egg long or flat and wide or broad we computed the median of the sizes (sample was not normally distributed p > 0.05), to set over and below the median categories. Method 2. English method: holding the chick by the skin of the neck or holding the beak between two fingers, the animal is suspended for 5 s to observe the reaction and posture that it acquires. If the chick kicks, it will be considered a female; if the animal remains motionless, it will be considered a male.
- (b)
- Method 3. Tail Inclination: if the direction of the tail feathers is towards the ground, the individual will be taken as a female. Instead, if the tail is straight, it will be taken as a male.
- (c)
- Method 4. Japanese method or cloaca examination: the basis for this method is the appreciation of morphological differences between the genital eminences and cloacal folds of males and females in newborn chicks. Once the cloaca is externalized applying light pressure with the fingers, attention is paid to the central and ventral part of it. If two small lumps are observed, it will be considered a female. If only one bulge is observed, it will be considered a male.
- (d)
- Method 5. General coloring of down feathers: if the color of down feathers of the sides of the chicken is heterogeneous, it will be considered to be female. If the coloration is homogeneous, it will be taken as a male.
- (e)
- Method 6. Development and changes in the combs from the month and a half onwards: a little developed comb could be characteristic of a female. A higher degree of development of this secondary character could be indicative of the chick being male. Noticeable from 4 to 6 weeks onwards, hence discarded as a sexing method and rather used as a sex confirmation method.
- (f)
- Method 7. Fan-shaped wings and general wing metrics determination: if all of the feathers of each wing are arranged in a well-defined fan shape, this characteristic is considered as specific to the female sex. On the other hand, if the feathers that comprise each wing are at different levels of growth and development, the chick is considered to be male.
- (g)
- Method 8. Body size and head morphology: it seems that males are larger than females within only a few days of life; in addition, his head is usually somewhat smaller and rounded. In females, the morphology of the head is considered to be angled and of greater size than in males. To state the limits to consider a chick big or small we computed the median of the sizes (sample was not normally distributed p > 0.05), to set over and below the median categories.
- (h)
- Method 9. Leg length: long legs are considered to be characteristics of male chickens. Short legs will be attributed to female chickens. To state the limits to consider legs long or short, we computed the median of the sizes (sample was not normally distributed p > 0.05), to set over and below the median categories. We considered the complete leg, not only the shanks.
- (i)
- Method 10. Behavior/coping styles or slap technique: hands are clapped at a prudent distance of 20 cm from the animal. This technique is applied individually for each chick in an isolated place, apart from the rest of chicks. Two different reactions can be observed: freezing (male) and fleeing or attempting to escape (female).
2.6. Sex Confirmation
2.7. Sexer Reliability Testing
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Somsen, D.; Capelle, A.; Tramper, J. Production yield analysis in the poultry processing industry. J. Food Eng. 2004, 65, 479–487. [Google Scholar] [CrossRef]
- Gerber, P.; Opio, C.; Steinfeld, H. Poultry production and the environment—A review. Anim. Prod. Health Div. 2007, 153, 1–27. [Google Scholar]
- Yegani, M. The future of poultry science: Student perspective. J. Poult. Sci. 2009, 88, 1339–1342. [Google Scholar] [CrossRef]
- Glatz, P.; Pym, R. Alojamiento y manejo de las aves de corral en los países en desarrollo. Revisión Desarro. Avícola 2013, 26, 1–5. (In Spanish) [Google Scholar]
- Mucksová, J.; Reinišová, M.; Kalina, J.; Lejčková, B.; Hejnar, J.; Trefil, P. Conservation of chicken male germline by orthotopic transplantation of primordial germ cells from genetically distant donors. Biol. Reprod. 2019, 101, 200–207. [Google Scholar] [CrossRef]
- Abdelqader, A.; Wollny, C.; Gauly, M. Characterization of local chicken production systems and their potential under different levels of management practice in Jordan. Trop. Anim. Health Prod. 2007, 39, 155–164. [Google Scholar] [CrossRef]
- Spalona, A.; Ranvig, H.; Cywa-Benko, K.; Zanon, A.; Sabbioni, A.; Szalay, I.; Benková, J.; Baumgartner, J.; Szwaczkowski, T. Population size in conservation of local chicken breeds in chosen European countries. Arch. Geflugelkd. 2007, 71, 49–55. [Google Scholar]
- Yosef, I.; Edry-Botzer, L.; Globus, R.; Shlomovitz, I.; Munitz, A.; Gerlic, M.; Qimron, U. A genetic system for biasing the sex ratio in mice. EMBO Rep. 2019, 20, e48269. [Google Scholar] [CrossRef]
- Jones, P.; Shearer, S.; Gates, R. Edge extraction algorithm for feather sexing poultry chicks. Trans. ASAE 1991, 34, 635–0640. [Google Scholar] [CrossRef]
- Wilson, H.; Jacob, J.; Mather, F. Métodos de Sexado en Pollitos de un día de Edad; University of Florida, IFAS: Gainesville, FL, USA, 2012; Volume 21, pp. 1–4. [Google Scholar]
- Manuel Llanos Company. Sexaje de pollitos. Hojas Divulgadoras Ministerio de Agricultura. Dir. Gen. Capacit. Agrar. 1963, 14, 63. [Google Scholar]
- Dakpogan, H.B.; Salifou, S.; Aboh, A.; Chrysostome, C. Effectiveness of a sexing technique on free-range day-old chick. J. Anim. Plant. Sci. 2012, 16, 2336–2342. [Google Scholar]
- Khosravinia, H.; Manafi, M. Broiler chicks with slow-feathering (K) or rapid-feathering (k+) genes: Effects of environmental stressors on physiological adaptive indicators up to 56 h posthatch. Poultry. Sci. 2016, 95, 1719–1725. [Google Scholar] [CrossRef]
- Gryzinska, M.; Batkowska, J.; Andraszek, K.; Horecka, B.; Jezewska-Witkowska, G. Changes in plumage color and patterns in Polbar breed chicks (Polish conservative breed) during their first weeks after hatching. Eur. Poult. Sci. 2014, 78, 1–10. [Google Scholar] [CrossRef]
- Alin, K.; Fujitani, S.; Kashimori, A.; Suzuki, T.; Ogawa, Y.; Kondo, N. Non-invasive broiler chick embryo sexing based on opacity value of incubated eggs. Comput. Electron. Agr. 2019, 158, 30–35. [Google Scholar] [CrossRef]
- Breuner, C.; Orchinik, M. Seasonal regulation of membrane and intracellular corticosteroid receptors in the house sparrow brain. J. Neuroendocrinol. 2001, 13, 412–420. [Google Scholar] [CrossRef]
- Gahr, M. Male Japanese quails with female brains do not show male sexual behaviors. Proc. Natl. Acad. Sci. USA 2003, 100, 7959–7964. [Google Scholar] [CrossRef]
- Eda-Fujiwara, H.; Satoh, R.; Hata, Y.; Yamasaki, M.; Watanabe, A.; Zandbergen, M.A.; Okamoto, Y.; Miyamoto, T.; Bolhuis, J.J. Sex differences in behavioural and neural responsiveness to mate calls in a parrot. Sci. Rep. 2016, 6, 18481. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Domjan, M. Differences in the sexual conditioned behavior of male and female Japanese quail (Coturnix japonica). J. Comp. Psychol. 1997, 111, 135. [Google Scholar] [CrossRef]
- Morinha, F.; Cabral, J.; Bastos, E. Molecular sexing of birds: A comparative review of polymerase chain reaction (PCR)-based methods. Theriogenology 2012, 78, 703–714. [Google Scholar] [CrossRef]
- Mataragka, A.; Balaskas, C.; Sotirakoglou, K.; Ikonomopoulos, J. Comparative evaluation of the performance of the PCR assays commonly used for the determination of sex in avian species. J. King Saud Univ. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Bruijnis, M.; Blok, V.; Stassen, E.; Gremmen, H. Moral Lock-In in responsible innovation: The ethical and social aspects of killing day-old chicks and its alternatives. J. Agric. Environ. Ethics. 2015, 28, 939–960. [Google Scholar] [CrossRef]
- Casana, C.F.D.; Ruíz, D.E.V.; Peña, G.A.S.; Effio, P.J.C. Molecular Sexing of the White-Winged Guan (Penelope albipennis) and Other Wild Birds of the North of Peru. Sex. Dev. 2019, 13, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Aerts, S.; Boonen, R.; Bruggeman, V.; De Tavernier, J.; Decuypere, E. Culling of day-old chicks: Opening the debates of moria. In Ethical futures: Bioscience and Food Horizons; Millar, K., Hobson West, P., Nerlich, B., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2009; pp. 117–122. [Google Scholar]
- Weissmann, A.; Förster, A.; Gottschalk, J.; Reitemeier, S.; Krautwald-Junghanns, M.-E.; Preisinger, R.; Einspanier, A. In ovo-gender identification in laying hen hybrids: Effects on hatching and production performance. Eur. Polut. Sci. 2014, 78, 199–205. [Google Scholar]
- Weissmann, A.; Reitemeier, S.; Hahn, A.; Gottschalk, J.; Einspanier, A. Sexing domestic chicken before hatch: A new method for in ovo gender identification. Theriogenology 2013, 80, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Witschi, E. Sex and secondary sexual characters. In Biology and Comparative Physiology of Birds; Marshall, A.J., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 1961; Volume 2, pp. 115–168. [Google Scholar]
- Western, P.S.; Sinclair, A.H. Sex, genes, and heat: Triggers of diversity. J. Exp. Zool. 2001, 290, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Punzalan, D.; Hosken, D.J. Sexual Dimorphism: Why the Sexes Are (and Are Not) Different. Curr. Biol. 2010, 20, R972–R973. [Google Scholar] [CrossRef]
- Smith, C.A.; Sinclair, A.H. Sex determination: Insights from the chicken. Bioessays 2004, 26, 120–132. [Google Scholar] [CrossRef]
- Nanda, I.; Schmid, M. Localization of the telomeric (TTAGGG) n sequence in chicken (Gallus domesticus) chromosomes. Cytogenet. Genome Res. 1994, 65, 190–193. [Google Scholar] [CrossRef]
- Schmid, M.; Nanda, I.; Guttenbach, M.; Steinlein, C.; Hoehn, M.; Schartl, M.; Haaf, T.; Weigend, S.; Fries, R.; Buerstedde, J. First report on chicken genes and chromosomes 2000. Cytogenet. Genome Res. 2000, 90, 169–218. [Google Scholar] [CrossRef]
- Wójcik, E.; Andraszek, K.; Gryzińska, M.; Witkowski, A.; Pałyszka, M.; Smalec, E. Sister chromatid exchange in Greenleg Partridge and Polbar hens covered by the gene-pool protection program for farm animals in Poland. Poult. Sci. 2012, 91, 2424–2430. [Google Scholar] [CrossRef]
- Cordero, P.J.; Griffith, S.C.; Aparicio, J.M.; Parkin, D.T. Sexual dimorphism in house sparrow eggs. Behav. Ecol. Sociobiol. 2000, 48, 353–357. [Google Scholar] [CrossRef]
- Mead, P.S.; Morton, M.L.; Fish, B.E. Sexual Dimorphism in Egg Size and Implications regarding Facultative Manipulation of Sex in Mountain White-Crowned Sparrows. Condor 1987, 89, 798–803. [Google Scholar] [CrossRef]
- Greenwood, A.; Crew, F.A.E. Studies on the relation of gonadic structure to plumage characterisation in the domestic fowl.—I. Henny-feathering in an ovariotomised hen with active testis grafts. Proc. R. Soc. Lond. 1926, 99, 232–241. [Google Scholar] [CrossRef]
- Cuervo, J.J.; Møller, A.P. Phenotypic variation and fluctuating asymmetry in sexually dimorphic feather ornaments in relation to sex and mating system. Biol. J. Linn. Soc. 1999, 68, 505–529. [Google Scholar] [CrossRef]
- Hutt, F. Sex dimorphism and variability in the appendicular skeleton of the Leghorn fowl. J. Poult. Sci. 1929, 8, 202–218. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U. Behavioral causes and consequences of sexual size dimorphism. Ethology 2005, 111, 977–1016. [Google Scholar] [CrossRef]
- Verdiglione, R.; Rizzi, C. A morphometrical study on the skull of Padovana chicken. Ital. J. Anim. Sci. 2018, 17, 785–796. [Google Scholar] [CrossRef]
- Gurusinghe, C.J.; Ehrlich, D. Sex-dependent structural asymmetry of the medial habenular nucleus of the chicken brain. Cell Tissue Res. 1985, 240, 149–152. [Google Scholar] [CrossRef]
- Fallahsharoudi, A.; de Kock, N.; Johnsson, M.; Ubhayasekera, S.J.K.A.; Bergquist, J.; Wright, D.; Jensen, P. Domestication Effects on Stress Induced Steroid Secretion and Adrenal Gene Expression in Chickens. Sci. Rep. 2015, 5, 15345. Available online: https://www.nature.com/articles/srep15345#supplementary-information (accessed on 13 September 2019). [CrossRef]
- Ajayi, F. Nigerian indigenous chicken: A valuable genetic resource for meat and egg production. Asian. J. Poultry. Sci. 2010, 4, 164–172. [Google Scholar] [CrossRef]
- Nie, Q.; Sun, B.; Zhang, D.; Luo, C.; Ishag, N.; Lei, M.; Yang, G.; Zhang, X. High diversity of the chicken growth hormone gene and effects on growth and carcass traits. J. Hered. 2005, 96, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Duguma, R. Phenotypic characterization of some indigenous chicken ecotypes of Ethiopia. Development. 2006, 18, 9. [Google Scholar]
- Kim, S.W.; Choi, J.S.; Sharma, N.; Ko, Y.G.; Do, Y.J.; Byun, M.; Seong, H.H.; Park, S.B.; Jeong, D.K. A novel approach for determination of chicken sexing at an early stage of development by using loop-mediated isothermal amplification method. Turk. J. Vet. Anim. Sci. 2015, 39, 583–588. [Google Scholar] [CrossRef]
- Zarazúa, G.M.S.; García, M.A.E.; Ayala, M.T.; Abraham, M.; Barrios, G. Algoritmo para sexar polluelos de un día de edad aplicando patrones morfométricos y análisis de imagen. In Proceedings of the Congreso Internacional de Investigación Academia Journals Celaya, Celaya, Guanajuato, México, 9–11 November 2016. [Google Scholar]
- Camacho Vallejo, M.E.; León Jurado, J.M.; Nogales Baena, S.; Doctor, J.; Navas González, F.J.; Arando Arbulu, A.; Delgado Bermejo, J.V.; Barba Capote, C. Primeros resultados de caracterización reproductiva en cutro variedades de gallinas Utreranas. In Proceedings of the 19th Simposio Iberoamericano sobre Conservación y Utilización de Recursos Zoogenéticos, Riobamba, Ecuador, 22–26 October 2019. [Google Scholar]
- Homma, K.; Siopes, T.D.; Wilson, W.O.; McFarland, L.Z. Identification of sex of day-old quail (Coturnix coturnix japonica) by cloacal examination. J. Poult. Sci. 1966, 45, 469–472. [Google Scholar] [CrossRef]
- González Ariza, A.; Navas González, F.J.; Arando Arbulu, A.; León Jurado, J.M.; Barba Capote, C.J.; Camacho Vallejo, M.E. Non-Parametrical Canonical Analysis of Quality-Related Characteristics of Eggs of Different Varieties of Native Hens Compared to Laying Lineage. Animals 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.P. Choosing an intraclass correlation coefficient. SPSS Keywords 1998, 67, 1–2. [Google Scholar]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Fleiss, J.L.; Cohen, J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ. Psychol. Meas. 1973, 33, 613–619. [Google Scholar] [CrossRef]
- McGraw, K.O.; Wong, S.P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1996, 1, 30. [Google Scholar] [CrossRef]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures, 1st ed.; CRC Press: Boca Ratón, FL, USA, 2003; p. 1926. [Google Scholar]
- Nolan, S.; Heinzen, T. Statistics for the Behavioral Sciences; Macmillan Learning: New York, NY, USA, 2017. [Google Scholar]
- Ng, C.S.; Li, W.-H. Genetic and Molecular Basis of Feather Diversity in Birds. Genome Biol. Evol. 2018, 10, 2572–2586. [Google Scholar] [CrossRef]
- Yilmaz-Dikmen, B.; Dikmen, S. A morphometric method of sexing white layer eggs. Bra. J. Poult. Sci. 2013, 15, 203–210. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Nätt, D.; Lindqvist, N.; Stranneheim, H.; Lundeberg, J.; Torjesen, P.A.; Jensen, P. Inheritance of acquired behaviour adaptations and brain gene expression in chickens. PLoS ONE 2009, 4, e6405. [Google Scholar] [CrossRef]
- Lindqvist, C.; Janczak, A.M.; Nätt, D.; Baranowska, I.; Lindqvist, N.; Wichman, A.; Lundeberg, J.; Lindberg, J.; Torjesen, P.A.; Jensen, P. Transmission of stress-induced learning impairment and associated brain gene expression from parents to offspring in chickens. PLoS ONE 2007, 2, e364. [Google Scholar] [CrossRef]
- Nätt, D.; Agnvall, B.; Jensen, P. Large sex differences in chicken behavior and brain gene expression coincide with few differences in promoter DNA-methylation. PLoS ONE 2014, 9, e96376. [Google Scholar] [CrossRef] [PubMed]
- Elfwing, M.; Nätt, D.; Goerlich-Jansson, V.C.; Persson, M.; Hjelm, J.; Jensen, P. Early stress causes sex-specific, life-long changes in behaviour, levels of gonadal hormones, and gene expression in chickens. PLoS ONE 2015, 10, e0125808. [Google Scholar] [CrossRef]
- Zappia, J.V.; Rogers, L.J. Sex differences and reversal of brain asymmetry by testosterone in chickens. Behav. Brain. Res. 1987, 23, 261–267. [Google Scholar] [CrossRef]
- Rosen, G.D.; Berrebi, A.S.; Yutzey, D.A.; Denenberg, V.H. Prenatal testosterone causes shift of asymmetry in neonatal tail posture of the rat. Brain. Res. Dev. 1983, 9, 99–101. [Google Scholar] [CrossRef]
- Voitkevich, A.A. The feathers and plumage of birds. In The Feathers and Plumage of Birds, 1st ed.; Sidgwick & Jackson Ltd.: London, UK, 1966; Volume 1, p. 335. [Google Scholar]
- Trinkaus, J.P. Estrogen, Thyroid Hormone and the Differentiation of Pigment Cells in the Brown Leghorn, 1st ed.; Academic Press: Waltham, MA, USA, 1953; p. 380. [Google Scholar]
- Merat, P. Major genes in fowls (Gallus gallus): Genes other than those affecting size. Anim. Prod. Sci. 1990, 3, 355–368. [Google Scholar]

| Method | Chi-Square | df | Asymp. Sig. | Cramér’s V |
|---|---|---|---|---|
| Method 1.1: Egg length test | 2.503 | 3 | 0.47 | N/A |
| Method 1.2: Egg width test | 8.042 | 3 | 0.04 | 0.317 |
| Method 2: English test | 7.736 | 3 | 0.05 | 0.311 |
| Method 3: Tail inclination | 0.918 | 3 | 0.82 | N/A |
| Method 4: Cloaca | 0.944 | 3 | 0.81 | N/A |
| Method 5: Down feathers | 6.269 | 3 | 0.10 | N/A |
| Method 6: Combs | 10.685 | 3 | 0.01 | 0.365 |
| Method 7: Wing fan | 6.382 | 3 | 0.09 | N/A |
| Method 8: Head size and morphology | 4.387 | 3 | 0.22 | N/A |
| Method 9: Legs | 7.974 | 3 | 0.05 | 0.316 |
| Method 10: Behavior/Coping styles | 7.403 | 3 | 0.06 | N/A |
| Method | Franciscan | Partridge | White | Black |
|---|---|---|---|---|
| Method 1.1: Egg length test | 2 | 2 | 2 | 2 |
| Method 1.2: Egg width test | 1 | 2 | 1 | 2 |
| Method 2: English test | 2 | 2 | 1 | 1 |
| Method 3: Tail inclination | 1 | 1 | 1 | 2 |
| Method 4: Cloaca | 2 | 1 | 1 | 1 |
| Method 5: Down feathers | 1 | 2 | 1 | 2 |
| Method 6: Combs | 2 | 2 | 2 | 1 |
| Method 7: Wing fan | 2 | 2 | 2 | 1 |
| Method 8: Head size and morphology | 2 | 2 | 1 | 1 |
| Method 9: Legs | 1 | 2 | 2 | 2 |
| Method 10: Behavior/Coping styles | 1 | 1 | 1 | 2 |
| Method | Assignation Criteria 1 (Literature) | Assignation Criteria 2 (Opposite to What is in Literature) | Assignation Criteria 3 (Combination of Best Performing Criteria) | |||
|---|---|---|---|---|---|---|
| Chi-Squared | Asymptotic Significance | Chi-Squared | Asymptotic Significance | Chi-Squared | Asymptotic Significance | |
| Method 1.1: Egg length test | 4.356 | 0.04 | 4.356 | 0.04 | 0.694 | 0.40 |
| Method 1.2: Egg width test | 0.089 | 0.77 | 0.089 | 0.77 | 0.694 | 0.40 |
| Method 2: English test | 35.220 | 0.00 | 35.220 | 0.00 | 21.025 | 0.00 |
| Method 3: Tail inclination | 3.698 | 0.05 | 0.893 | 0.34 | 0.893 | 0.34 |
| Method 4: Cloaca | 11.605 | 0.01 | 11.605 | 0.01 | 3.349 | 0.07 |
| Method 5: Down feathers | 10.256 | 0.01 | 10.256 | 0.01 | 20.024 | 0.00 |
| Method 7: Wing fan | 25.689 | 0.00 | 25.689 | 0.00 | 17.361 | 0.00 |
| Method 8: Head size and morphology | 3.841 | 0.05 | 3.841 | 0.05 | 0.432 | 0.51 |
| Method 9: Legs | 2.132 | 0.14 | 2.132 | 0.14 | 0.000 | 1.00 |
| Method 10: Behavior/Coping styles | 12.033 | 0.01 | 12.033 | 0.01 | 15.373 | 0.00 |
| Method | B | S.E. | Wald | df | Significance | Exp(B) | 95% C.I. for Exp(B) |
|---|---|---|---|---|---|---|---|
| Method 1.1: Egg length test | 0.226 | 0.626 | 0.130 | 1 | 0.72 | 1.253 | 0.367–4.274 |
| Method 1.2: Egg width test | −0.923 | 0.616 | 2.241 | 1 | 0.13 | 0.397 | 0.119–1.330 |
| Method 2: English test | 1.289 | 1.353 | 0.908 | 1 | 0.34 | 3.630 | 0.256–51.458 |
| Method 3: Tail inclination | −1.859 | 0.650 | 8.176 | 1 | 0.01 | 0.156 | 0.044–0.557 |
| Method 4: Cloaca | 1.443 | 0.747 | 3.737 | 1 | 0.05 | 4.235 | 0.980-18.301 |
| Method 5: Down feathers | −0.431 | 0.703 | 0.375 | 1 | 0.54 | 0.650 | 0.164–2.580 |
| Method 7: Wing fan | −0.651 | 1.031 | 0.398 | 1 | 0.53 | 0.522 | 0.069–3.933 |
| Method 8: Head size and morphology | 0.194 | 0.583 | 0.110 | 1 | 0.74 | 1.214 | 0.387–3.805 |
| Method 9: Legs | 0.851 | 0.576 | 2.186 | 1 | 0.14 | 2.342 | 0.758–7.237 |
| Method 10: Behavior/Coping styles | 1.971 | 0.787 | 6.266 | 1 | 0.01 | 7.176 | 1.534–33.579 |
| Constant | −1.338 | 1.757 | 0.580 | 1 | 0.45 | 0.262 |
| Method | B | S.E. | Wald | df | Significance | Exp(B) | 95% C.I. for Exp(B) |
|---|---|---|---|---|---|---|---|
| Method 1.1: Egg length test | −0.226 | 0.626 | 0.130 | 1 | 0.719 | 0.798 | 0.234–2.722 |
| Method 1.2: Egg width test | 0.923 | 0.616 | 2.241 | 1 | 0.134 | 2.516 | 0.752–8.420 |
| Method 2: English test | −1.289 | 1.353 | 0.908 | 1 | 0.341 | 0.276 | 0.019–3.906 |
| Method 3: Tail inclination | 1.859 | 0.650 | 8.176 | 1 | 0.004 | 6.420 | 1.795–22.961 |
| Method 4: Cloaca | −1.443 | 0.747 | 3.737 | 1 | 0.053 | 0.236 | 0.055–1.020 |
| Method 5: Down feathers | 0.431 | 0.703 | 0.375 | 1 | 0.540 | 1.538 | 0.388–6.104 |
| Method 7: Wing fan | 0.651 | 1.031 | 0.398 | 1 | 0.528 | 1.917 | 0.254–14.445 |
| Method 8: Head size and morphology | −0.194 | 0.583 | 0.110 | 1 | 0.740 | 0.824 | 0.263–2.583 |
| Method 9: Legs | −0.851 | 0.576 | 2.186 | 1 | 0.139 | 0.427 | 0.138–1.319 |
| Method 10: Behavior/Coping styles | −1.971 | 0.787 | 6.266 | 1 | 0.012 | 0.139 | 0.030–0.652 |
| Constant | 0.772 | 0.982 | 0.618 | 1 | 0.432 | 2.164 |
| Method | B | S.E. | Wald | df | Significance | Exp(B) | 95% C.I. for Exp(B) |
|---|---|---|---|---|---|---|---|
| Method 1: Egg length test | 0.226 | 0.626 | 0.130 | 1 | 0.71 | 1.253 | 0.367–4.274 |
| Method 1.2: Egg width test | −0.923 | 0.616 | 2.241 | 1 | 0.13 | 0.397 | 0.119–1.330 |
| Method 2: English test | 1.289 | 1.353 | 0.908 | 1 | 0.34 | 3.630 | 0.256–51.458 |
| Method 3: Tail inclination | 1.859 | 0.650 | 8.176 | 1 | 0.01 | 6.420 | 1.795–22.961 |
| Method 4: Cloaca | 1.443 | 0.747 | 3.737 | 1 | 0.05 | 4.235 | 0.980–18.301 |
| Method 5: Down feathers | −0.431 | 0.703 | 0.375 | 1 | 0.54 | 0.650 | 0.164–2.580 |
| Method 7: Wing fan | −0.651 | 1.031 | 0.398 | 1 | 0.53 | 0.522 | 0.069–3.933 |
| Method 8: Head size and morphology | 0.194 | 0.583 | 0.110 | 1 | 0.74 | 1.214 | 0.387–3.805 |
| Method 9: Legs | 0.851 | 0.576 | 2.186 | 1 | 0.14 | 2.342 | 0.758–7.237 |
| Method 10: Behavior/Coping styles | 1.971 | 0.787 | 6.266 | 1 | 0.01 | 7.176 | 1.534–33.579 |
| Constant | −9.027 | 4.568 | 3.906 | 1 | 0.05 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias Pastrana, C.; Navas González, F.J.; Marín Navas, C.; Arando Arbulu, A.; González Ariza, A.; León Jurado, J.M.; Pizarro Inostroza, M.G.; Camacho Vallejo, M.E. Sexual Dimorphism for Coping Styles Complements Traditional Methods for Sex Determination in a Multivariety Endangered Hen Breed. Animals 2019, 9, 1165. https://doi.org/10.3390/ani9121165
Iglesias Pastrana C, Navas González FJ, Marín Navas C, Arando Arbulu A, González Ariza A, León Jurado JM, Pizarro Inostroza MG, Camacho Vallejo ME. Sexual Dimorphism for Coping Styles Complements Traditional Methods for Sex Determination in a Multivariety Endangered Hen Breed. Animals. 2019; 9(12):1165. https://doi.org/10.3390/ani9121165
Chicago/Turabian StyleIglesias Pastrana, Carlos, Francisco Javier Navas González, Carmen Marín Navas, Ander Arando Arbulu, Antonio González Ariza, José Manuel León Jurado, María Gabriela Pizarro Inostroza, and Maria Esperanza Camacho Vallejo. 2019. "Sexual Dimorphism for Coping Styles Complements Traditional Methods for Sex Determination in a Multivariety Endangered Hen Breed" Animals 9, no. 12: 1165. https://doi.org/10.3390/ani9121165
APA StyleIglesias Pastrana, C., Navas González, F. J., Marín Navas, C., Arando Arbulu, A., González Ariza, A., León Jurado, J. M., Pizarro Inostroza, M. G., & Camacho Vallejo, M. E. (2019). Sexual Dimorphism for Coping Styles Complements Traditional Methods for Sex Determination in a Multivariety Endangered Hen Breed. Animals, 9(12), 1165. https://doi.org/10.3390/ani9121165





