Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps
Simple Summary
Abstract
1. Introduction
2. Methods
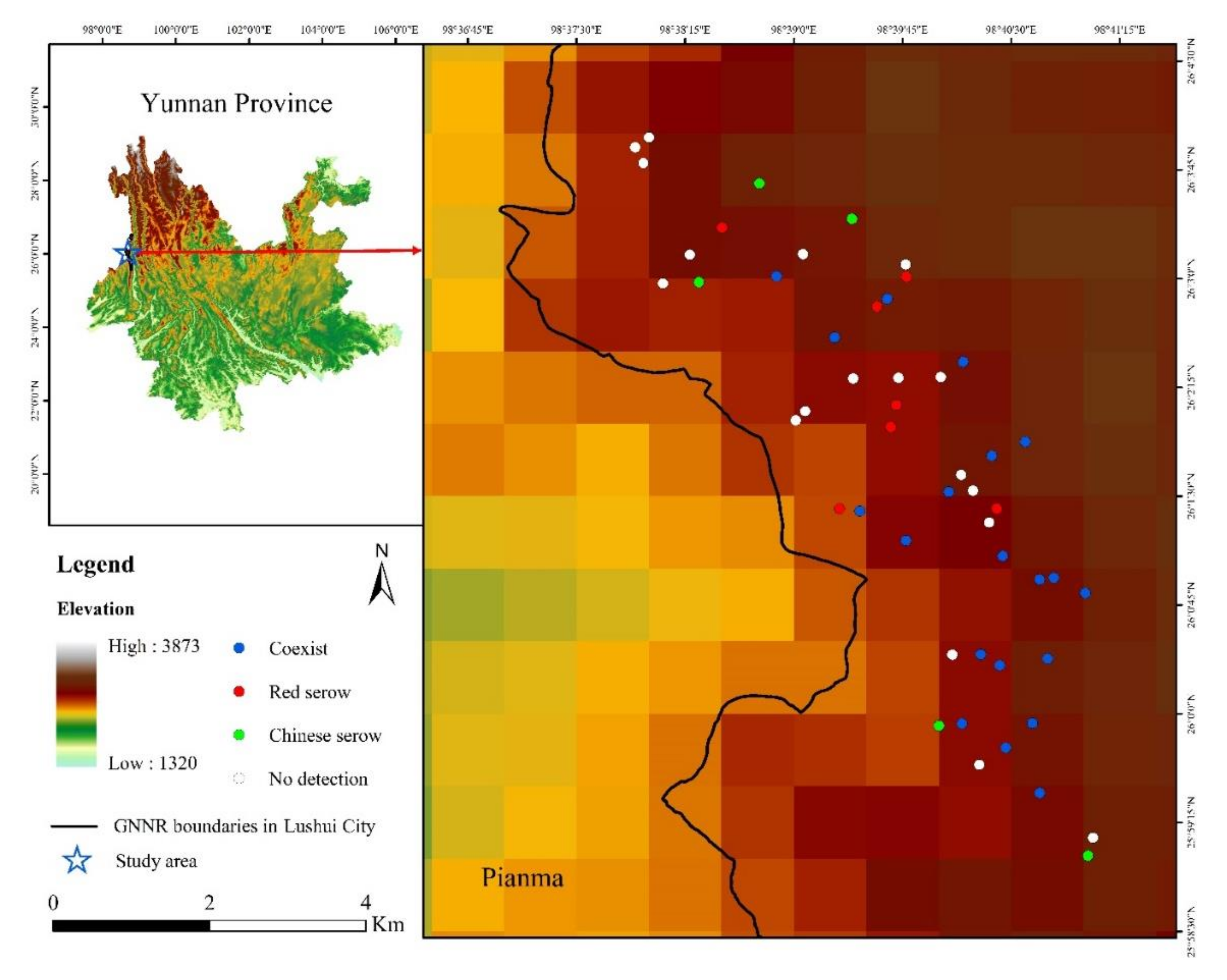
2.1. Study Site
2.2. Infrared Camera-Trapping
2.3. Data Analysis
2.3.1. Identification of Serows
2.3.2. Independent Detections and Behaviors
2.3.3. Circular Kernel Density Models
3. Results
3.1. Survey Results
3.2. Overall Daily Activity Rhythm
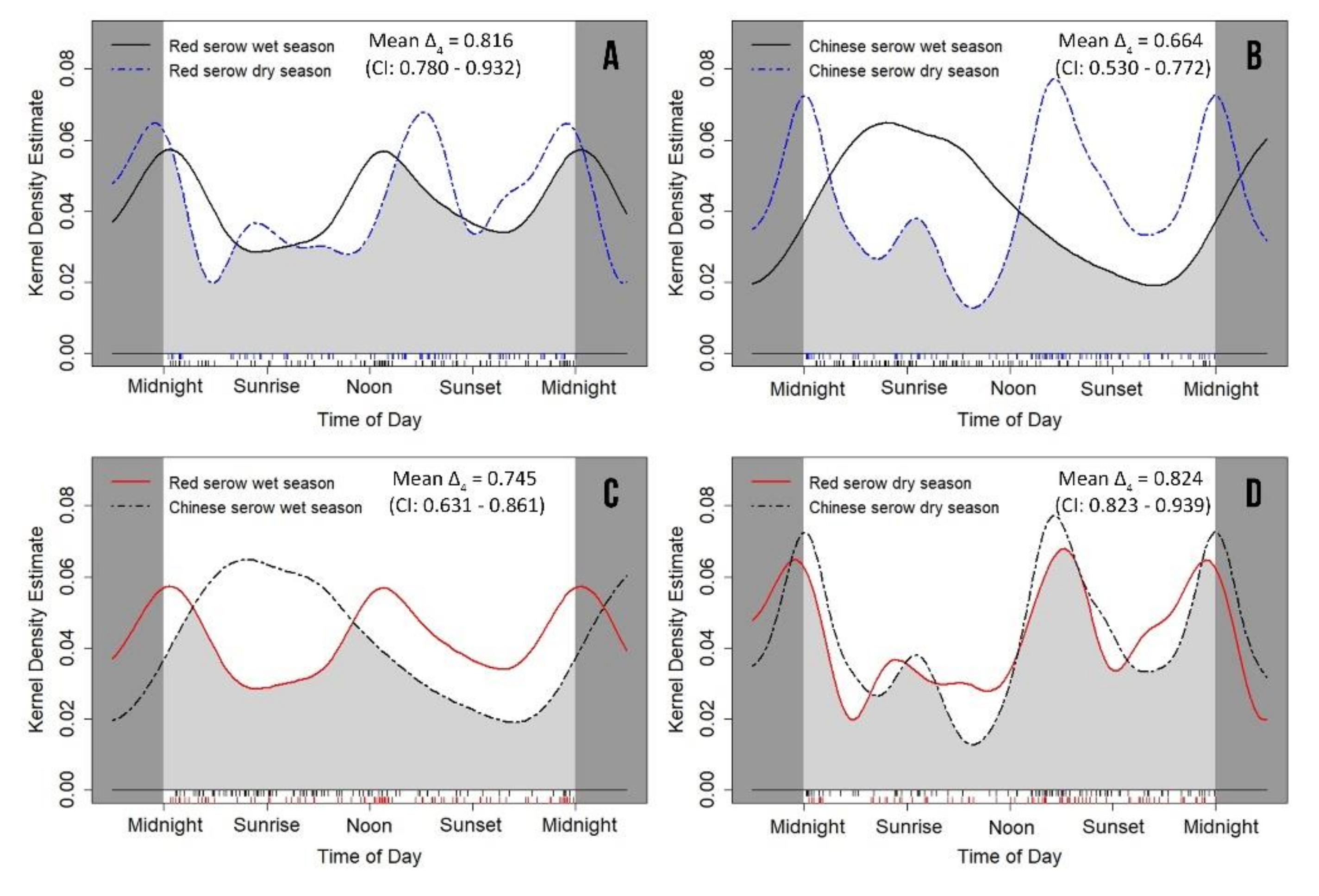
3.3. Seasonal Variation of Daily Activity Rhythms
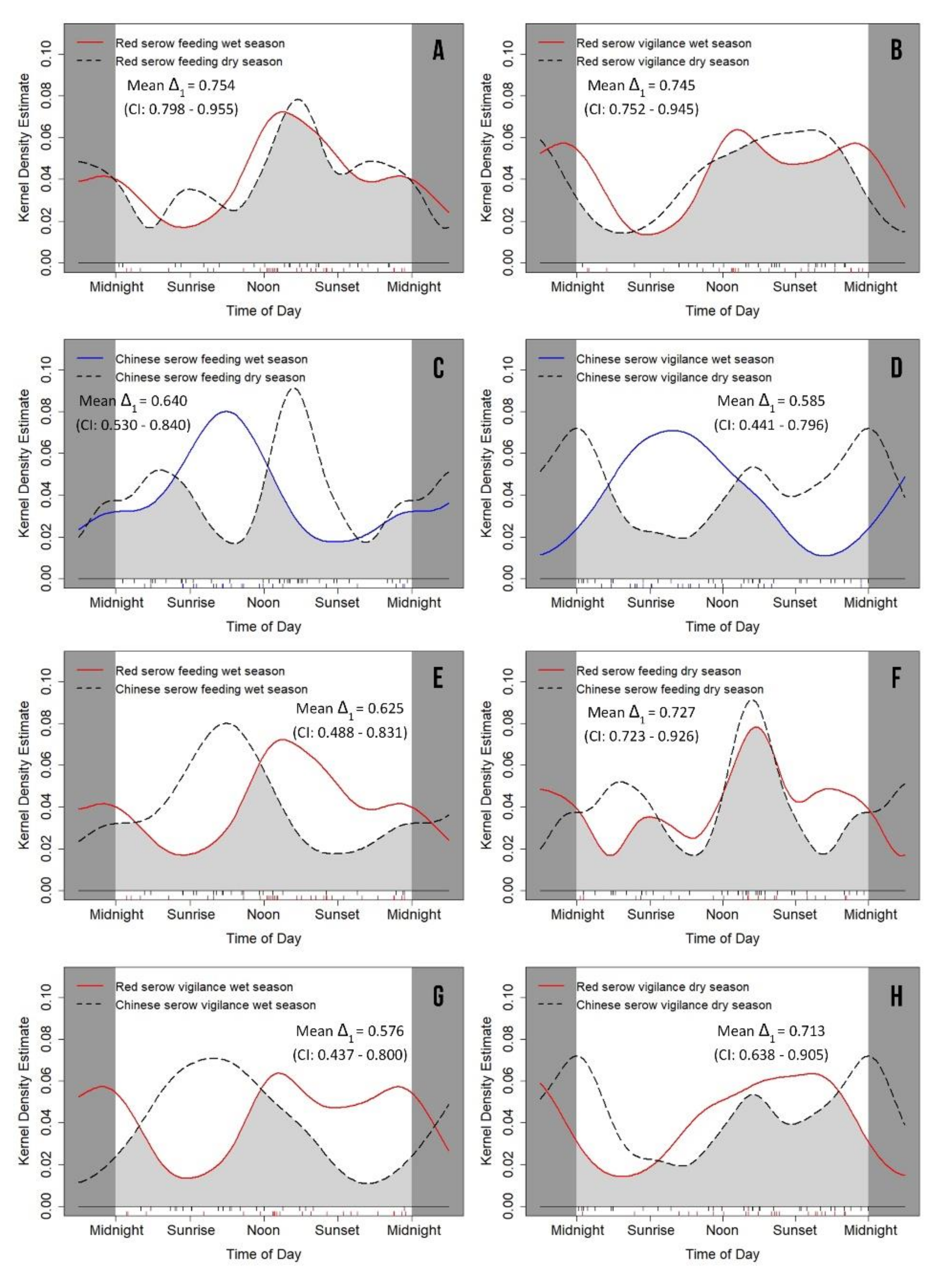
3.4. Seasonal Variation of Feeding and Vigilance Behaviors
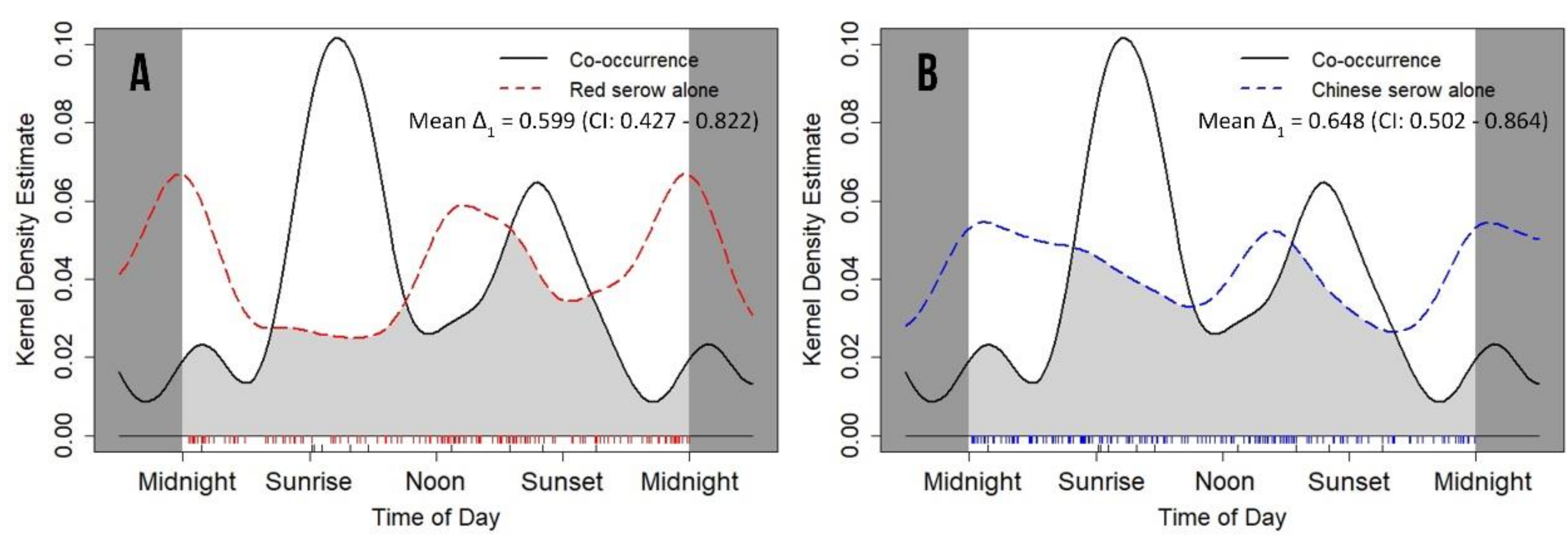
3.5. Coexistence and Associated Daily Activity Rhythms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Frey, S.; Fisher, J.T.; Burton, A.C.; Volpe, J.P. Investigating animal activity patterns and temporal niche partitioning using camera-trap data: Challenges and opportunities. Remote Sens. Ecol. Conserv. 2017, 3, 123–132. [Google Scholar] [CrossRef]
- Liu, Z.S.; Wang, X.M.; Li, Z.G.; Cui, D.Y.; Li, X.Q. Seasonal variation of diurnal activity budgets by blue sheep (Pseudois nayaur) with different age-sex classes in Helan Mountain. Zool. Res. 2005, 26, 350–357, (In Chinese with an English Abstract). [Google Scholar]
- Zhou, Q.H.; Wei, H.; Tang, H.X.; Huang, Z.H.; Krzton, A.; Huang, C.M. Niche separation of sympatric macaques, Macaca assamensis and M. mulatta, in limestone habitats of Nonggang, China. Primates 2014, 55, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.F.; Ai, H.S.; Fei, H.L.; Zhang, D.; Yuan, S.D. Seasonal variation of diet and time budget of Eastern hoolock gibbons (Hoolock leuconedys) living in a northern montane forest. Primates 2013, 54, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Wu, P.F.; Songer, M.; Cai, Q.; He, X.B.; Zhu, Y.; Shao, X.M. Monitoring wildlife abundance and diversity with infra-red camera traps in Guanyinshan Nature Reserve of Shaanxi Province, China. Ecol. Indic. 2013, 33, 121–128. [Google Scholar] [CrossRef]
- Hofmann, G.S.; Coelho, I.P.; Bastazini, V.A.G.; Cordeiro, J.L.P.; de Oliveira, L.F.B. Implications of climatic seasonality on activity patterns and resource use by sympatric peccaries in northern Pantanal. Int. J. Biometeorol. 2016, 60, 421–433. [Google Scholar] [CrossRef]
- Ensing, E.P.; Ciuti, S.; de Wijs, F.A.L.M.; Lentferink, D.H.; ten Hoedt, A.; Boyce, M.S.; Hut, R.A. GPS based daily activity patterns in European red deer and north American elk (Cervus elaphus): Indication for a weak circadian clock in ungulates. PLoS ONE 2014, 9, e106997. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Powell, G. Habitat use, activity patterns and use of mineral licks by five species of ungulate in south-eastern Peru. J. Trop. Ecol. 2009, 25, 261–270. [Google Scholar] [CrossRef]
- Šprem, N.; Zanella, D.; Ugarković, D.; Prebanić, I.; Gančević, P.; Corlatti, L. Unimodal activity pattern in forest-dwelling chamois: Typical behaviour or interspecific avoidance? Eur. J. Wildl. Res. 2015, 61, 789–794. [Google Scholar] [CrossRef]
- Ferreguetti, Á.C.; Tomás, W.M.; Bergallo, H.G. Density, occupancy, and activity pattern of two sympatric deer (Mazama) in the Atlantic Forest, Brazil. J. Mammal. 2015, 96, 1245–1254. [Google Scholar] [CrossRef]
- Gerber, B.D.; Karpanty, S.M.; Randrianantenaina, J. Activity patterns of carnivores in the rain forests of Madagascar: Implications for species coexistence. J. Mammal. 2012, 93, 667–676. [Google Scholar] [CrossRef]
- Macarthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Abrams, P. The theory of limiting similarity. Annu. Rev. Ecol. Syst. 1983, 14, 359–376. [Google Scholar] [CrossRef]
- Hardin, G. The competitive exclusion principle. Science 1960, 131, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Belovsky, G.E.; Slade, J.B. Time budgets of grassland herbivores: Body size similarities. Oecologia 1986, 70, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Li, S.; Mcshea, W.J.; Wang, D.J.; Shao, L.K.; Shi, X.G. The use of infrared-triggered cameras for surveying phasianids in Sichuan Province, China. Ibis 2010, 152, 299–309. [Google Scholar] [CrossRef]
- Meek, P.D.; Zewe, F.; Falzon, G. Temporal activity patterns of the swamp rat (Rattus lutreolus) and other rodents in north-eastern New South Wales, Australia. Aust. Mammal. 2012, 34, 223–233. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Bashir, T.; Poudyal, K.; Sathyakumar, S.; Saha, G.K. Distribution, occupancy and activity patterns of goral (Nemorhaedus goral) and serow (Capricornis thar) in Khangchendzonga Biosphere Reserve, Sikkim, India. Mammal Study 2012, 37, 173–181. [Google Scholar] [CrossRef]
- Li, X.Y.; Bleisch, W.V.; Jiang, X.L. Unveiling a wildlife haven: Occupancy and activity patterns of mammals at a Tibetan sacred mountain. Eur. J. Wildl. Res. 2018, 64, 53. [Google Scholar] [CrossRef]
- Galetti, M.; Camargo, H.; Siqueira, T.; Keuroghlian, A.; Donatti, C.I.; Jorge, M.L.S.P.; Pedrosa, F.; Kanda, C.Z.; Ribeiro, M.C. Diet overlap and foraging activity between feral pigs and native peccaries in the Pantanal. PLoS ONE 2015, 10, e0141459. [Google Scholar] [CrossRef] [PubMed]
- Darmon, G.; Bourgoin, G.; Marchand, P.; Garel, M.; Dubray, D.; Jullien, J.; Loison, A. Do ecologically close species shift their daily activities when in sympatry? A test on chamois in the presence of mouflon. Biol. J. Linn. Soc. 2014, 111, 621–626. [Google Scholar] [CrossRef]
- Ferretti, F.; Sforzi, A.; Lovari, S. Behavioural interference between ungulate species: Roe are not on velvet with fallow deer. Behav. Ecol. Sociobiol. 2011, 65, 875–887. [Google Scholar] [CrossRef]
- Valeix, M.; Chamaillé-Jammes, S.; Fritz, H. Interference competition and temporal niche shifts: Elephants and herbivore communities at waterholes. Oecologia 2007, 153, 739–748. [Google Scholar] [CrossRef]
- Grubb, P. Artiodactyla. In Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Wilson, D.E., Reeder, D.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 637–722. [Google Scholar]
- Groves, C.P.; Grubb, P. Capricornis Ogilby, 1837. In Ungulate Taxonomy; Groves, C.P., Grubb, P., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2011; pp. 255–261. [Google Scholar]
- Groves, C.P.; Leslie, D.M., Jr. Family Bovidae (Hollow-horned ruminants). In Handbook of the Mammals of the World Vol. 2 Hoofed Mammals; Wilson, D.E., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2011; pp. 444–779. [Google Scholar]
- Castelló, J.R. Tribe caprini: Sheep, goats, and relatives. In Bovids of the World: Antelopes, Gazelles, Cattle, Goats, Sheep, and Relatives; Castelló, J.R., Huffman, B., Groves, C.P., Eds.; Princeton University Press: Princeton, NJ, USA, 2016; pp. 302–465. [Google Scholar]
- Mori, E.; Nerva, L.; Lovari, S. Reclassification of the serows and gorals: The end of a neverending story? Mammal Rev. 2019, 49, 256–262. [Google Scholar] [CrossRef]
- Duckworth, J.W.; Than, Z. Capricornis rubidus. The IUCN Red List of Threatened Species 2008: e.T3815A10102774. Available online: https://www.iucnredlist.org/species/3815/10102774 (accessed on 6 June 2019).
- IUCN. The IUCN Red List of Threatened Species Version 2019-2. Available online: http://www.iucnredlist.org/ (accessed on 6 June 2019).
- Smith, A.; Xie, Y. Genus Capricornis. In A Guide to the Mammals of China; Smith, A.T., Xie, Y., Hoffmann, R.S., Lunde, D., MacKinnon, J., Wilson, D.E., Gemma, F., Wang, S., Eds.; Hunan Education Publishing House: Changsha, China, 2009; pp. 493–495, (Chinese Version). [Google Scholar]
- Sun, J.X.; Li, J.Q.; Wang, Y.Q.; Li, S.; Guan, T.P.; Wang, J.; Xia, W.C.; Xu, H.G. Study on the activity rhythms of nine ungulates in summer and autumn in Sichuan. J. Ecol. Rural Env. 2018, 34, 1003–1009, (In Chinese with an English Abstract). [Google Scholar]
- Chen, W.; Hu, J.C.; Lu, X. Habitat use and separation between the Chinese serow (Capricornis milneedwardsi) and the Chinese goral (Naemorhedus griseus) in winter. Mammalia 2009, 73, 249–252. [Google Scholar] [CrossRef]
- Song, Y.L.; Gong, H.S.; Zeng, Z.G.; Wang, X.Z.; Zhu, L.; Zhao, N.X. Food habits of serow. Chin. J. Zool. 2005, 40, 50–56, (In Chinese with an English Abstract). [Google Scholar]
- Paudel, P.K.; Kindlmann, P. Distribution pattern of the threatened Himalayan serow (Capricornis thar) in western midhills of Nepal: An insight for conservation along an altitudinal gradient. J. Nat. Conserv. 2012, 20, 177–180. [Google Scholar] [CrossRef]
- Aryal, A. Habitat ecology of Himalayan serow (Capricornis sumatraensis ssp. thar) in Annapurna Conservation Area of Nepal. Tigerpaper 2009, 36, 12–20. [Google Scholar]
- Maita, K. Radio tracking of Japanese serow in Japan. In The Biology and Management of Capricornis and Related Mountain Antelopes; Soma, H., Ed.; Croom Helm: New York, NY, USA, 1987; pp. 119–124. [Google Scholar]
- Ochiai, K. Territorial behavior of the Japanese serow in Kusoudomari, Wakinosawa Village. J. Mammal Soc. Jpn. 1983, 9, 253–259, (In Japanese with an English Abstract). [Google Scholar]
- Kishimoto, R.; Kawamichi, T. Territoriality and monogamous pairs in a solitary ungulate, the Japanese serow, Capricornis crispus. Anim. Behav. 1996, 52, 673–682. [Google Scholar] [CrossRef]
- Lue, K.Y. A preliminary study on the ecology of Formosan serow Capricornis crispus swinhoei. In The Biology and Management of Capricornis and Related Mountain Antelopes; Soma, H., Ed.; Croom Helm: New York, NY, USA, 1987; pp. 125–133. [Google Scholar]
- Choudhury, A.U. Status of serow (Capricornis sumatraensis) in Assam. Tigerpaper 2003, 30, 1–2. [Google Scholar]
- Zhao, P.R. Endangered red serows have been found in southwest China’s Yunnan Province China Xinhua News, 2017–12–06, Xinhua News Agency. Available online: http://www.facebook.com/XinhuaNewsAgency/videos/2233497140011051/ (accessed on 6 June 2019).
- Li, F.; Huang, X.Y.; Zhang, X.C.; Zhao, X.X.; Yang, J.H.; Chan, B.P.L. Mammals of Tengchong Section of Gaoligongshan National Nature Reserve in Yunnan Province, China. J. Threat. Taxa 2019, 11, 14402–14414. [Google Scholar] [CrossRef]
- Xue, J.R. Chapter IV: Resources of wild animals. In Gaoligong Mountain National Nature Reserve; Xue, J.R., Tang, J.S., Xu, Z.H., Yang, Y.M., Chen, Y.S., Wang, J.H., Eds.; China Forestry Publishing House: Beijing, China, 1995; pp. 277–379, (In Chinese Text). [Google Scholar]
- Chen, Y.X.; Xiao, Z.S.; Li, M.; Wang, X.W.; He, C.X.; He, G.P.; Li, H.S.; Shi, S.J.; Xiang, Z.F. Preliminary survey for the biodiversity of mammal and bird using camera traps in the west of mid-section Mt. Gaoligong. Acta Theriol. Sin. 2016, 36, 302–312, (In Chinese with an English Abstract). [Google Scholar]
- Dou, H.L.; Zhang, Y.S.; Feng, L.M. Complete mitochondrial genome of the Himalayan serow (Capricornis thar) and its phylogenetic status within the genus Capricornis. Biochem. Syst. Ecol. 2016, 65, 115–123. [Google Scholar] [CrossRef]
- Chen, Y.X.; Xiang, Z.F.; Wang, X.W.; Xiao, W.; Xiao, Z.S.; Ren, B.P.; He, C.X.; Sang, C.H.; Li, H.S.; Li, M. Preliminary study of a newly discovered primate species Rhinopithecus strykeri at Pianma, Yunnan, China using infra-red camera traps. Int. J. Primatol. 2015, 36, 679–690. [Google Scholar] [CrossRef]
- Chen, Y.X. Preliminary study of behavior ecology of Burmese snub-nosed monkey (Rhinopithecus strykeri). Master’s Thesis, Science Central South University of Forestry and Technology, Changsha, China, May 2015. (In Chinese with an English Abstract). [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Lan, D.Y.; Dunbar, R. Bird and mammal conservation in Gaoligongshan Region and Jingdong County, Yunnan, China: Patterns of species richness and nature reserves. Oryx 2000, 34, 275–286. [Google Scholar] [CrossRef]
- Chaplin, G. Physical geography of the Gaoligong Shan area of southwest China in relation to biodiversity. Proc. Calif. Acad. Sci. 2005, 56, 527–556. [Google Scholar]
- Xue, J.R. Chapter I: Summary of natural and economic status. In Gaoligong Mountain National Nature Reserve; Xue, J.R., Tang, J.S., Xu, Z.H., Yang, Y.M., Chen, Y.S., Wang, J.H., Eds.; China Forestry Publishing House: Beijing, China, 1995; pp. 1–55, (In Chinese Text). [Google Scholar]
- Xue, J.R. Chapter II: Vegetation in the reserve. In Gaoligong Mountain National Nature Reserve; Xue, J.R., Tang, J.S., Xu, Z.H., Yang, Y.M., Chen, Y.S., Wang, J.H., Eds.; China Forestry Publishing House: Beijing, China, 1995; pp. 59–126, (In Chinese Text). [Google Scholar]
- Yang, Y.; Ren, G.P.; Li, W.J.; Huang, Z.P.; Anug, K.L.; Garber, P.A.; Ma, C.; Yi, S.L.; Momberg, F.; Gao, Y.; et al. Identifying transboundary conservation priorities in a biodiversity hotspot of China and Myanmar: Implications for data poor mountainous regions. Glob. Ecol. Conserv. 2019, 20, e00732. [Google Scholar] [CrossRef]
- Sino BON-Mammals. Sino Biodiversity Online Network-Mammals. Available online: http://www.nsii.org.cn/chinabiodiv (accessed on 25 June 2019).
- CameraData Team for Wildlife Diversity Monitoring. CameraData Network of Wildlife Diversity Monitoring: An Online Database. Available online: http://cameradata.ioz.ac.cn/sampleloc/list.shtml#Wzg4LFswLDIwLDEsMF0sW10sW10sOTld (accessed on 25 August 2019).
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. Forum 2003, 6, 131–139. [Google Scholar] [CrossRef]
- Wang, C.Y.; Hu, X.B. Serow behavior observation. Chin. J. Wild 2012, 33, 5–7, (In Chinese with an English Abstract). [Google Scholar]
- Takada, H.; Nakamura, K.; Takatsuki, S.; Minami, M. Freezing behavior of the Japanese serow (Capricornis crispus) in relation to habitat and group size. Mammal Res. 2018, 63, 107–112. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Bateson, P. Chapter 5: Recording methods. In Measuring Behaviour: An Introductory Guide, 3rd ed.; Martin, P., Bateson, P., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 48–61. [Google Scholar]
- Li, Z.Q.; Jiang, Z.G.; Beauchamp, G. Vigilance in Przewalski’s gazelle: Effects of sex, predation risk and group size. J. Zool. 2009, 277, 302–308. [Google Scholar] [CrossRef]
- Hunter, L.T.B.; Skinner, J.D. Vigilance behaviour in African ungulates: The role of predation pressure. Behaviour 1998, 135, 195–211. [Google Scholar]
- Meek, P.D.; Ballard, G.; Fleming, P.J.S.; Schaefer, M.; Williams, W.; Falzon, G. Camera traps can be heard and seen by animals. PLoS ONE 2014, 9, e110832. [Google Scholar] [CrossRef]
- Meek, P.; Ballard, G.; Fleming, P.; Falzon, G. Are we getting the full picture? Animal responses to camera traps and implications for predator studies. Ecol. Evol. 2016, 6, 3216–3225. [Google Scholar] [CrossRef]
- Tan, C.L.; Yang, Y.Q.; Niu, K.F. Into the night: Camera traps reveal nocturnal activity in a presumptive diurnal primate, Rhinopithecus brelichi. Primates 2013, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Oliveira-Santos, L.G.R.; Zucco, C.A.; Agostinelli, A. Using conditional circular kernel density functions to test hypotheses on animal circadian activity. Anim. Behav. 2013, 85, 269–280. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Kays, R.; Kranstauber, B.; Carbone, C.; Jansen, P.A. Quantifying levels of animal activity using camera trap data. Methods Ecol. Evol. 2014, 5, 1170–1179. [Google Scholar] [CrossRef]
- Rowcliffe, J.M. Package ‘Activity’, Version 1.1. Available online: https://cran.r-project.org/web/packages/activity/index.html (accessed on 10 June 2019).
- Meredith, M.; Ridout, M. Overview of the overlap package, Version 0.3.2. Available online: https://cran.r-project.org/web/packages/overlap/index.html (accessed on 10 June 2019).
- R Development Core Team. R: A Language and Environment for Statistical Computing, Version 3.6.1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Nouvellet, P.; Rasmussen, G.S.A.; Macdonald, D.W.; Courchamp, F. Noisy clocks and silent sunrises: Measurement methods of daily activity pattern. J. Zool. 2012, 286, 179–184. [Google Scholar] [CrossRef]
- Weitzman, M.S. Measure of the overlap of income distribution of white and negro families in the United States; Technical report No.22. Bureau of the Census, U.S. Department of Commerce: Washington DC, USA, 1970. [Google Scholar]
- Wu, P.J.; Zhang, N.D. Habitat selection and its seasonal change of serow (Capricornis sumatraensis) in Cibagou Nature Reserve, Tibet. Acta Theriol. Sin. 2004, 24, 6–12, (In Chinese with an English Abstract). [Google Scholar]
- Bai, D.F.; Chen, P.J.; Atzeni, L.; Cering, L.; Li, Q.; Shi, K. Assessment of habitat suitability of the snow leopard (Panthera uncia) in Qomolangma National Nature Reserve based on MaxEnt modeling. Zool. Res 2018, 39, 373–386. [Google Scholar]
- Kobayashi, K.; Takatsuki, S. A comparison of food habits of two sympatric ruminants of Mt. Yatsugatake, central Japan: Sika deer and Japanese serow. Acta Theriol. 2012, 57, 343–349. [Google Scholar] [CrossRef]
- Treydte, A.C.; Trumpf, P.; Langenberger, G.; Yang, Y.; Liu, F. Wild ungulate distribution in the Naban River Watershed National Nature Reserve, southwest China. J. Trop. For. Env. 2013, 2, 53–65. [Google Scholar] [CrossRef]


| Trapping Periods | Camera-Trapping Sites | Trapping Days | Elevation (m) |
|---|---|---|---|
| November 2013 to November 2014 | 29 | 4298 | 2570–3240 |
| November 2014 to October 2015 | 24 | 5039 | 2570–3425 |
| October 2015 to October 2016 | 20 | 4053 | 2620–3425 |
| November 2016 to November 2017 | 32 | 9827 | 2570–3447 |
| November 2017 to January 2019 | 26 | 8025 | 2620–3447 |
| Total camera-trapping sites: 50 | Total trapping days: 31,242 | ||
| Trait | Red Serow (Capricornis Rubidus) | Chinese Serow (C. Milneedwardsii Milneedwardsii) |
|---|---|---|
| Overall coloration | Reddish-brown | Black, dark brown |
| Overall coloration in monochrome images | Light grey | Black, dark grey |
| Hair | Slick, very short | Coarse rather thin |
| Mane | Very short, dark red | Medium, mixed black to pale yellow |
| Jaw streak | White | White to golden-brown |
| Throat patch | Large and continuous, white | Usually discrete, white |
| Upper half of legs | Reddish-brown | Black |
| Lower half of legs | Buffy red | Reddish tan, creamy white |
| Category | Red Serow (Capricornis Rubidus) | Chinese Serow (C. Milneedwardsii Milneedwardsii) | Uncertain Serow (Capricornis Spp.) |
|---|---|---|---|
| Number of photos | 956 | 1125 | 106 |
| Number of videos | 263 | 225 | 36 |
| Camera-trapping sites | 27 | 25 | 3 |
| Independent detections | 157 | 179 | 7 |
| Detections in wet season | 82 | 86 | 0 |
| Detections in dry season | 75 | 93 | 7 |
| Feeding in wet season | 31 | 23 | 0 |
| Feeding in dry season | 20 | 35 | 0 |
| Vigilance in wet season | 22 | 21 | 0 |
| Vigilance in dry season | 23 | 31 | 0 |
| Co-occurring detections | 13 | 13 | 0 |
| Single-species detections | 144 | 166 | 0 |
| Category | Red Serow (Capricornis Rubidus) | Chinese Serow (C. Milneedwardsii Milneedwardsii) | R. Test | Wald Test | |||
|---|---|---|---|---|---|---|---|
| Estimate | CI | Estimate | CI | p | W | p | |
| Overall | 0.573 ± 0.074 | 0.449–0.737 | 0.696 ± 0.069 | 0.526–0.795 | 0.119 | 1.476 ± 0.101 | 0.224 |
| Wet season | 0.592 ± 0.082 | 0.342–0.666 | 0.605 ± 0.073 | 0.380–0.665 | 0.002 * | 0.014 ± 0.110 | 0.905 |
| Dry season | 0.538 ± 0.077 | 0.324–0.623 | 0.457 ± 0.070 | 0.287–0.560 | 0.678 | 0.618 ± 0.104 | 0.432 |
| Feeding (w) | 0.475 ± 0.094 | 0.223–0.588 | 0.449 ± 0.095 | 0.219–0.581 | 0.034 * | 0.039 ± 0.133 | 0.844 |
| Feeding (d) | 0.461 ± 0.101 | 0.208–0.598 | 0.380 ± 0.089 | 0.217–0.561 | 0.885 | 0.359 ± 0.135 | 0.549 |
| Vigilance (w) | 0.517 ± 0.105 | 0.203–0.602 | 0.530 ± 0.090 | 0.209–0.557 | 0.017 * | 0.009 ± 0.138 | 0.926 |
| Vigilance (d) | 0.563 ± 0.095 | 0.236–0.604 | 0.478 ± 0.108 | 0.234–0.645 | 0.380 | 0.350 ± 0.143 | 0.554 |
| Category | Red Serow (Capricornis Rubidus) | Chinese Serow (C. Milneedwardsii Milneedwardsii) | ||||
|---|---|---|---|---|---|---|
| R. Test | Wald Test | R. Test | Wald Test | |||
| p | W | p | p | W | p | |
| Wet season/Dry season | 0.382 | 0.222 ± 0.113 | 0.637 | <0.0001 * | 2.148 ± 0.101 | 0.143 |
| Feeding (w)/Feeding (d) | 0.894 | 0.011 ± 0.138 | 0.917 | 0.046 * | 0.283 ± 0.130 | 0.595 |
| Vigilance (w)/Vigilance (d) | 0.751 | 0.107 ± 0.141 | 0.744 | 0.014 * | 0.135 ± 0.141 | 0.713 |
| Category | Single-Species | Co-Occurring | R. Test | Wald Test | |||
|---|---|---|---|---|---|---|---|
| Estimate | CI | Estimate | CI | p | W | p | |
| Red serow (Capricornis rubidus) | 0.547 ± 0.073 | 0.431–0.715 | 0.383 ± 0.103 | 0.147–0.548 | 0.052 | 1.590 ± 0.127 | 0.207 |
| Chinese serow (C. milneedwardsii milneedwardsii) | 0.683 ± 0.071 | 0.517–0.795 | 0.149 | 5.689 ± 0.126 | 0.017 * | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xiao, Z.; Zhang, L.; Wang, X.; Li, M.; Xiang, Z. Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps. Animals 2019, 9, 1071. https://doi.org/10.3390/ani9121071
Chen Y, Xiao Z, Zhang L, Wang X, Li M, Xiang Z. Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps. Animals. 2019; 9(12):1071. https://doi.org/10.3390/ani9121071
Chicago/Turabian StyleChen, Yixin, Zhishu Xiao, Long Zhang, Xinwen Wang, Ming Li, and Zuofu Xiang. 2019. "Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps" Animals 9, no. 12: 1071. https://doi.org/10.3390/ani9121071
APA StyleChen, Y., Xiao, Z., Zhang, L., Wang, X., Li, M., & Xiang, Z. (2019). Activity Rhythms of Coexisting Red Serow and Chinese Serow at Mt. Gaoligong as Identified by Camera Traps. Animals, 9(12), 1071. https://doi.org/10.3390/ani9121071





