Anticoagulant Rodenticides, Islands, and Animal Welfare Accountancy
Abstract
Simple Summary
Abstract
1. Rodents, People, and Unwanted Consequences
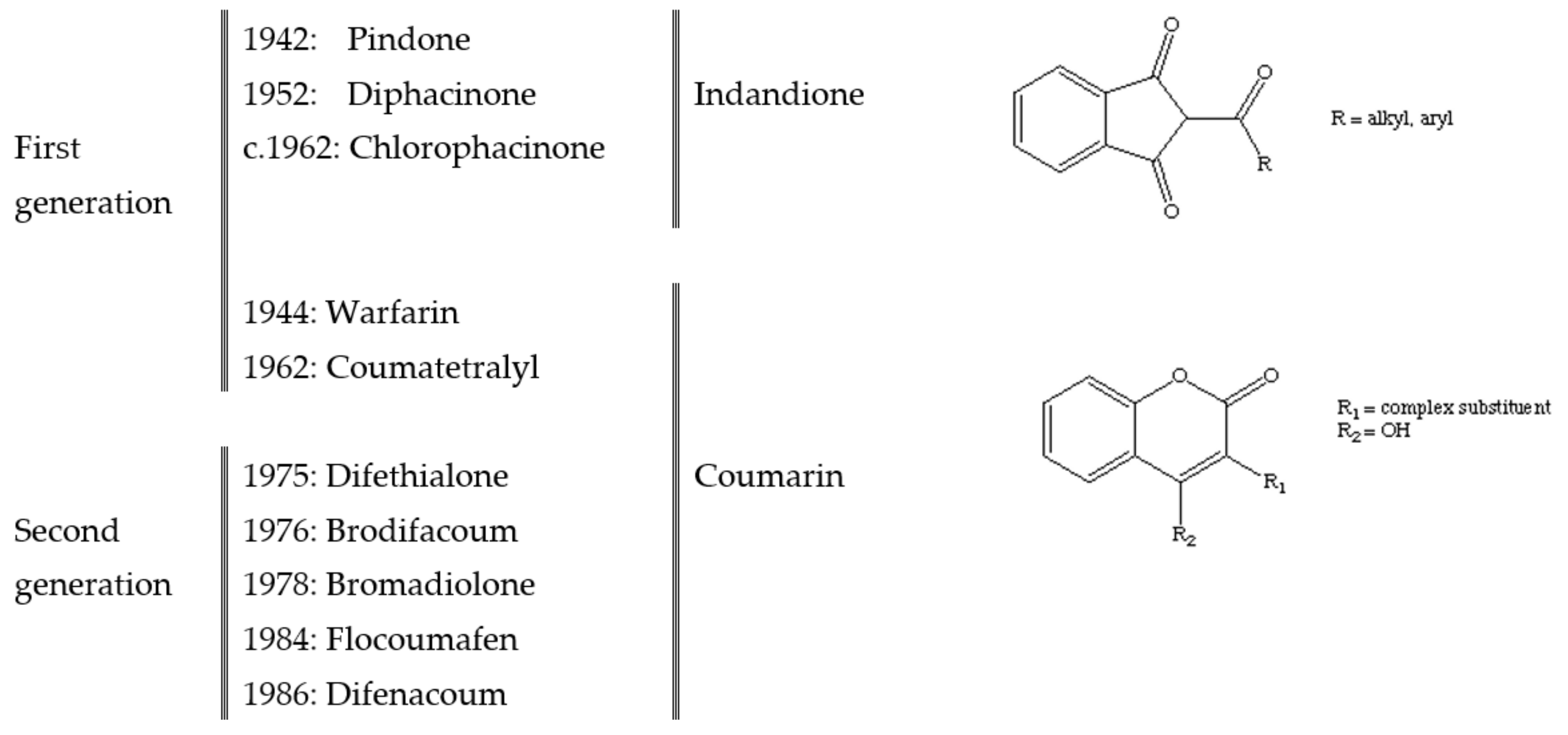
2. Anticoagulant Rodenticides
3. Toxic Action of Anticoagulants
4. Animal Welfare Outcomes of Anticoagulant Poisoning
5. Nontarget Effects of Anticoagulant Use
6. The Difference between Rodent Control and Rodent Eradication
Author Contributions
Funding
Conflicts of Interest
References
- Van den Brink, N.W.; Elliott, J.E.; Shore, R.F.; Rattner, B.A. Anticoagulant rodenticides and wildlife: Introduction. In Anticoagulant Rodenticides and Wildlife; Springer: Cham, Switzerland, 2018; pp. 1–9. [Google Scholar]
- Brooks, J.E.; Jackson, W.B. A review of commensal rodents and their control. CRC Crit. Rev. Environ. Control 1973, 3, 405–453. [Google Scholar] [CrossRef]
- Meyer, A.; Kaukeinen, D. 11 rodent control in practice: Protection of humans and animal health. In Rodent Pests and Their Control; CABI: Wallingford, UK, 2015; p. 231. [Google Scholar]
- Renner, M.; Nelson, E.; Watson, J.; Haynie, A.; Poe, A.; Robards, M.; Hess, S.C. The risk of rodent introductions from shipwrecks to seabirds on Aleutian and Bering Sea islands. Biol. Invasions 2018, 20, 2679–2690. [Google Scholar] [CrossRef]
- Atkinson, I.A. The spread of commensal species of Rattus to oceanic islands and their effects on island avifaunas. ICPB Tech. Publ. 1985, 3, 35–81. [Google Scholar]
- Macdonald, D.; Fenn, M.; Gelling, M. The natural history of rodents: Preadaptations to pestilence. In Rodent Pests and Their Control; CAB International: Oxon, UK, 1994; pp. 1–21. [Google Scholar]
- Landry, S.O., Jr. The Rodentia as omnivores. Q. Rev. Biol. 1970, 45, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.; Hilton, G. Introduced house mice Mus musculus: A significant predator of threatened and endemic birds on Gough Island, South Atlantic Ocean? Biol. Conserv. 2004, 117, 483–489. [Google Scholar] [CrossRef]
- Jones, M.; Ryan, P. Evidence of mouse attacks on albatross chicks on sub-Antarctic Marion Island. Antarct. Sci. 2010, 22, 39–42. [Google Scholar] [CrossRef]
- Hamer Environmental L.P.; Planning Solutions Inc. Midway Seabird Protection Project Draft Environmetal Assessment; US Fish and Wildlife Service: Washington, DC, USA, 2018; p. 198. [Google Scholar]
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive predators and global biodiversity loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef]
- Fukami, T.; Wardle, D.A.; Bellingham, P.J.; Mulder, C.P.; Towns, D.R.; Yeates, G.W.; Bonner, K.I.; Durrett, M.S.; Grant-Hoffman, M.N.; Williamson, W.M. Above-and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecol. Lett. 2006, 9, 1299–1307. [Google Scholar] [CrossRef]
- Graham, N.A.; Wilson, S.K.; Carr, P.; Hoey, A.S.; Jennings, S.; MacNeil, M.A. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 2018, 559, 250. [Google Scholar] [CrossRef]
- Jacob, J.; Buckle, A. Use of anticoagulant rodenticides in different applications around the world. In Anticoagulant Rodenticides and Wildlife; Springer: Cham, Switzerland, 2018; pp. 11–43. [Google Scholar]
- Lim, G.B. Warfarin: From rat poison to clinical use. Nat. Rev. Cardiol. 2017, 10. [Google Scholar] [CrossRef]
- Tomlin, C.D. The Pesticide Manual: A World Compendium; British Crop Production Council: Aldershot, UK, 2009. [Google Scholar]
- Jackson, W.B.; Ashton, A.D. Case histories of anticoagulant resistance. In Pesticide Resistance: Strategies and Tactics for Management; National Academies Press: Washington, DC, USA, 1986; pp. 355–369. [Google Scholar]
- Buckle, A.P.; Prescott, C.V.; Ward, K.J. Resistance to the first and second generation anticoagulant rodenticides-a new perspective. In Proceedings of the Sixteenth Vertebrate Pest Conference, Santa Clara, CA, USA, 1–3 March 1994. [Google Scholar]
- Berny, P.; Esther, A.; Jacob, J.; Prescott, C. Development of resistance to anticoagulant rodenticides in rodents. In Anticoagulant Rodenticides and Wildlife; Springer: Cham, Switzerland, 2018; pp. 259–286. [Google Scholar]
- Horak, K.E.; Fisher, P.M.; Hopkins, B. Pharmacokinetics of anticoagulant rodenticides in target and non-target organisms. In Anticoagulant Rodenticides and Wildlife; Springer: Cham, Switzerland, 2018; pp. 87–108. [Google Scholar]
- Fisher, P. Review of House Mouse (Mus musculus) Susceptibility to Anticoagulant Poisons; Department of Conservation: Wellington, New Zealand, 2005. [Google Scholar]
- Parmar, G.; Bratt, H.; Moore, R.; Batten, P. Evidence for a common binding site in vivo for the retention of anticoagulants in rat liver. Hum. Toxicol. 1987, 6, 431–432. [Google Scholar]
- Huckle, K.; Hutson, D.; Warburton, P. Elimination and accumulation of the rodenticide flocoumafen in rats following repeated oral administration. Xenobiotica 1988, 18, 1465–1479. [Google Scholar] [CrossRef] [PubMed]
- Ashton, A.; Jackson, W.; Peters, H. Comparative evaluation of LD50 values for various anticoagulant rodenticides. In Control of Mammal Pests; Richards, C.G.J., Ku, T.Y., Eds.; Taylor & Francis: London, UK, 1987. [Google Scholar]
- Garcia, A.A.; Reitsma, P.H. VKORC1 and the vitamin K cycle. Vitam. Horm. 2008, 78, 23–33. [Google Scholar] [PubMed]
- Lowenthal, J.; Birnbaum, H. Vitamin K and coumarin anticoagulants: Dependence of anticoagulant effect on inhibition of vitamin K transport. Science 1969, 164, 181–183. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.A. Vitamin K and the oral anticoagulant drugs. Annu. Rev. Med. 1976, 27, 245–261. [Google Scholar] [CrossRef]
- Gebauer, M. Synthesis and structure–activity relationships of novel warfarin derivatives. Bioorg. Med. Chem. 2007, 15, 2414–2420. [Google Scholar] [PubMed]
- Wittkowsky, A.K. Warfarin and other coumarin derivatives: Pharmacokinetics, pharmacodynamics, and drug interactions. Semin. Vasc. Med. 2003, 3, 221–230. [Google Scholar]
- Prakash, I. Bait shyness and poison aversion. In Rodent Pest Management; CRC Press: Boca Raton, FL, USA, 1988; pp. 321–329. [Google Scholar]
- Fisher, P.; O’Connor, C.; Wright, G.; Eason, C. Anticoagulant residues in rats and secondary non-target risk. DOC Sci. Intern. Ser. 2004, 188, 29. [Google Scholar]
- Pelfrène, A.F. Rodenticides. In Hayes’ Handbook of Pesticide Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 2153–2217. [Google Scholar]
- Desheesh, M. Effects of anticoagulant poison-baits on the behaviour of white rats (Rattus norvegicus). Alex. Sci. Exch. 1983, 4, 49–56. [Google Scholar]
- Bai, K.M.; Krishnakumari, M.K.; Majumder, S.K. Toxicity of calciferol, warfarin and their combinations to Rattus norvegicus (albino) and R. rattus. Pestic. Sci. 1978, 9, 44–50. [Google Scholar] [CrossRef]
- Bentley, E.; Rowe, M. Pival, an anti-coagulant rodenticide. Epidemiol. Infect. 1956, 54, 20–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Girish, G.; Singh, K.; Srivastava, P.; Krishnamurthy, K. Studies on rodents and their control. VIII. Susceptibility of Rattus to different anticoagulants. Bull. Grain Technol. 1972, 113–115. [Google Scholar]
- Krishnamurthy, K.; Uniyal, V.; Pingale, S. Studies on rodents and their control. IV. Susceptibility of Rattus rattus to warfarin. Bull. Grain Technol. 1968, 6, 133–137. [Google Scholar]
- Rowe, F.; Redfern, R. The toxicity of 0·025% warfarin to wild house-mice (Mus musculus L.). Epidemiol. Infect. 1964, 62, 389–393. [Google Scholar] [CrossRef]
- Littin, K.; O′Connor, C.; Eason, C. Comparative effects of brodifacoum on rats and possums. N. Z. Plant Prot. 2000, 53, 310–315. [Google Scholar] [CrossRef]
- Wheeler, R.; Priddel, D.; O’Dwyer, T.; Carlile, N.; Portelli, D.; Wilkinson, I. Evaluating the susceptibility of invasive black rats (Rattus rattus) and house mice (Mus musculus) to brodifacoum as a prelude to rodent eradication on Lord Howe Island. Biol. Invasions 2019, 21, 833–845. [Google Scholar] [CrossRef]
- O′Connor, C.E.; Booth, L. Palatability of Rodent Baits to Wild House Mice; Department of Conservation: Wellington, New Zealand, 2001. [Google Scholar]
- Morriss, G. Susceptibility of Rangitoto and Motutapu Island House Mice to 20R Brodifacoum Baits; Landcare Research Contract Report: LC0607/155; Landcare Research: Lincoln, New Zealand, 2007; p. 9. [Google Scholar]
- Cleghorn, M.; Griffiths, R. Palatability and Efficacy of Pestoff 20R Bait on Mice From Mokoia Island, Rotorua; Department of Conservation: Wellington, New Zealand, 2002. [Google Scholar]
- Cuthbert, R.; Visser, P.; Louw, H.; Ryan, P. Palatability and efficacy of rodent baits for eradicating house mice (Mus musculus) from Gough Island, Tristan da Cunha. Wildl. Res. 2011, 38, 196–203. [Google Scholar] [CrossRef]
- Fisher, P.M.; Zhang, M.; Campion, M.; Pech, R. Anticoagulant rodenticides in the environment: Excretion as a residue transfer pathway (abstract). In Proceedings of the 17th Australasian Vertebrate Pest Conference, Canberra, Australia, 1–4 May 2017. [Google Scholar]
- Rowe, F. The toxicity and acceptability of the sodium salt of pindone, an anti-coagulant rodenticide, to the house-mouse (Mus musculus L.). Epidemiol. Infect. 1961, 59, 335–341. [Google Scholar] [CrossRef]
- Mason, G.; Littin, K. The humaneness of rodent pest control. Anim. Welf. 2003, 12, 1–37. [Google Scholar]
- Littin, K.; Fisher, P.; Beausoleil, N.; Sharp, T. Welfare aspects of vertebrate pest control and culling: Ranking control techniques for humaneness. Rev. Sci. Tech. Int. Off. Epizoot. 2014, 33, 281–289. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Brom, F.W.; Kijlstra, A. The ethics of rodent control. Pest Manag. Sci. 2008, 64, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Paparella, M. Rodenticides-an animal welfare paradox? ALTEX Altern. Anim. Exp. 2006, 23, 51–52. [Google Scholar]
- Leitschuh, C.M.; Kanavy, D.; Backus, G.A.; Valdez, R.X.; Serr, M.; Pitts, E.A.; Threadgill, D.; Godwin, J. Developing gene drive technologies to eradicate invasive rodents from islands. J. Responsib. Innov. 2018, 5, S121–S138. [Google Scholar] [CrossRef]
- Guitart, R.; Sachana, M.; Caloni, F.; Croubels, S.; Vandenbroucke, V.; Berny, P. Animal poisoning in Europe. Part 3: Wildlife. Vet. J. 2010, 183, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Erickson, W.A.; Urban, D.J. Potential risks of Nine Rodenticides to Birds and Nontarget Mammals: A Comparative Approach; US Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Harradine, J.P. Anticoagulant Rodenticides And Non-Target Wildlife: An Ecological Evaluation Of Permanent Baiting In Rural Rat Control. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 1976. [Google Scholar]
- Eason, C.; Spurr, E. Review of the toxicity and impacts of brodifacoum on non-target wildlife in New Zealand. N. Z. J. Zool. 1995, 22, 371–379. [Google Scholar] [CrossRef]
- Eadsforth, C.V.; Gray, A.; Harrison, E.G. Monitoring the exposure of barn owls to second-generation rodenticides in Southern Eire. Pestic. Sci. 1996, 47, 225–233. [Google Scholar] [CrossRef]
- Berny, P.J.; Buronfosse, T.; Buronfosse, F.; Lamarque, F.; Lorgue, G. Field evidence of secondary poisoning of foxes (Vulpes vulpes) and buzzards (Buteo buteo) by bromadiolone, a 4-year survey. Chemosphere 1997, 35, 1817–1829. [Google Scholar] [CrossRef]
- Stone, W.B.; Okoniewski, J.C.; Stedelin, J.R. Poisoning of wildlife with anticoagulant rodenticides in New York. J. Wildl. Dis. 1999, 35, 187–193. [Google Scholar] [CrossRef]
- Elliott, J.E.; Rattner, B.A.; Shore, R.F.; Van Den Brink, N.W. Paying the pipers: Mitigating the impact of anticoagulant rodenticides on predators and scavengers. Bioscience 2016, 66, 401–407. [Google Scholar] [CrossRef]
- López-Perea, J.J.; Mateo, R. Secondary exposure to anticoagulant rodenticides and effects on predators. In Anticoagulant Rodenticides and Wildlife; Springer: Cham, Switzerland, 2018; pp. 159–193. [Google Scholar]
- Lohr, M.T.; Davis, R.A. Anticoagulant rodenticide use, non-target impacts and regulation: A case study from Australia. Sci. Total Environ. 2018, 634, 1372–1384. [Google Scholar] [CrossRef]
- Nakayama, S.M.; Morita, A.; Ikenaka, Y.; Mizukawa, H.; Ishizuka, M. A review: Poisoning by anticoagulant rodenticides in non-target animals globally. J. Vet. Med Sci. 2018, 81, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.; Ward, N. Contaminants in Estuarine and Riverine Sediments and Biota in Southland; Environment Southland: Invercargill, New Zealand, 2014. [Google Scholar]
- Regnery, J.; Friesen, A.; Geduhn, A.; Göckener, B.; Kotthoff, M.; Parrhysius, P.; Petersohn, E.; Reifferscheid, G.; Schmolz, E.; Schulz, R.S. Rating the risks of anticoagulant rodenticides in the aquatic environment: A review. Environ. Chem. Lett. 2019, 17, 215–240. [Google Scholar] [CrossRef]
- Siers, S.R.; Foster, D.K.; Neibuhr, C.N.; Lienbach, I.; Shiles, A.B.; Volker, S.F. Monitoring Diphacinone Residues After an Eradication of Polynesian Rats From Lehua Island, Hawaii; Final Report QA-2802; USDA, APHIS, WS, NWRC: Hilo, HI, USA, 2018; p. 14. [Google Scholar]
- DIISE. The Database of Island Invasive Species Eradications; Island Conservation, Coastal Conservation Action Laboratory UCSC, IUCN SSC Invasive Species Specialist Group, University of Auckland and Landcare Research: Auckland, New Zealand, 2015. [Google Scholar]
- Simberloff, D.; Keitt, B.; Will, D.; Holmes, N.; Pickett, E.; Genovesi, P. Yes we can! Exciting progress and prospects for controlling invasives on islands and beyond. West. North Am. Nat. 2018, 78, 942–958. [Google Scholar] [CrossRef]
- Howald, G.; Donlan, C.J.; Galvan, J.P.; Russell, J.C.; Parkes, J.; Samaniego, A.; Wang, Y.; Veitch, D.; Genovesi, P.; Pascal, M. Invasive rodent eradication on islands. Conserv. Biol. 2007, 21, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Parkes, J.; Fisher, P.; Forrester, G. Diagnosing the cause of failure to eradicate introduced rodents on islands: Brodifacoum versus diphacinone and method of bait delivery. Conserv. Evid. 2011, 8, 100–106. [Google Scholar]
- Oppel, S.; Beaven, B.M.; Bolton, M.; Vickery, J.; Bodey, T.W. Eradication of invasive mammals on islands inhabited by humans and domestic animals. Conserv. Biol. 2011, 25, 232–240. [Google Scholar] [CrossRef]
- Glen, A.S.; Atkinson, R.; Campbell, K.J.; Hagen, E.; Holmes, N.D.; Keitt, B.S.; Parkes, J.P.; Saunders, A.; Sawyer, J.; Torres, H. Eradicating multiple invasive species on inhabited islands: The next big step in island restoration? Biol. Invasions 2013, 15, 2589–2603. [Google Scholar] [CrossRef]
- Cowan, P.; Warburton, B. Animal welfare and ethical issues in island pest eradication. In Island Invasives: Eradication and Management; Veitch, C.R., Clout, M.N., Towns, D.R., Eds.; IUCN: Gland, Switzerland, 2011; pp. 418–421. [Google Scholar]
- Russell, J.C.; Jones, H.P.; Armstrong, D.P.; Courchamp, F.; Kappes, P.J.; Seddon, P.J.; Oppel, S.; Rauzon, M.J.; Cowan, P.E.; Rocamora, G. Importance of lethal control of invasive predators for island conservation. Conserv. Biol. 2016, 30, 670–672. [Google Scholar] [CrossRef]
- Valdez, R.; Peterson, M.; Pitts, E.; Delborne, J. International news media framing of invasive rodent eradications. Biol. Invasions 2019, 21, 1439–1449. [Google Scholar] [CrossRef]
- Morzillo, A.T.; Mertig, A.G. Urban resident attitudes toward rodents, rodent control products, and environmental effects. Urban Ecosyst. 2011, 14, 243–260. [Google Scholar] [CrossRef]
| Anticoagulant/Rodent Species | Time to Observed Illness (days) | Time to Death (days) | Reference |
|---|---|---|---|
| Warfarin | |||
| Laboratory R. norvegicus | mean 5.5 | mean 6.0 | [33] |
| Laboratory R. norvegicus | - | range 4.2–17 | [34] |
| Laboratory R. norvegicus | - | mean 3.0 ± 0.45 | [31] |
| Wild-caught R. norvegicus | - | mean 6.2–6.6 range 4.0–10.0 | [35] |
| Wild-caught R. rattus | - | mean 7.1 range 3.0–13.0 | [36] |
| Wild-caught R. rattus | - | mean (males) 7.9 range 4.0–13.0 mean (females) 7.0 range 2.0–13.0 | [37] |
| Wild-caught R. rattus | - | means 7.0 and 7.5 | [34] |
| Wild-caught R. rattus | - | mean 8.8 range 5.0–12.0 | [35] |
| Wild-caught Mus musculus | - | range 3.0–30.0 | [38] |
| Coumatetralyl | |||
| Laboratory R. norvegicus | mean 1.2 | mean 5.7 | [33] |
| Laboratory R. norvegicus | - | mean 6.6 ± 0.5 | [31] |
| Wild-caught R. rattus | - | mean 8.1 range 5.0–13.0 | [36] |
| Difenacoum | |||
| Laboratory R. norvegicus | mean 8.4 | mean 11.0 | [33] |
| Brodifacoum | |||
| Laboratory R. norvegicus | mean 4.0 | mean 13.3 | [33] |
| Laboratory R. norvegicus | mean 3.0 | mean 7.2 range 5.6-8.5 | [39] |
| Laboratory R. norvegicus | - | mean 4.3 ± 0.5 | [31] |
| Wild-caught R. rattus | - | mean 6.9 ± 1.9 range 3.0–13.0 | [40] |
| Wild-caught M. musculus | - | mean 9.9 range 6.0–18.0 | [41] |
| Wild-caught M. musculus | - | mean 9.0 ± 0.6 range 3.0–21.0 | [42] |
| Wild-caught M. musculus | - | range 4.0–19.0 | [43] |
| Wild-caught M. musculus | mean 5.5± 2.5 range 1.0–16.0 median 6.0 | [44] | |
| Wild-caught M. musculus | mean 7.3 ± 3.9 range 1.0–18.0 | [40] | |
| Bromadiolone | |||
| Wild-caught R. norvegicus | - | range 4.0–6.2 | [45] |
| Pindone | |||
| Laboratory R. norvegicus | - | mean 4.0 ± 0.5 | [31] |
| Wild-caught R. rattus | - | mean 7.9 range 3.0–19.0 | [36] |
| Wild-caught R. norvegicus | - | means 4.2 and 5.0 range 4.0–9.0 | [35] |
| Wild-caught R. rattus | - | means 8.7 and 8.7 range 4.0–13.0 | [35] |
| Wild-caught M. musculus | - | range 4.0–6.0 | [46] |
| Diphacinone | |||
| Laboratory R. norvegicus | - | mean 2.9 ± 1.8 | [31] |
| Chlorophacinone | |||
| Wild-caught R. rattus | - | mean 9.7 range 4.0–19.0 | [36] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, P.; Campbell, K.J.; Howald, G.R.; Warburton, B. Anticoagulant Rodenticides, Islands, and Animal Welfare Accountancy. Animals 2019, 9, 919. https://doi.org/10.3390/ani9110919
Fisher P, Campbell KJ, Howald GR, Warburton B. Anticoagulant Rodenticides, Islands, and Animal Welfare Accountancy. Animals. 2019; 9(11):919. https://doi.org/10.3390/ani9110919
Chicago/Turabian StyleFisher, Penny, Karl J. Campbell, Gregg R. Howald, and Bruce Warburton. 2019. "Anticoagulant Rodenticides, Islands, and Animal Welfare Accountancy" Animals 9, no. 11: 919. https://doi.org/10.3390/ani9110919
APA StyleFisher, P., Campbell, K. J., Howald, G. R., & Warburton, B. (2019). Anticoagulant Rodenticides, Islands, and Animal Welfare Accountancy. Animals, 9(11), 919. https://doi.org/10.3390/ani9110919





