Identification of a Type-Specific Epitope in the ORF2 Protein of Duck Astrovirus Type 1
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Cells, and Antibodies
2.2. Reactivity Analysis of the mAb 3D2
2.3. Neutralization Assay for mAb 3D2 Against DAstV-1
2.4. Identification of the B-Cell Linear Epitope to 3D2
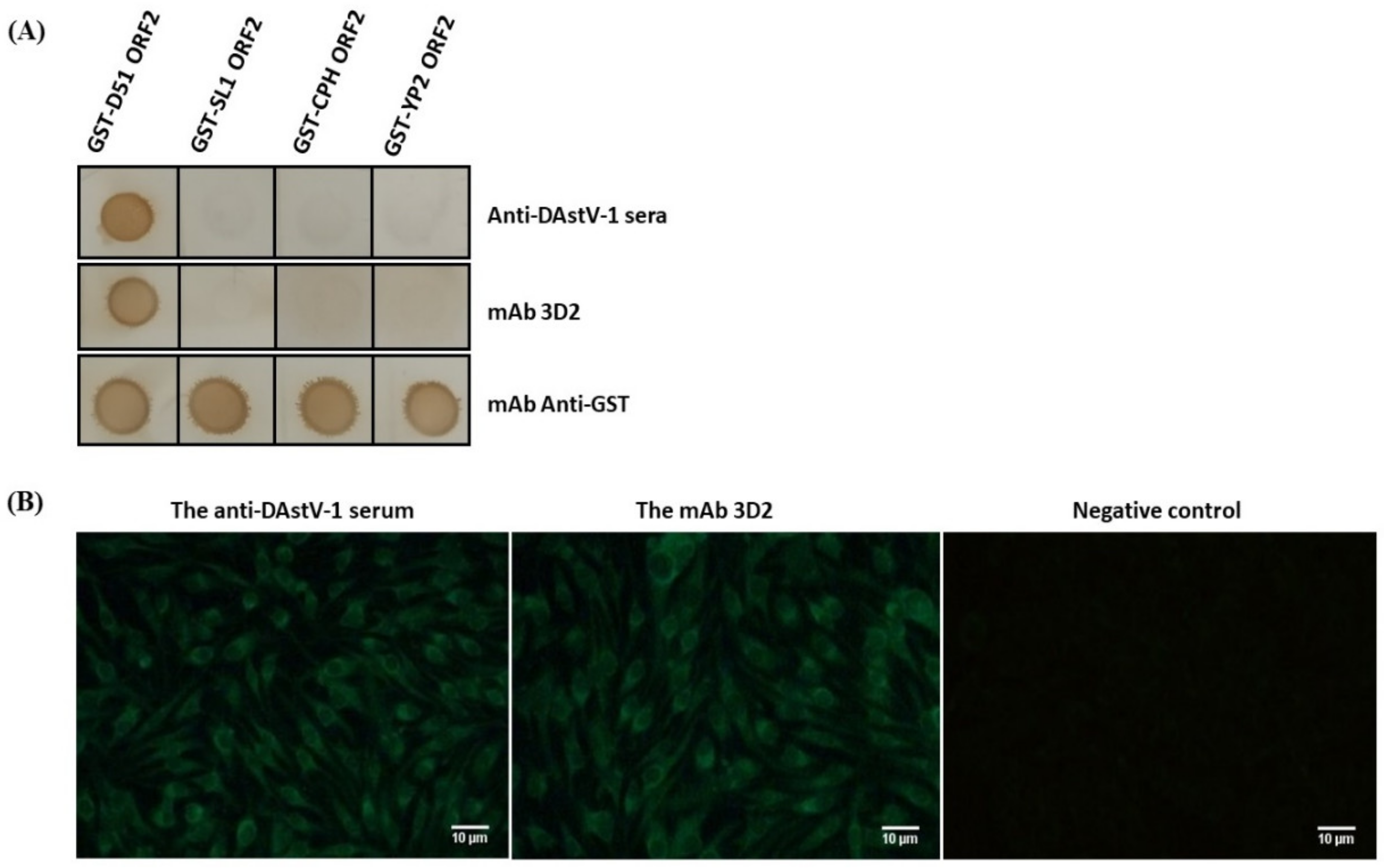
2.5. Cross-Reactivity of the mAb 3D2
3. Results
3.1. Reactivity Analysis of mAb 3D2
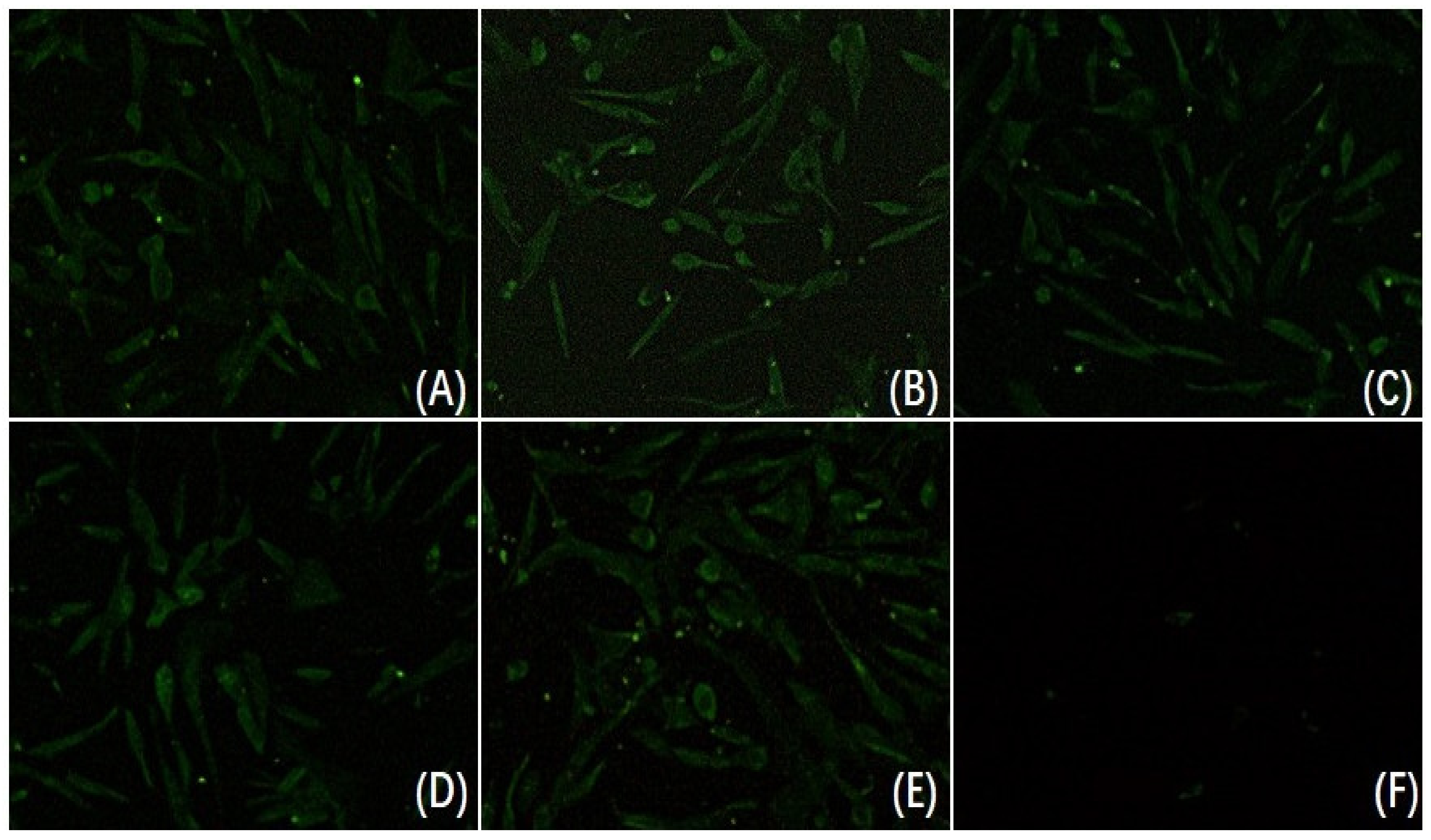
3.2. Neutralization Analysis
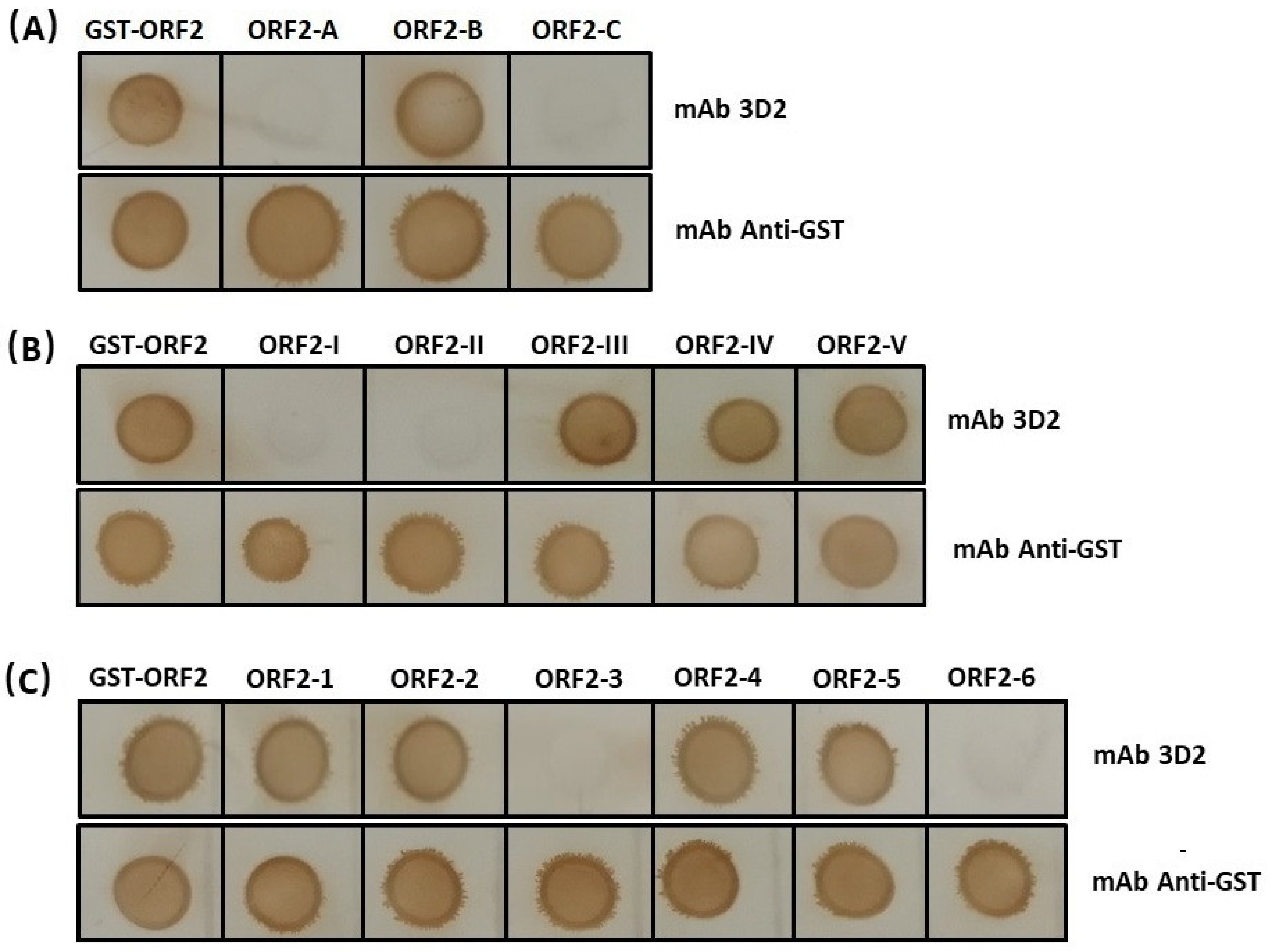
3.3. The Accurate Position of the Linear Epitope in DAstV-1 ORF2 to mAb 3D2
3.4. Epitope Mapping of the mAb 3D2
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Madeley, C.R.; Cosgrove, B.P. Letter: 28 nm particles in faeces in infantile gastroenteritis. Lancet 1975, 2, 451–452. [Google Scholar] [CrossRef]
- Walter, J.E.; Mitchell, D.K. Astrovirus infection in children. Curr. Opin. Infect. Dis. 2003, 16, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Poon, L.L.; Guan, Y.; Peiris, J.S. Novel astroviruses in insectivorous bats. J. Virol. 2008, 82, 9107–9114. [Google Scholar] [CrossRef] [PubMed]
- Coppo, P.; Scieux, C.; Ferchal, F.; Clauvel, J.; Lassoued, X. Astrovirus enteritis in a chronic lymphocytic leukemia patient treated with fludarabine monophosphate. Ann. Hematol. 2000, 79, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Guix, S.; Caballero, S.; Villena, C.; Bartolomé, R.; Latorre, C.; Rabella, N.; Simó, M.; Bosch, A.; Pintó, R.M. Molecular epidemiology of astrovirus infection in Barcelona, Spain. J. Clin. Microbiol. 2002, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Li, L.; Victoria, J.; Oderinde, B.; Mason, C.; Pandey, P.; Zaidi, S.Z.; Delwart, E. Multiple novel astrovirus species in human stool. J. Gen. Virol. 2009, 90, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Wagner, T.A.; Briese, T.; Torgerson, T.R.; Hornig, M.; Tashmukhamedova, A.; Firth, C.; Palacios, G.; Baisre-De-Leon, A.; Paddock, C.D.; et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 2010, 16, 918–925. [Google Scholar] [CrossRef]
- Schlottau, K.; Schulze, C.; Bilk, S.; Hanke, D.; Hoper, D.; Beer, M.; Hoffmann, B. Detection of a novel bovine astrovirus in a cow with encephalitis. Transbound. Emerg. Dis. 2016, 63, 253–259. [Google Scholar] [CrossRef]
- Seuberlich, T.; Wüthrich, D.; Selimovic-Hamza, S.; Drögemüller, C.; Oevermann, A.; Bruggmann, R.; Bouzalas, I. Identification of a second encephalitis-associated astrovirus in cattle. Emerg. Microbes Infect. 2016, 5, e71. [Google Scholar] [CrossRef]
- Koci, M.D.; Seal, B.S.; Schultz-Cherry, S. Molecular characterization of an avian astrovirus. J. Virol. 2000, 74, 6173–6177. [Google Scholar] [CrossRef]
- Yu, M.; Tang, Y.; Guo, M.; Zhang, Q.; Saif, Y.M. Characterization of a small round virus associated with the poult enteritis and mortality syndrome. Avian Dis. 2000, 44, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Imada, T.; Yamaguchi, S.; Mase, M.; Tsukamoto, K.; Kubo, M.; Morooka, A. Avian nephritis virus (ANV) as a new member of the family Astroviridae and construction of infectious ANV cDNA. J. Virol. 2000, 74, 8487–8493. [Google Scholar] [CrossRef] [PubMed]
- Gough, R.E.; Collins, M.S.; Borland, E.; Keymer, L.F. Astrovirus-like particles associated with hepatitis in ducklings. Vet. Rec. 1984, 114, 279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, Y.; Wang, J.; Fu, G.; Sun, M.; Zhang, L.; Meng, L.; Cui, G.; Huang, Y.; Hu, X.; et al. Isolation and characterization of an astrovirus causing fatal visceral gout in domestic goslings. Emerg. Microbes Infect. 2018, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.A.; Calnek, B.W. In vitro isolation, propagation, and characterization of duck hepatitis virus type III. Avian Dis. 1979, 23, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, F.; Shi, J.; Zheng, L.; Wang, X.; Zhang, D. Molecular characterization of a duck hepatitis virus 3-like astrovirus. Vet. Microbiol. 2014, 170, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Liu, N.; Wang, X.; Wang, F.; Zhang, D. Genetic characterization of a novel astrovirus in Pekin ducks. Infect. Genet. Evol. 2015, 32, 60–67. [Google Scholar] [CrossRef]
- Gough, R.E.; Borland, E.D.; Keymer, I.F.; Stuart, J.C. An outbreak of duck hepatitis type II in commercial ducks. Avian Pathol. 1985, 14, 227–236. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q.; Zhang, R.; Li, J.; Xie, Z.; Wang, Y.; Zhu, Y.; Jiang, S. Complete genome sequence of a duck astrovirus discovered in eastern China. J. Virol. 2012, 86, 13833–13834. [Google Scholar] [CrossRef]
- Matsui, S.M.; Greenberg, H.B. Astroviruses. In Fields Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 875–893. [Google Scholar]
- Méndez, E.; Muñoz-Yañez, C.; Sánchez-San, M.C.; Aguirre-Crespo, G.; del Rocio Baños-Lara, M.; Gutierrez, M.; Espinosa, R.; Acevedo, Y.; Arias, C.F.; López, S. Characterization of human astrovirus cell entry. J. Virol. 2014, 88, 2452–2460. [Google Scholar]
- Krishna, N.K.; Cunnion, K.M. Human astrovirus coat protein: A novel C1 inhibitor. Adv. Exp. Med. Biol. 2008, 632, 237–251. [Google Scholar] [PubMed]
- Bogdanoff, W.A.; Campos, J.; Perez, E.I.; Yin, L.; Alexander, D.L.; DuBois, R.M. Structure of a human astrovirus capsid-antibody complex and mechanistic insights into virus neutralization. J. Virol. 2017, 91, e01859-16. [Google Scholar] [CrossRef] [PubMed]
- De Nova-Ocampo, M.; Soliman, M.C.; Espinosa-Hernandez, W.; Velez-Del Valle, C.; Salas-Benito, J.; Valdes-Flores, J.; Garcia-Morales, L. Human astroviruses: In silico analysis of the untranslated region and putative binding sites of cellular proteins. Mol. Biol. Rep. 2019, 46, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Bullard, D.E.; Bigner, D.D. Applications of monoclonal antibodies in the diagnosis and treatment of primary brain tumors. J. Neurosurg. 1985, 63, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Koprowski, H. Plant biopharming of monoclonal antibodies. Virus Res. 2005, 111, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Huygen, K. Development of human monoclonal antibodies to diphtheria toxin: A solution for the increasing lack of equine DAT for therapeutic use? Virulence 2016, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lan, J.; Li, H.; Chen, J.; Yang, Y.; Lin, S.; Xie, Z.; Jiang, S. A novel method to rescue and culture duck astrovirus type 1 in Vitro. Virol. J. 2019, 16, 112. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, G.; Xin, Y.; Chen, J.; Lin, S.; Tian, Y.; Xie, Z.; Jiang, S. Identification of a conserved neutralizing linear B-cell epitope in the VP1 proteins of duck hepatitis A virus type 1 and 3. Vet. Microbiol. 2015, 180, 196–204. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Xie, X.; Zhang, B.; Zhang, D. Expression of the C-terminal ORF2 protein of duck astrovirus for application in a serological test. J. Virol. Methods 2011, 171, 8–12. [Google Scholar] [CrossRef]
- Chen, L.; Ma, M.; Zhang, R.; Xu, Q.; Si, X.; Wang, Y.; Xie, Z.; Jiang, S. Simultaneous detection of duck hepatitis A virus types 1 and 3, and of duck astrovirus type 1, by multiplex RT-PCR. Virol. Sin. 2014, 29, 196–198. [Google Scholar] [CrossRef]
- Fu, Y.; Pan, M.; Wang, X.; Xu, Y.; Xie, X.; Knowles, N.J.; Yang, H.; Zhang, D. Complete sequence of a duck astrovirus associated with fatal hepatitis in ducklings. J. Gen. Virol. 2009, 90, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.E.; Nowak, N.A.; Perron-Henry, D.M.; Hudson, R.W.; Cubitt, W.D.; Blacklow, N.R. Diagnosis of astrovirus gastroenteritis by antigen detection with monoclonal antibodies. J. Infect. Dis. 1990, 161, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, W.; Mebatsion, T. The isolation and characterisation of astroviruses from chickens. Avian Pathol. 2004, 33, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Strain, E.; Kelley, L.A.; Schultz-Cherry, S.; Muse, S.V.; Koci, M.D. Genomic analysis of closely related astroviruses. J. Virol. 2008, 82, 5099–5103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kakizawa, J.; Wen, L.Y.; Shimizu, M.; Nishio, O.; Fang, Z.Y.; Ushijima, H. Genetic analysis of the capsid region of astroviruses. J. Med. Virol. 2001, 64, 245–255. [Google Scholar] [CrossRef]
- Wei, J.C.; Huang, Y.Z.; Zhong, D.K.; Kang, L.; Ishag, H.; Mao, X.; Cao, R.B.; Zhou, B.; Chen, P.Y. Design and evaluation of a multi-epitope peptide against Japanese encephalitis virus infection in BALB/c mice. Biochem. Biophys. Res. Commun. 2010, 396, 787–792. [Google Scholar] [CrossRef]
- Sakarellos-Daitsiotis, M.; Krikorian, D.; Panou-Pomonis, E.; Sakarellos, C. Artificial carriers: A strategy for constructing antigenic/immunogenic conjugates. Curr. Top. Med. Chem. 2006, 6, 1715–1735. [Google Scholar] [CrossRef]
- Takaiwa, F. A rice-based edible vaccine expressing multiple T-cell epitopes to induce oral tolerance and inhibit allergy. Immunol. Allergy Clin. North. Am. 2007, 27, 129–139. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.N.; Wang, X.; Tang, J.N.; Lu, D.; Zhang, Y.F.; Guo, Z.C.; Li, Y.L.; Gao, R.; Kang, R.M. The protective immune response against infectious bronchitis virus induced by multi-epitope based peptide vaccines. Biosci. Biotechnol. Biochem. 2009, 73, 1500–1504. [Google Scholar] [CrossRef]
- Zeng, Y.; You, X.; Liu, L.; He, J.; Zhu, C.; Yu, M.; Ma, X.; Wu, Y. The immune effects of multiple antigen peptides containing the mimic epitopes of the adhesion protein of Mycoplasma genitalium. Can. J. Microbiol. 2013, 59, 479–484. [Google Scholar] [CrossRef]
- Xue, W.; Zhao, Q.; Li, P.; Zhang, R.; Lan, J.; Wang, J.; Yang, X.; Xie, Z.; Jiang, S. Identification and characterization of a novel nanobody against duck hepatitis A virus type 1. Virology 2019, 528, 101–109. [Google Scholar] [CrossRef] [PubMed]
- DuBois, R.M.; Freiden, P.; Marvin, S.; Reddivari, M.; Heath, R.J.; White, S.W.; Schultz-Cherry, S. Crystal structure of the avian astrovirus capsid spike. J. Virol. 2013, 87, 7853–7863. [Google Scholar] [CrossRef] [PubMed]
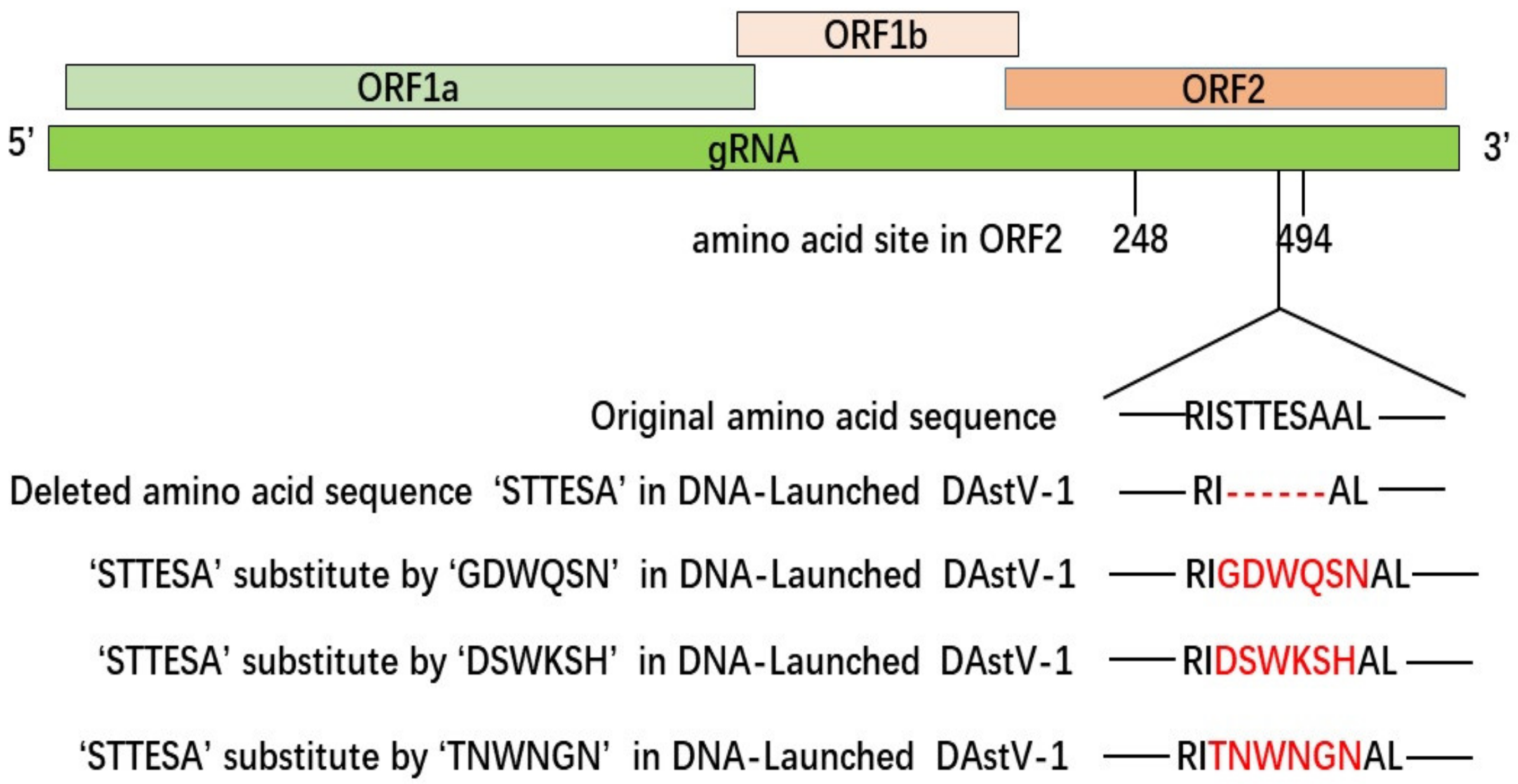
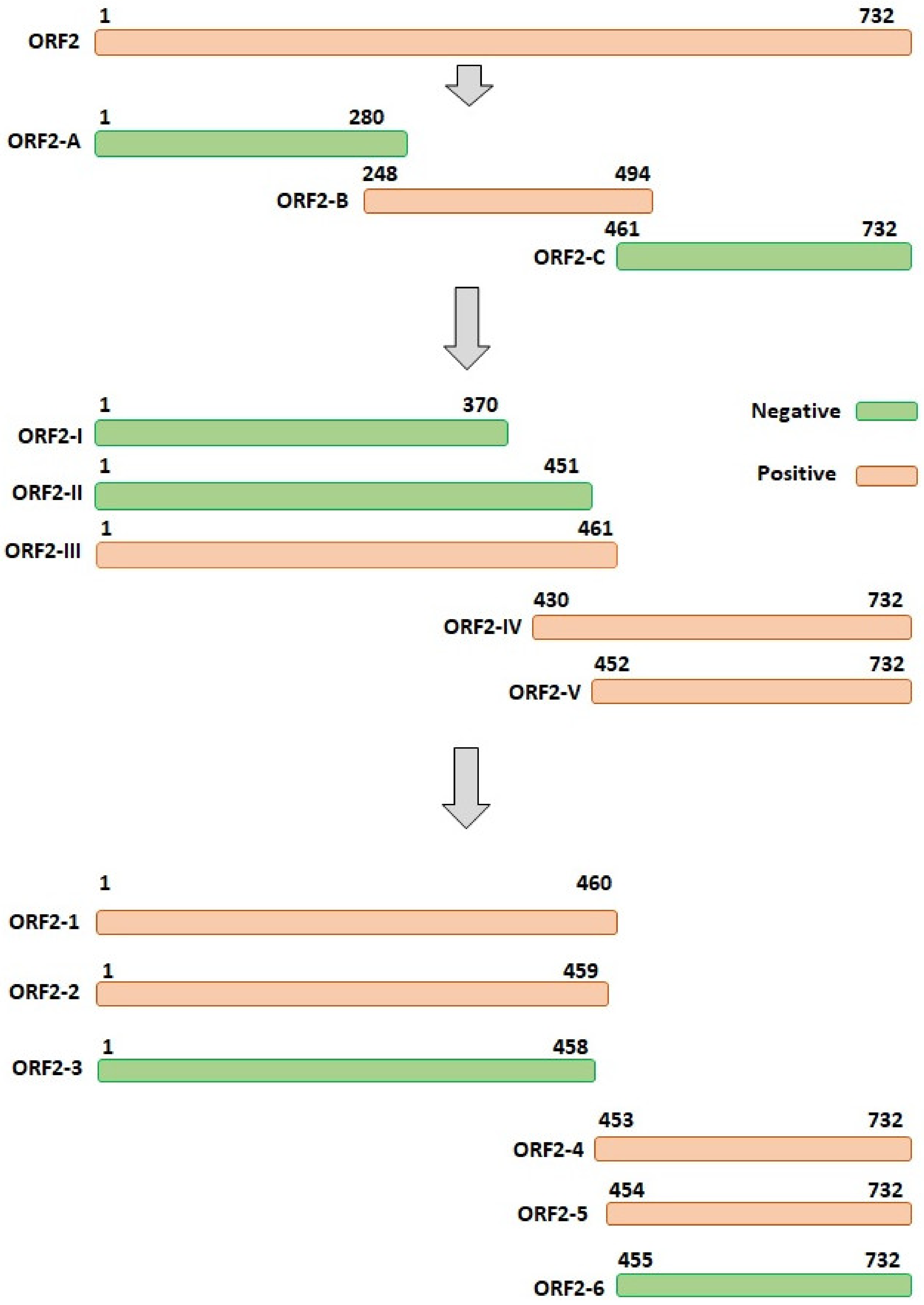
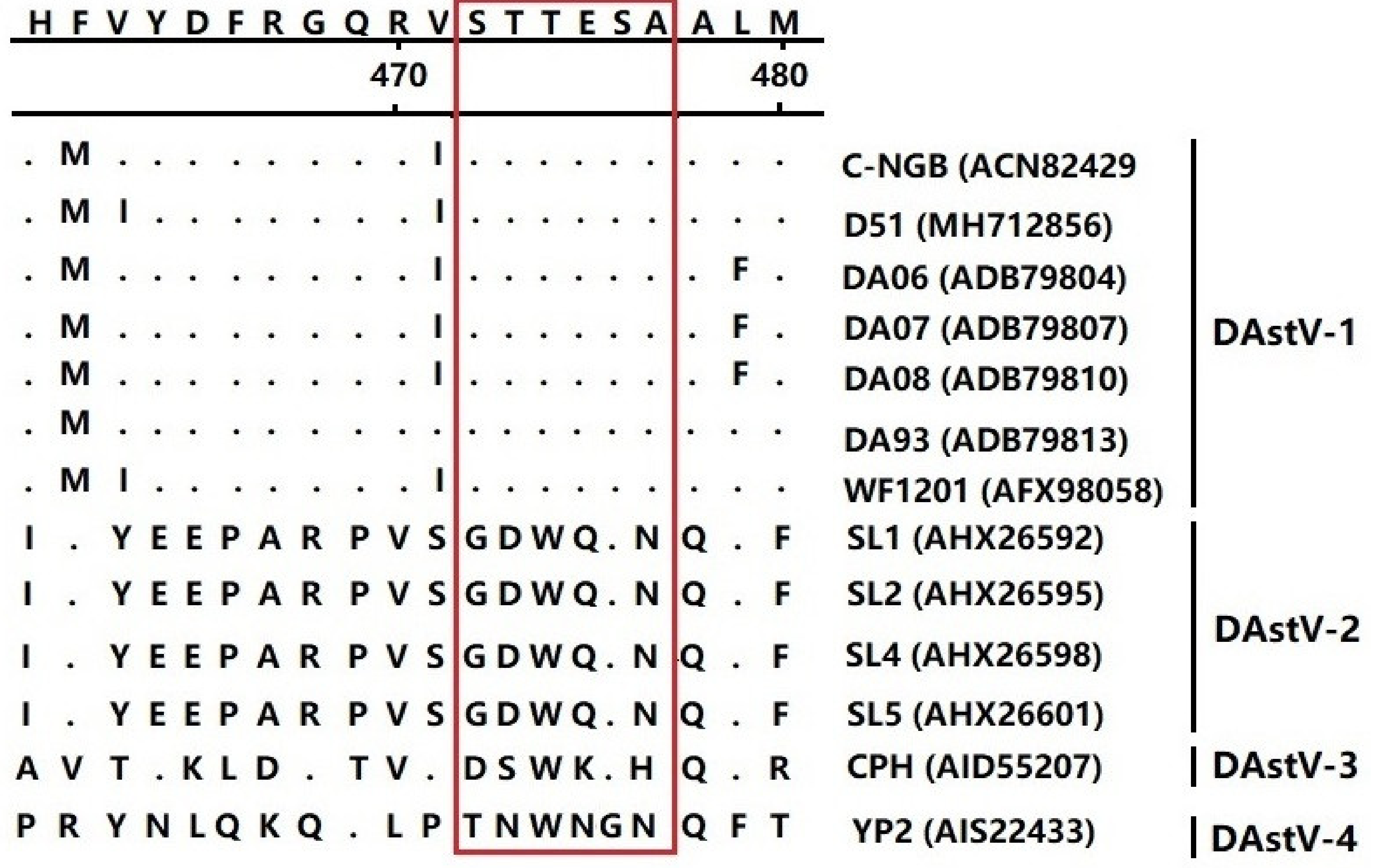
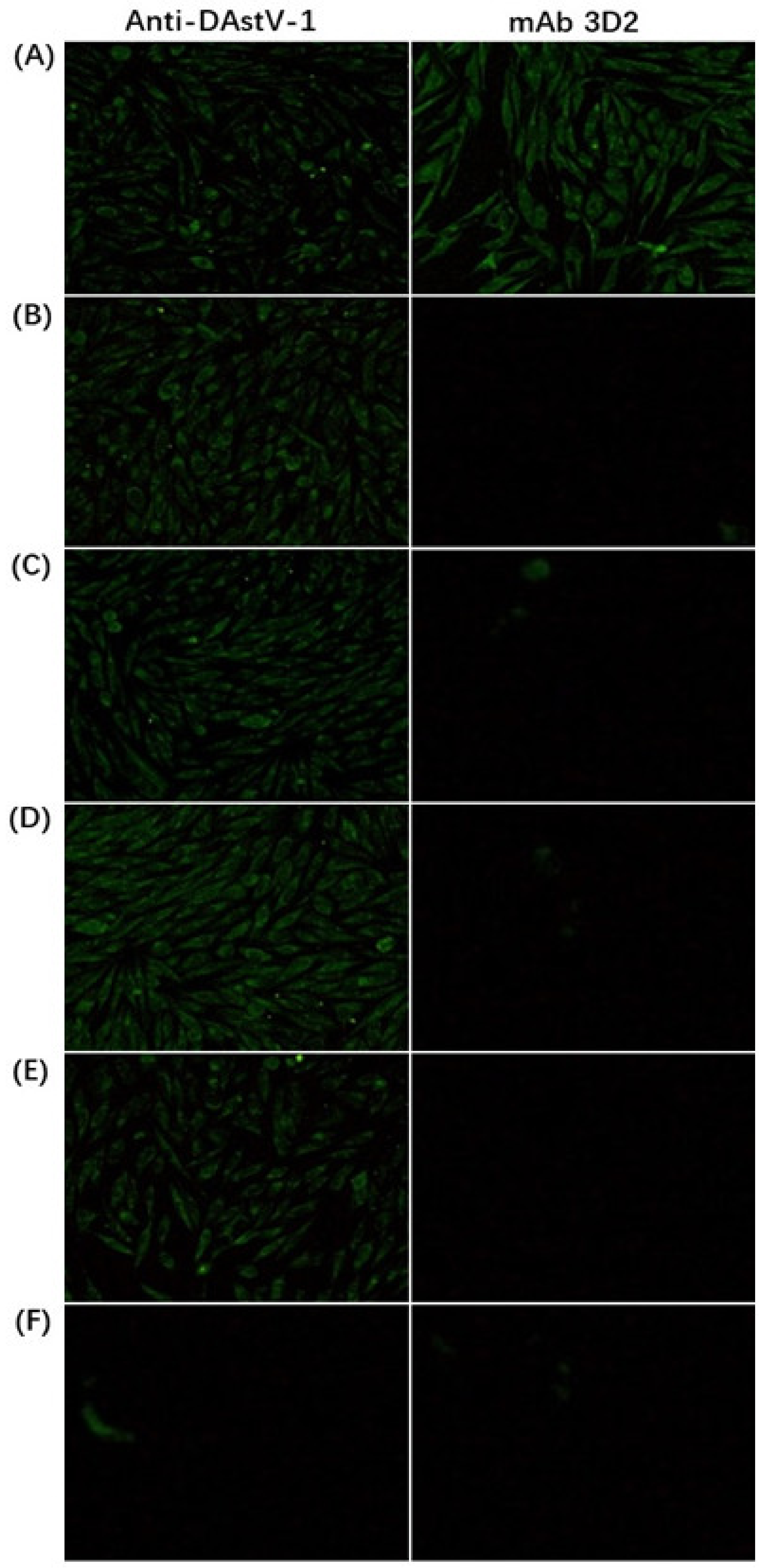
| Primers | Sequence (5′→3′) | AA Position in ORF2 Protein |
|---|---|---|
| D51-ORF2-F | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–732 |
| D51-ORF2-R | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT | |
| SL1-ORF2-F | CCGGAATTCATGGTGGCGGCGATGGCCGA | 1–727 |
| SL1-ORF2-R | CCGCTCGAGCTACTCGGCGTGGCCTCCAC | |
| CPH-ORF2-F | CCGGAATTCATGGCCGATAAGGCTGTTGT | 1–746 |
| CPH-ORF2-R | CCGCTCGAGCTACTCGGCGTGGCCACGGC | |
| YP2-ORF2-F | CCGGAATTCATGACTGGGGCCACCCCAA | 1–728 |
| YP2-ORF2-R | CCGCTCGAGCTACTCGGCGTGGCCGCGGC |
| Primers | Sequence (5′→3′) | AA Position in ORF2 Protein |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–280 |
| ORF2-AR | CCGCTCGAGAGTGTAGTTATTTGAAATC | |
| ORF2-BF | CCGGAATTCACCAGTGTTCTCTTTTTGGT | 248–494 |
| ORF2-BR | CCGCTCGAGAGACTTCTGTCTGCCATTGT | |
| ORF2-CF | CCGGAATTCCTAATGATTGCAGCTATACC | 461–732 |
| ORF2-CR | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT | |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–370 |
| ORF2-IR | CCGCTCGAGGGCATCATTGGCAGCACCA | |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–451 |
| ORF2-IIR | CCGCTCGAGTTGACCACGAAAGTCATAAAT | |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–461 |
| ORF2-IIIR | CCGCTCGAGTAGTGCTGCTGATTCTGTG | |
| ORF2-IVF | CCGGAATTCTACTTGCCCTTGCCTCTCGC | 430–732 |
| ORF2-CR | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT | |
| ORF2-VF | CCGGAATTCAGAATCAGCACCACAGAATC | 452–732 |
| ORF2-CR | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT | |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–460 |
| ORF2-1R | CCGCTCGAGTGCTGCTGATTCTGTGGTGC | |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–459 |
| ORF2-2R | CCGCTCGAGTGCTGATTCTGTGGTGCTGA | |
| ORF2-AF | CCGGAATTCATGGCTGGTGAGGCCCTTG | 1–458 |
| ORF2-3R | CCGCTCGAGTGATTCTGTGGTGCTGATTC | |
| ORF2-4F | CCGGAATTCATCAGCACCACAGAATCAG | 453–732 |
| ORF2-CR | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT | |
| ORF2-5F | CCGGAATTCAGCACCACAGAATCAGCAG | 454–732 |
| ORF2-CR | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT | |
| ORF2-6F | CCGGAATTCACCACAGAATCAGCAGCACT | 455–732 |
| ORF2-CR | CCGCTCGAGCTACTCGGCGTGGCCGCGGCT |
| Product Name | Primers | Sequence (5′→3′) |
|---|---|---|
| Upstream | U-F | GGAAGATCTGGAGGCTGTTGAACCG |
| U-R | GATTCTTTGACCACGAAAGTCATAA | |
| Downstream | D-F | GCACTAATGATTGCAGCTATACCAC |
| D-R | TCCCCGCGGCTGCTGGCAGCTTGTTGT | |
| Delete epitope | UF-1R | ATTTGCTTGTGGTATAGCTGCAATCATTAGTGCTGCACCACGAAAGTCATAAATCA |
| DR-1F | CAGTATACACCACACATGATTTATGACTTTCGTGGTGCAGCACTAATGATTGCAGC | |
| STTESA-GDWQSN | UF-2R | GTGCTGCTGATTCTGTGGTGCTATTACTCTGCCAATCACCGATTCTTTGACCACGAAAG |
| DR-2F | TGACTTTCGTGGTCAAAGAATCGGTGATTGGCAGAGTAATGCACTAATGATTGCAGCTA | |
| STTESA-DSWKSH | UF-3R | GGTATAGCTGCAATCATTAGTGCGTGGCTCTTCCATGAATCGATTCTTTGACCACGAAAG |
| DR-3F | ATGACTTTCGTGGTCAAAGAATCGATTCATGGAAGAGCCACGCACTAATGATTGCAGCT | |
| STTESA-TNWNGN | UF-4R | TGGTATAGCTGCAATCATTAGTGCATTGCCATTCCAGTTGGTGATTCTTTGACCACGAA |
| DR-4F | ATGACTTTCGTGGTCAAAGAATCACCAACTGGAATGGCAATGCACTAATGATTGCAGCT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Zhang, R.; Li, P.; Chen, J.; Xie, Z.; Jiang, S. Identification of a Type-Specific Epitope in the ORF2 Protein of Duck Astrovirus Type 1. Animals 2019, 9, 1069. https://doi.org/10.3390/ani9121069
Lan J, Zhang R, Li P, Chen J, Xie Z, Jiang S. Identification of a Type-Specific Epitope in the ORF2 Protein of Duck Astrovirus Type 1. Animals. 2019; 9(12):1069. https://doi.org/10.3390/ani9121069
Chicago/Turabian StyleLan, Jingjing, Ruihua Zhang, Pengfei Li, Junhao Chen, Zhijing Xie, and Shijin Jiang. 2019. "Identification of a Type-Specific Epitope in the ORF2 Protein of Duck Astrovirus Type 1" Animals 9, no. 12: 1069. https://doi.org/10.3390/ani9121069
APA StyleLan, J., Zhang, R., Li, P., Chen, J., Xie, Z., & Jiang, S. (2019). Identification of a Type-Specific Epitope in the ORF2 Protein of Duck Astrovirus Type 1. Animals, 9(12), 1069. https://doi.org/10.3390/ani9121069





