Effect of the Addition of Humic Substances as Growth Promoter in Broiler Chickens Under Two Feeding Regimens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the EHS
2.2. Animal, Treatments, and Diets
2.3. Sample Collection and Laboratory Determinations
2.4. Statistical Analysis
3. Results
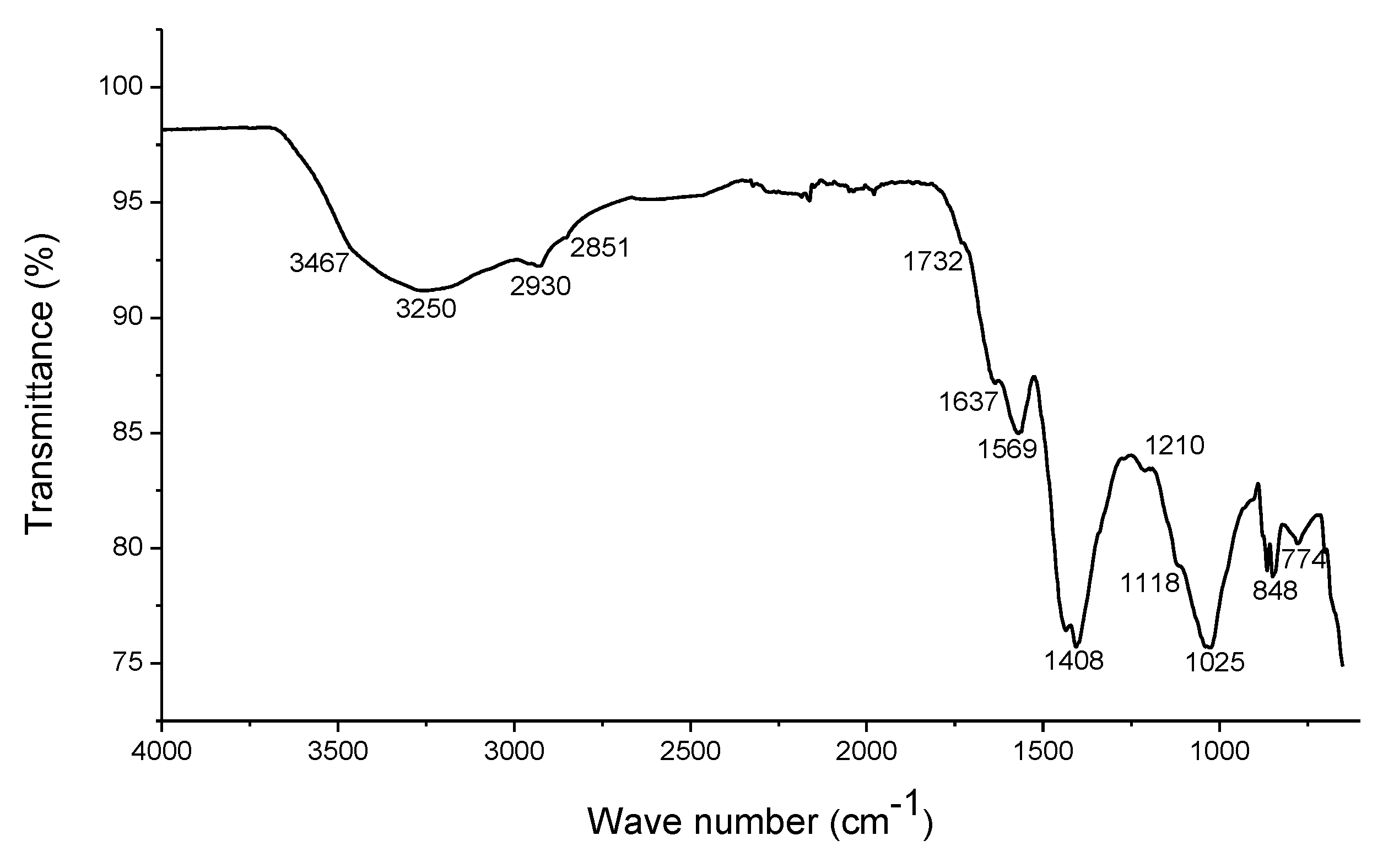
3.1. Composition of Humic Substances
3.2. Effect of Dietary Treatments
3.3. Effect of Feeding Regimen
4. Discussion
4.1. Composition of Humic Substances
4.2. Effect of Dietary Treatments
4.3. Effect of Feeding Regimen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. World Health Statistics 2015. Available online: http://www.who.int/gho/publications/world_health_statistics/ 2015/en/ (accessed on 14 September 2019).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140083. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rosales, S.; de L. Angeles, M. Addition of a Worm Leachate as Source of Humic Substances in the Drinking Water of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2015, 28, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Maguey-Gonzalez, J.A.; Michel, M.A.; Baxter, M.F.A.; Tellez, G.; Moore, P.A.; Solis-Cruz, B.; Hernández-Patlan, D.; Merino-Guzman, R.; Hernandez-Velasco, X.; Latorre, J.D.; et al. Effect of Humic Acids on Intestinal Viscosity, Leaky Gut and Ammonia Excretion in a 24 h Feed Restriction Model to Induce Intestinal Permeability in Broiler Chickens. Anim. Sci. J. 2018, 89, 1002–1010. [Google Scholar] [CrossRef]
- Maguey-Gonzalez, J.A.; Michel, M.A.; Baxter, M.F.A.; Solis-Cruz, B.; Hernandez-Patlan, D.; Merino-Guzman, R.; Hernandez-Velasco, X.; Latorre, J.D.; Hargis, B.M.; Tellez, G.; et al. Effects of Humic Acids on Recovery of Salmonella Enterica Serovar Enteritidis. Ann. Anim. Sci. 2018, 18, 387–399. [Google Scholar] [CrossRef]
- EMEA. Committee for Veterinary Medical Products. Humic Acids and Their Sodium Salts, Summary Report. Committee for Veterinary Medicinal Products 1999. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014416.pdf (accessed on 29 April 2014).
- Arif, M.; Alagawany, M.; Abd El-Hack, M.E.; Saeed, M.; Arain, M.A.; Elnesr, S.S. Humic Acid as a Feed Additive in Poultry Diets: A Review. Iran. J. Vet. Res. 2019, 20, 167–172. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Peña-Méndez, E.M.; Havel, J.; Patočka, J. Humic Substances—Compounds of Still Unknown Structure: Applications in Agriculture, Industry, Environment, and Biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef]
- Kamel, M.M.; Elhady, M. Biologyical Immune Stimulants Effects on Immune Response, Behavioural and Productive Performance of Broilers. Oxidative Stress. 2015, 35, 691–702. [Google Scholar]
- Vaskova, J.; Patlevič, P.; Žatko, D.; Marcinčák, S.; Vaško, L.; Krempaská, K.; Nagy, J. Effects of Humic Acids on Poultry Under Stress Conditions. Slov. Vet. Res. 2018, 55, 245–253. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Berghman, L.R.; Vicuña, E.A.; Latorre, J.D.; Menconi, A.; Wolchok, J.D.; Wolfenden, A.D.; Faulkner, O.B.; Tellez, G.I.; Hargis, B.M.; et al. Poultry Enteric Inflammation Model with Dextran Sodium Sulfate Mediated Chemical Induction and Feed Restriction in Broilers. Poult. Sci. 2015, 94, 1220–1226. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Vicuña, E.A.; Latorre, J.D.; Wolfenden, A.D.; Téllez, G.I.; Hargis, B.M.; Bielke, L.R. Evaluation of Gastrointestinal Leakage in Multiple Enteric Inflammation Models in Chickens. Front. Vet. Sci. 2015, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, M. Soil Organic Matter—The Next 75 Years. Soil Sci. 1991, 151, 41. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis. 21st Edition. 2019. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 30 October 2019).
- Díaz-Alonso, J.A.; Gómez-Rosales, S.; de L. Angeles, M.; Ávila-González, E.; López-Coello, C. Effects of the Level and Relationship of Calcium and Available Phosphorus on the Growth and Tibia Mineralization of Broiler Starter Chickens. J. Appl. Poult. Res. 2019, 28, 339–349. [Google Scholar] [CrossRef]
- Grau, R.; Hamm, R. Eine einfache Methode zur Bestimmung der Wasserbindung im Muskel. Naturwissenschaften 1953, 40, 29–30. [Google Scholar] [CrossRef]
- Earl, L.A.; King, A.J.; Fitzpatrick, D.P.; Cooper, J.E. A Modification of a Method to Determine Expressible Moisture in Ground, Dark Poultry Meat. Poult. Sci. 1996, 75, 1433–1436. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total Antioxidant Capacities of Raw and Cooked Meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Maraschiello, C.; Sárraga, C.; García Regueiro, J.A. Glutathione Peroxidase Activity, TBARS, and α-Tocopherol in Meat from Chickens Fed Different Diets. J. Agric. Food Chem. 1999, 47, 867–872. [Google Scholar] [CrossRef]
- Rondón, A.J.; Samaniego, L.M.; Bocourt, R.; Rodríguez, S.; Milián, G.; Ranilla, M.J.; Laurencio, M. Isolation, identification and partial characterization of the probiotic properties of lactobacillus sp. strains obtained from the gastrointestinal tract of broilers. Cienc. Tecnol. Aliment. 2008, 6, 56–63. [Google Scholar] [CrossRef]
- Miah, M.S.; Asaduzzaman, M.; Sufian, M.A.; Hossain, M.M. Isolation of Clostridium Perfringens, Causal Agents of Necrotic Enteritis in Chickens. J. Bangladesh Agric. Univ. 2011, 9, 97–102. [Google Scholar] [CrossRef]
- Long, P.L.; Rowell, J.G. Sampling Broiler House Litter for Coccidial Oocysts. Br. Poult. Sci. 1975, 16, 583–592. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS User’s Guide: Statistics, Version, 64th ed.; SAS Inst Inc.: Cary, NC, USA, 1990. [Google Scholar]
- Piccolo, A.; Celano, G.; Conte, P. Adsorption of Glyphosate by Humic Substances. J. Agric. Food Chem. 1996, 44, 2442–2446. [Google Scholar] [CrossRef]
- Rupiasih, N.N.; Vidyasagar, P.B. Analytical Study of Humic Acid from Various Sources Commonly Used as Fertilizer: Emphasis on Heavy Metal Content. Int. J. Des. Nat. Ecodynamics 2009, 4, 32–46. [Google Scholar] [CrossRef]
- Jayaganesh, S.; Senthurpandian, V.K. Extraction and Characterization of Humic and Fulvic Acids from Latosols under Tea Cultivation in South India. Asian J. Earth Sci. 2010, 3, 130–135. [Google Scholar] [CrossRef]
- Zara, M.; Ahmad, Z.; Akhtar, J.; Shahzad, K.; Sheikh, N.; Munir, S. Extraction and Characterization of Humic Acid from Pakistani Lignite Coals. Energy Sources Part Recovery Util. Environ. Eff. 2017, 39, 1159–1166. [Google Scholar] [CrossRef]
- Tan, W.F.; Norde, W.; Koopal, L.K. Humic Substance Charge Determination by Titration with a Flexible Cationic Polyelectrolyte. Geochim. Cosmochim. Acta 2011, 75, 5749–5761. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant Properties of Humic Substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Canellas, L.P.; Piccolo, A.; Dobbss, L.B.; Spaccini, R.; Olivares, F.L.; Zandonadi, D.B.; Façanha, A.R. Chemical Composition and Bioactivity Properties of Size-Fractions Separated from a Vermicompost Humic Acid. Chemosphere 2010, 78, 457–466. [Google Scholar] [CrossRef]
- Prakash, P.; Chitradevi, A.; Anand, T.A.; Arasu, R.; Narendrakumar, G.; Masilamani Selvam, M. Optimization of Humic acid Production using RSM-CCD, its Characterization and Applications on Vigna mungo. Int. J. ChemTech Res. 2014, 6, 1531–1537. [Google Scholar]
- Disetlhe, A.R.P.; Marume, U.; Mlambo, V. Humic Acid and Enzymes Inclusion in Canola-Based Diets Generate Different Responses in Growth Performance, Protein Utilization Dynamics, and Hemato-Biochemical Parameters in Broiler Chickens. Poult. Sci. 2018, 97, 2745–2753. [Google Scholar] [CrossRef]
- Proctor, A.; Phillips, G.J. Differential Effects of Bacitracin Methylene Disalicylate (BMD) on the Distal Colon and Cecal Microbiota of Young Broiler Chickens. Front. Vet. Sci. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Eren, M.; Cengiz, S.; Deniz, G.; Orhan, F. Effects of Liquid Humate Supplemented to Drinking Water on the Performance and Eggshell Quality of Hens in Different Laying Periods. Rev. Med. Vet. 2008, 159, 91–95. [Google Scholar]
- Ozturk, E.; Ocak, N.; Turan, A.; Erener, G.; Altop, A.; Cankaya, S. Performance, Carcass, Gastrointestinal Tract and Meat Quality Traits, and Selected Blood Parameters of Broilers Fed Diets Supplemented with Humic Substances. J. Sci. Food Agric. 2012, 92, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yasar, S.; Gokcimen, A.; Altuntas, I.; Yonden, Z.; Petekkaya, E. Performance and Ileal Histomorphology of Rats Treated with Humic Acid Preparations. J. Anim. Physiol. Anim. Nutr. 2002, 86, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y. Modulation of the Growth Performance, Meat Composition, Oxidative Status, and Immunity of Broilers by Dietary Fulvic Acids. Poult. Sci. 2019, 10, 4509–4513. [Google Scholar] [CrossRef] [PubMed]
- Taklimi, S.M.S.M.; Ghahri, H.; Isakan, M.A. Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agric. Sci. 2012, 3, 663–668. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel Electrochemical Approach to Assess the Redox Properties of Humic Substances. Environ. Sci. Technol. 2010, 44, 87–93. [Google Scholar] [CrossRef]
- Ratasuk, N.; Nanny, M.A. Characterization and Quantification of Reversible Redox Sites in Humic Substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef]
- Chatli, M.K.; Joseph, S. Augmentation of Shelf Life of Meat with Natural Antioxidants: An Overview. J. Meat Sci. Technol. 2014, 2, 16–30. [Google Scholar]
- Guban, J.; Korver, D.R.; Allison, G.E.; Tannock, G.W. Relationship of Dietary Antimicrobial Drug Administration with Broiler Performance, Decreased Population Levels of Lactobacillus Salivarius, and Reduced Bile Salt Deconjugation in the Ileum of Broiler Chickens. Poult. Sci. 2006, 85, 2186–2194. [Google Scholar] [CrossRef]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Bozkurt, A.S. Etçi Piliçlerde Esansiyel Yağlar ve/Veya Humatın Yaz Sezonunda Performans, İnce Bağırsak Mikrobiyel Populasyonu ve Antikor Titreleri Üzerine Etkisi. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 185–190. [Google Scholar] [CrossRef]
- Shermer, C.L.; Maciorowski, K.G.; Baile, C.A. Caecal Metabolites and Microbial Populations in Chickens Consuming Diets Containing a Mined Humate Compound. J. Sci. Food Agric. 1998, 77, 479–486. [Google Scholar] [CrossRef]
- LaVorgna, M.; Schaeffer, J.L.; Bade, D.; Dickson, J.; Cookson, K.; Davis, S.W. Performance of Broilers Fed a Broader Spectrum Antibiotic (Virginiamycin) or a Narrower Spectrum Antibiotic (Bacitracin Methylene Disalicylate) over 3 Consecutive Grow-out Cycles. J. Appl. Poult. Res. 2013, 22, 574–582. [Google Scholar] [CrossRef]
- Díaz Carrasco, J.M.; Redondo, E.A.; Pin Viso, N.D.; Redondo, L.M.; Farber, M.D.; Fernández Miyakawa, M.E. Tannins and Bacitracin Differentially Modulate Gut Microbiota of Broiler Chickens. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Kaevska, M.; Lorencova, A.; Videnska, P.; Sedlar, K.; Provaznik, I.; Trckova, M. Effect of Sodium Humate and Zinc Oxide Used in Prophylaxis of Post-Weaning Diarrhoea on Faecal Microbiota Composition in Weaned Piglets. Veterinární Med. 2016, 61, 328–336. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Reißhauer, A.; Göktas, O.; Krüger, M.; Neuhaus, J.; Schrödl, W. Impact of Humic Acids on the Colonic Microbiome in Healthy Volunteers. World J. Gastroenterol. 2017, 7, 885–890. [Google Scholar] [CrossRef]
- Hernández-Velasco, X.; Chapman, H.D.; Owens, C.M.; Kuttappan, V.A.; Fuente-Martínez, B.; Menconi, A.; Latorre, J.D.; Kallapura, G.; Bielke, L.R.; Rathinam, T.; et al. Absorption and Deposition of Xanthophylls in Broilers Challenged with Three Dosages of Eimeria Acervulina Oocysts. Br. Poult. Sci. 2014, 55, 167–173. [Google Scholar] [CrossRef]
- Mathis, G.; Schaeffer, J.; Cookson, K.; Dickson, J.; LaVorgna, M.; Waldrip, D. Effect of Lasalocid or Salinomycin Administration on Performance and Immunity Following Coccidia Vaccination of Commercial Broilers1. J. Appl. Poult. Res. 2014, 23, 577–585. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Jiang, L.; Chen, X.; Griffiths, B.S.; Li, H.; Hu, F. Vermicompost Increases Defense against Root-Knot Nematode (Meloidogyne Incognita) in Tomato Plants. Appl. Soil Ecol. 2016, 105, 177–186. [Google Scholar] [CrossRef]
- Khalil, M.; Awad-Allah, S. Effects of Vermicompost, Vermicompost Tea and a Bacterial Bioagent against Meloidogyne Incognita on Banana in Egypt. Pak. J. Nematol. 2019, 37, 25–33. [Google Scholar] [CrossRef]
- Meinelt, T.; Schreckenbach, K.; Pietrock, M.; Heidrich, S.; Steinberg, C.E.W. Humic Substances: Part 1: Dissolved Humic Substances (HS) in Aquaculture and Ornamental Fish Breeding. Environ. Sci. Pollut. Res. 2008, 15, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Meinelt, T.; Playle, R.; Schreckenbach, K.; Pietrock, M. The toxicity of the antiparasitic mixture FMC is changed by humic substances and calcium. Aquacult. Res. 2001, 32, 405–410. [Google Scholar] [CrossRef]
- Kodama, H.; Denso; Okazaki, F.; Ishida, S. Protective Effect of Humus Extract against Trypanosoma Brucei Infection in Mice. J. Vet. Med. Sci. 2008, 70, 1185–1190. [Google Scholar] [CrossRef]
- Jansen van Rensburg, C.E.; Naude, P.J. Potassium Humate Inhibits Complement Activation and the Production of Inflammatory Cytokines in Vitro. Inflammation 2009, 32, 270–276. [Google Scholar] [CrossRef]
- Tohid, T.; Hasan, G.; Alireza, T. Efficacy of Mannanoligosaccharides and Humate on Immune Response to Avian Influenza (H9) Disease Vaccination in Broiler Chickens. Vet. Res. Commun. 2010, 34, 709–717. [Google Scholar] [CrossRef]
- Mehdi, A.; Hasan, G. Immune Response of Broiler Chicks Fed Yeast Derived Mannan Oligosaccharides and Humate against Newcastle Disease. World Appl. Sci. J. 2012, 7, 779–785. [Google Scholar]
- Moore, R.W.; Holt, P.S. The Effect of Feed Deprivation on Tissue Invasion by Salmonella Enteritidis. Poult. Sci. 2006, 85, 1333–1337. [Google Scholar] [CrossRef]
- Najafi, P.; Zulkifli, I.; Soleimani, A.F.; Goh, Y.M. Acute Phase Proteins Response to Feed Deprivation in Broiler Chickens. Poult. Sci. 2016, 95, 760–763. [Google Scholar] [CrossRef]
| Item % | From 1–13 d of Age | From 14 to 35 d of Age | ||
|---|---|---|---|---|
| Positive Control | Negative Control | Extract of Humic Substances | ||
| Ground corn | 50.2 | 63.8 | 63.8 | 63.5 |
| Soybean meal | 40.9 | 29.7 | 29.7 | 29.7 |
| Vegetable oil | 4.22 | 2.33 | 2.36 | 2.41 |
| Calcium orthophosphate | 1.70 | 1.23 | 1.23 | 1.23 |
| Calcium carbonate | 1.49 | 1.45 | 1.45 | 1.45 |
| Vitamins and minerals 1 | 0.70 | 0.70 | 0.70 | 0.70 |
| Sodium bicarbonate | 0.25 | 0.20 | 0.20 | 0.20 |
| Salt | 0.28 | 0.45 | 0.45 | 0.45 |
| DL-Methionine | 0.15 | 0.10 | 0.10 | 0.10 |
| L-Lysine·HCl 2 | 0.12 | 0.09 | 0.09 | 0.09 |
| L-Threonine | 0.02 | 0.00 | 0.00 | 0.00 |
| Antibiotic | 0.05 | 0.05 | 0.00 | 0.00 |
| Coccidiostat | 0.05 | 0.05 | 0.00 | 0.00 |
| Extract of humic substances | 0.00 | 0.00 | 0.00 | 0.25 |
| Calculated nutrient content | ||||
| Metabolizable energy, kcal/kg | 3000 | 3100 | 3100 | 3100 |
| Digestible Lys % | 1.19 | 1.00 | 1.00 | 1.00 |
| Digestible Met % | 0.46 | 0.38 | 0.38 | 0.38 |
| Digestible Thr % | 0.79 | 0.65 | 0.65 | 0.65 |
| Ca % | 1.00 | 0.90 | 0.90 | 0.90 |
| Available P % | 0.50 | 0.45 | 0.45 | 0.45 |
| Element | Atomic Content % | Standard Deviation | Standard Error |
|---|---|---|---|
| O | 45.77 | 0.59 | 0.34 |
| Na | 27.45 | 1.386 | 0.80 |
| C | 13.79 | 0.982 | 0.567 |
| Si | 4.39 | 0.538 | 0.311 |
| K | 3.88 | 1.601 | 0.924 |
| Cl | 2.88 | 0.489 | 0.282 |
| S | 1.50 | 0.143 | 0.083 |
| P | 0.81 | 0.059 | 0.034 |
| Ca | 0.43 | 0.119 | 0.069 |
| Feeding Regimen (FR) | Ad Libitum | Restricted Feeding | SEM c | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Treatment (DT) | PC b | NG | EHS | PC | NG | EHS | FR | DT | DT * FR | |
| Initial weight g | 342.2 | 326.2 | 336.9 | 337.2 | 341.8 | 331.7 | 14.284 | 0.88 | 0.91 | 0.69 |
| Final weight g | 1967.9 | 1885.5 | 1909.6 | 1817.6 | 1806.3 | 1791.0 | 52.465 | 0.01 | 0.62 | 0.79 |
| Feed intake g/d | 76.6 | 73.5 | 74.2 | 70.1 | 69.1 | 68.8 | 1.234 | 0.01 | 0.81 | 0.37 |
| Weight gain g/d | 98.7 | 97.6 | 97.2 | 92.0 | 94.2 | 93.1 | 2.054 | 0.01 | 0.55 | 0.87 |
| Feed conversion ratio | 1.32 | 1.34 | 1.33 | 1.34 | 1.38 | 1.37 | 0.034 | 0.20 | 0.66 | 0.94 |
| Legs % | 8.3 | 8.3 | 8.3 | 8.4 | 8.4 | 8.5 | 0.179 | 0.53 | 0.82 | 0.84 |
| Thighs % | 9.0 | 8.8 | 8.9 | 8.9 | 8.8 | 9.0 | 0.137 | 0.72 | 0.53 | 0.84 |
| Breast % | 24.2 | 23.6 | 23.8 | 23.0 | 22.8 | 23.3 | 0.266 | 0.01 | 0.17 | 0.47 |
| Carcass % | 41.5 | 40.7 | 41.0 | 40.4 | 40.0 | 40.8 | 0.314 | 0.01 | 0.05 | 0.31 |
| Feeding Regimen (FR) | Ad Libitum | Restricted Feeding | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Treatment (DT) | PC b | NG | EHS | PC | NG | EHS | SEM c | FR | DT | DT * FR |
| Tibia Measurements | ||||||||||
| Fresh weight g | 12.1 | 13.3 | 13.0 | 11.7 | 12.3 | 12.1 | 0.444 | 0.02 | 0.14 | 0.84 |
| Dried matter % | 40.7 | 39.8 | 42.1 | 39.0 | 39.9 | 39.6 | 0.871 | 0.04 | 0.45 | 0.26 |
| Dried weight g | 4.9 | 5.3 | 5.4 | 4.6 | 4.9 | 4.8 | 0.201 | 0.01 | 0.19 | 0.77 |
| Ashes % | 62.8 | 62.3 | 64.3 | 63.3 | 64.6 | 62.8 | 0.918 | 0.57 | 0.9 | 0.13 |
| Ashes weight g | 3.1 | 3.1 | 3.5 | 2.9 | 3.1 | 3.0 | 0.153 | 0.03 | 0.29 | 0.18 |
| Balance of Dietary Components | ||||||||||
| Dry matter | ||||||||||
| Intake g/d | 127 | 132 | 129 | 130 | 134 | 132 | 2.855 | 0.25 | 0.37 | 0.97 |
| Excretion g/d | 36.7 | 37.9 | 37.7 | 39.3 | 39.3 | 39.1 | 0.854 | 0.02 | 0.75 | 0.73 |
| Retention % | 70.8 | 71.1 | 70.7 | 69.8 | 70.5 | 70.5 | 0.719 | 0.3 | 0.76 | 0.87 |
| Ashes | ||||||||||
| Intake, g/d | 13.6 | 14.0 | 13.8 | 13.9 | 14.2 | 14.1 | 0.288 | 0.25 | 0.38 | 0.97 |
| Excretion, g/d | 11.3 | 12.2 | 11.5 | 11.5 | 12.2 | 11.6 | 0.327 | 0.17 | 0.88 | 0.56 |
| Retention, % | 15.5 | 13.3 | 16.7 | 17.3 | 14.1 | 17.7 | 0.365 | 0.42 | 0.46 | 0.39 |
| Nitrogen | ||||||||||
| Intake g/d | 3.6 | 3.7 | 3.7 | 3.7 | 3.8 | 3.8 | 0.081 | 0.25 | 0.37 | 0.97 |
| Excretion g/d | 1.3 | 1.3 | 1.4 | 1.4 | 1.4 | 1.4 | 0.037 | 0.01 | 0.24 | 0.45 |
| Retention % | 64.5 | 65.4 | 63.1 | 63.0 | 62.2 | 62.7 | 0.947 | 0.03 | 0.51 | 0.30 |
| Energy | ||||||||||
| Intake Kcal/d | 524 | 543 | 532 | 536 | 550 | 545 | 11.746 | 0.25 | 0.37 | 0.97 |
| Excretion Kcal/d | 133.9 | 142.9 | 138.3 | 146.2 | 146.2 | 146.1 | 3.566 | 0.01 | 0.43 | 0.43 |
| Retention % | 74.1 | 73.6 | 73.9 | 72.7 | 73.4 | 73.2 | 0.743 | 0.18 | 0.98 | 0.67 |
| AMEn Kcal/kg of feed | 2903 | 2879 | 2899 | 2847 | 2880 | 2869 | 29.067 | 0.22 | 0.96 | 0.60 |
| Feeding Regimen (FR) | Ad Libitum | Restricted Feeding | SEM c | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Treatment (DT) | PC b | NG | EHS | PC | NG | EHS | FR | DT | DT * FR | |
| pH | 6.3 | 6.3 | 6.3 | 6.3 | 6.1 | 6.2 | 0.077 | 0.05 | 0.25 | 0.42 |
| Exterior breast meat color | ||||||||||
| L* (Brightness) | 74.4 | 74.1 | 73.8 | 74.3 | 75.7 | 75.4 | 0.480 | 0.02 | 0.47 | 0.14 |
| a* (redness) | 4.9 | 4.9 | 5.1 | 4.7 | 5.1 | 5.3 | 0.259 | 0.76 | 0.39 | 0.71 |
| b* (yellowness) | 10.2 | 9.7 | 9.7 | 9.9 | 10.3 | 9.9 | 0.605 | 0.73 | 0.92 | 0.71 |
| Water holding capacity % | ||||||||||
| Filtration | 15.3 | 14.5 | 15.7 | 14.4 | 14.0 | 18.7 | 1.848 | 0.51 | 0.26 | 0.71 |
| Centrifugation | 32.8 | 37.2 | 34.2 | 29.6 | 23.2 | 29.3 | 4.677 | 0.05 | 0.94 | 0.97 |
| Dripping | 1.3 | 0.9 | 0.9 | 1.0 | 1.0 | 1.0 | 0.159 | 0.93 | 0.26 | 0.48 |
| Antioxidant status d | ||||||||||
| TBARS, mg MDA/kg meat | 0.14 | 0.12 | 0.14 | 0.16 | 0.16 | 0.14 | 0.023 | 0.22 | 0.97 | 0.74 |
| DPPH, mmol Trolox/kg meat | 16.8 | 19.1 | 17.5 | 16.6 | 17.4 | 15.8 | 2.516 | 0.18 | 0.26 | 0.71 |
| FRAP, mmol Trolox/kg meat | 14.5 | 15.7 | 16.0 | 14.7 | 14.6 | 13.5 | 1.065 | 0.57 | 0.98 | 0.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Negrete, A.; Gómez-Rosales, S.; Angeles, M.d.L.; López-Hernández, L.H.; Reis-de Souza, T.C.; López-García, Y.; Zavala-Franco, A.; Téllez-Isaias, G. Effect of the Addition of Humic Substances as Growth Promoter in Broiler Chickens Under Two Feeding Regimens. Animals 2019, 9, 1101. https://doi.org/10.3390/ani9121101
Domínguez-Negrete A, Gómez-Rosales S, Angeles MdL, López-Hernández LH, Reis-de Souza TC, López-García Y, Zavala-Franco A, Téllez-Isaias G. Effect of the Addition of Humic Substances as Growth Promoter in Broiler Chickens Under Two Feeding Regimens. Animals. 2019; 9(12):1101. https://doi.org/10.3390/ani9121101
Chicago/Turabian StyleDomínguez-Negrete, Alejandra, Sergio Gómez-Rosales, María de Lourdes Angeles, Luis Humberto López-Hernández, Tercia Cesaria Reis-de Souza, Yair López-García, Anai Zavala-Franco, and Guillermo Téllez-Isaias. 2019. "Effect of the Addition of Humic Substances as Growth Promoter in Broiler Chickens Under Two Feeding Regimens" Animals 9, no. 12: 1101. https://doi.org/10.3390/ani9121101
APA StyleDomínguez-Negrete, A., Gómez-Rosales, S., Angeles, M. d. L., López-Hernández, L. H., Reis-de Souza, T. C., López-García, Y., Zavala-Franco, A., & Téllez-Isaias, G. (2019). Effect of the Addition of Humic Substances as Growth Promoter in Broiler Chickens Under Two Feeding Regimens. Animals, 9(12), 1101. https://doi.org/10.3390/ani9121101






