Toxoplasma gondii Tetravalent Chimeric Proteins as Novel Antigens for Detection of Specific Immunoglobulin G in Sera of Small Ruminants
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Toxoplasma gondii Chimeric Recombinant Proteins and Toxoplasma Lysate Antigen
2.2. Serums Samples of Small Ruminants
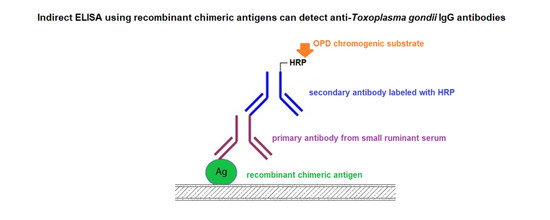
2.3. IgG ELISA
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Dubey, J.P. Toxoplasmosis in sheep-the last 20 years. Vet. Parasitol. 2009, 163, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Rajendran, C.; Ferreira, L.R.; Martins, J.; Kwok, O.C.; Hill, D.E.; Villena, I.; Zhou, H.; Su, C.; Jones, J.L. High prevalence and genotypes of Toxoplasma gondii isolated from goats, from a retail meat store, destined for human consumption in the USA. Int. J. Parasitol 2011, 41, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.; Maley, S.W.; Wright, S.E.; Rodger, S.; Bartley, P.; Innes, E.A. Toxoplasma gondii and ovine toxoplasmosis: New aspects of an old story. Vet. Parasitol. 2007, 149, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Innes, E.A.; Bartley, P.M.; Maley, S.; Katzer, F.; Buxton, D. Veterinary vaccines against Toxoplasma gondii. Mem. Inst. Oswaldo Cruz 2009, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gasior, L. Toxoplasma gondii recombinant antigens as tools for serodiagnosis of human toxoplasmosis: Current status of studies. Clin. Vaccine Immunol. 2013, 20, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Drapała, D.; Holec-Gąsior, L.; Kur, J. New recombinant chimeric antigens, P35-MAG1, MIC1-ROP1, and MAG1-ROP1, for the serodiagnosis of human toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2015, 82, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. A new Toxoplasma gondii chimeric antigen containing fragments of SAG2, GRA1, and ROP1 proteins-impact of immunodominant sequences size on its diagnostic usefulness. Parasitol. Res. 2015, 114, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- Ferra, B.T.; Holec-Gąsior, L.; Gatkowska, J.; Dziadek, B.; Dzitko, K.; Grąźlewska, W.; Lautenbach, D. The first study on the usefulness of recombinant tetravalent chimeric proteins containing fragments of SAG2, GRA1, ROP1 and AMA1 antigens in the detection of specific anti-Toxoplasma gondii antibodies in mouse and human sera. PLoS ONE 2019, 14, e0217866. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D.; Lautenbach, D.; Kur, J. A new MIC1-MAG1 recombinant chimeric antigen can be used instead of the Toxoplasma gondii lysate antigen in serodiagnosis of human toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gąsior, L.; Ferra, B.; Drapała, D. MIC1-MAG1-SAG1 chimeric protein, a most effective antigen for detection of human toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 1977–1979. [Google Scholar] [CrossRef] [PubMed]
- Ferra, B.; Holec-Gąsior, L.; Kur, J. Serodiagnosis of Toxoplasma gondii infection in farm animals (horses, swine, and sheep) by enzyme-linked immunosorbent assay using chimeric antigens. Parasitol. Int. 2015, 64, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Hiszczyńska-Sawicka, E.; Brillowska-Dąbrowska, A.; Dąbrowski, S.; Pietkiewicz, H.; Myjak, P.; Kur, J. High yield expression and single-step purification of Toxoplasma gondii SAG1, GRA1, and GRA7 antigens in Escherichia coli. Protein Exp. Purif. 2003, 27, 150–157. [Google Scholar] [CrossRef]
- Hiszczyńska-Sawicka, E.; Kur, J.; Pietkiewicz, H.; Holec, L.; Gąsior, A.; Myjak, P. Efficient production of the Toxoplasma gondii GRA6, p35 and SAG2 recombinant antigens and their applications in the serodiagnosis of toxoplasmosis. Acta Parasitol. 2005, 50, 249–254. [Google Scholar]
- Holec-Gąsior, L.; Kur, J.; Hiszczynska-Sawicka, E. GRA2 and ROP1 recombinant antigens as potential markers for detection of Toxoplasma gondii-specific immunoglobulin G in human with acute toxoplasmosis. Clin. Vaccine Immunol. 2009, 16, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gąsior, L.; Ferra, B.; Hiszczyńska-Sawicka, E.; Kur, J. The optimal mixture of Toxoplasma gondii recombinant antigens (GRA1, P22, ROP1) for diagnosis of ovine toxoplasmosis. Vet. Parasitol. 2014, 206, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, B.; Gatkowska, J.; Brzostek, A.; Dziadek, J.; Dzitko, K.; Dlugonska, H. Toxoplasma gondii: The immunogenic and protective efficacy of recombinant ROP2 and ROP4 rhoptry proteins in murine experimental toxoplasmosis. Exp. Parasitol. 2009, 123, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Holec-Gąsior, L.; Dominiak-Górski, B.; Kur, J. First report of seroprevalence of Toxoplasma gondii infection in sheep in Pomerania, northern Poland. Ann. Agric. Environ. Med. 2015, 22, 604–607. [Google Scholar] [CrossRef] [PubMed]
| Chimeric Proteins | Amino Acid Residues | Protein Characteristic |
|---|---|---|
| AMA1N-SAG2-GRA1-ROP1 | 68–287 AMA1 31–170 SAG2 26–190 GRA1 85–396 ROP1 | 899 aa Mw 97.95 kDa |
| AMA1C-SAG2-GRA1-ROP1 | 287–569 AMA1 31–170 SAG2 26–190 GRA1 85–396 ROP1 | 961 aa Mw 103.20 kDa |
| AMA1-SAG2-GRA1-ROP1 | 68–569 AMA1 31–170 SAG2 26–190 GRA1 85–396 ROP1 | 1182 aa Mw 128.42 kDa |
| SAG2-GRA1-ROP1-GRA2 | 31–170 SAG2 26–190 GRA1 185–396 ROP1 51–185 GRA2 | 806 aa Mw 86.32 kDa |
| Results of IgG ELISA | Chimeric Recombinant Antigens and TLA | |||||
|---|---|---|---|---|---|---|
| AMA1N-SAG2-GRA1-ROP1 | AMA1C-SAG2-GRA1-ROP1 | AMA1-SAG2-GRA1-ROP1 | SAG2-GRA1-ROP1-GRA2 | TLA | ||
| Sheep Sera | Group IS = 48 A | 1.457 (0.175–2.700) | 1.808 (0.081–2.308) | 1.572 (0.150–2.611) | 1.509 (0.108–2.897) | 1.349 (0.237–2.256) |
| Group IIS = 42 A | 0.222 (0.096–0.469) | 0.132 (0.077–0.364) | 0.212 (0.100–0.440) | 0.232 (0.101–0.968) | 0.269 (0.133–0.438) | |
| Cutoff | 0.388 | 0.238 | 0.467 | 0.502 | 0.442 | |
| Sensitivity | 97.92% | 95.83% | 97.92% | 97.92% | 97.92% | |
| Specificity | 97.62% | 95.24% | 100% | 97.62% | 100% | |
| AUC (ROC analysis) | 0.9836 | 0.9678 | 0.9836 | 0.9777 | 0.9881 | |
| PPV B | 97.92% | 95.83% | 100% | 97.92% | 100% | |
| NPV C | 97.62% | 95.24% | 97.67% | 97.62% | 97.67% | |
| Goat Sera | Group IG = 45 A | 0.991 (0.184–2.668) | 1.201 (0.304–2.382) | 1.142 (0.343–2.668) | 1.160 (0.179–2.311) | 1.046 (0.427–2.258) |
| Group IIG = 41 A | 0.256 (0.127–0.795) | 0.620 (0.304–1.128) | 0.267 (0.127–0.535) | 0.198 (0.109–0.353) | 0.314 (0.196–0.464) | |
| Cutoff | 0.546 | 0.336 | 0.534 | 0.533 | 0.489 | |
| Sensitivity | 88.89% | 95.56% | 95.56% | 57.78% | 97.78% | |
| Specificity | 100% | 97.56% | 100% | 95.12% | 100% | |
| AUC (ROC analysis) | 0.9981 | 0.9897 | 0.9900 | 0.8425 | 0.9989 | |
| PPV B | 100% | 89.58% | 100% | 92.86% | 100% | |
| NPV C | 89.13% | 95.24% | 95.35% | 67.24% | 97.62% | |
| All Sera | Sensitivity D | 93.55% | 95.70% | 96.77% | 78.49% | 97.85% |
| Specificity E | 98.8% | 97.59% | 100% | 96.39% | 100% | |
| PPV B | 98.86% | 97.80% | 100% | 96.05% | 100% | |
| NPV C | 93.18% | 95.29% | 97.65% | 80% | 97.65% | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holec-Gąsior, L.; Ferra, B.; Grąźlewska, W. Toxoplasma gondii Tetravalent Chimeric Proteins as Novel Antigens for Detection of Specific Immunoglobulin G in Sera of Small Ruminants. Animals 2019, 9, 1146. https://doi.org/10.3390/ani9121146
Holec-Gąsior L, Ferra B, Grąźlewska W. Toxoplasma gondii Tetravalent Chimeric Proteins as Novel Antigens for Detection of Specific Immunoglobulin G in Sera of Small Ruminants. Animals. 2019; 9(12):1146. https://doi.org/10.3390/ani9121146
Chicago/Turabian StyleHolec-Gąsior, Lucyna, Bartłomiej Ferra, and Weronika Grąźlewska. 2019. "Toxoplasma gondii Tetravalent Chimeric Proteins as Novel Antigens for Detection of Specific Immunoglobulin G in Sera of Small Ruminants" Animals 9, no. 12: 1146. https://doi.org/10.3390/ani9121146
APA StyleHolec-Gąsior, L., Ferra, B., & Grąźlewska, W. (2019). Toxoplasma gondii Tetravalent Chimeric Proteins as Novel Antigens for Detection of Specific Immunoglobulin G in Sera of Small Ruminants. Animals, 9(12), 1146. https://doi.org/10.3390/ani9121146