MTOR Variation Related to Heat Resistance of Chinese Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
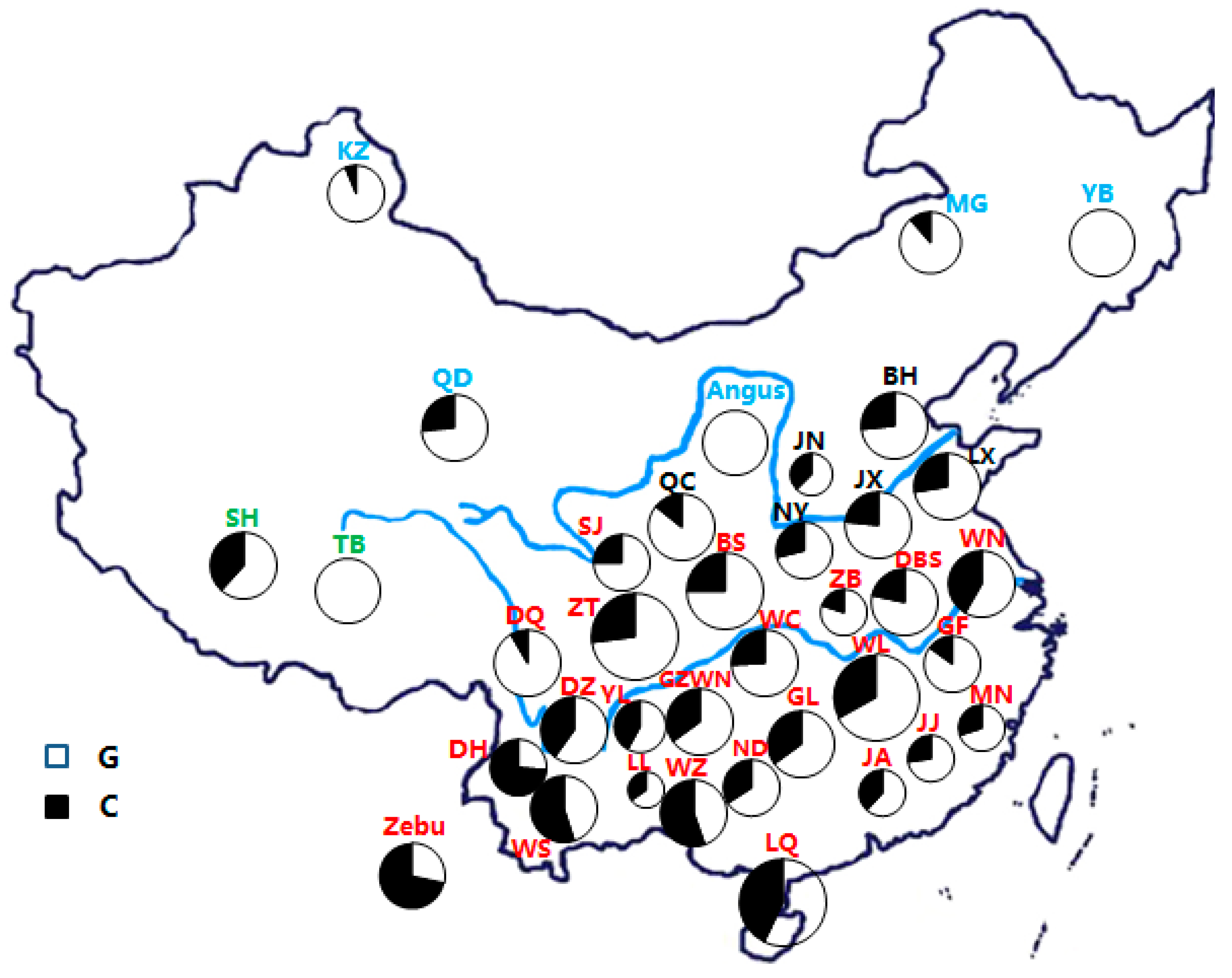
2.2. Animal Samples Information, DNA Extraction, and Data Collection
2.3. Primers Information, PCR Amplification and PCR Product Processing
2.4. Statistic Analysis of MTOR Gene Polymorphism
3. Results
3.1. Diversity Analysis
3.2. Statistical Analysis of Genotypic and Allele Frequencies
3.3. Correlation Analysis of MTOR Gene Polymorphism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fuquay, J.W. Heat Stress as it Affects Animal Production. J. Anim. Sci. 1981, 52, 164. [Google Scholar] [CrossRef]
- Morrison, S.R. Ruminant Heat Stress: Effect on Production and Means of Alleviation. J. Anim. Sci. 1983, 57, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Gabler, N.K.; Pearce, S.C. The impact of heat stress on intestinal function and productivity in grow-finish pigs. Anim. Prod. Sci. 2015, 55, 1403–1410. [Google Scholar] [CrossRef]
- Naqvi, S.M.K.; Kumar, D.; Paul, R.K.; Sejian, V. Heat Stress Impact on Livestock Production. In Environmental Stresses and Livestock Reproduction, 3.; Daramola, J.O., Abioja, M.O., Onagbesan, O.M., Eds.; Springer: New York, NY, USA, 2012; Volume 3, pp. 53–73. [Google Scholar]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic Losses from Heat Stress by US Livestock Industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef]
- West, J.W. Effects of Heat Stress on Production in Dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013.
- Karimaian, A.; Majidinia, M.; Baghi, H.B.; Yousefi, B. The crosstalk between Wnt/b-catenin signaling pathway with DNA damage response and oxidative stress: Implications in cancer therapy. DNA Repair 2017, 51, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Dominick, G.; Bowman, J.; Li, X.; Miller, R.A.; Garcia, G.G. mTOR regulates the expression of DNA damage response enzymes in long-lived Snell dwarf, GHRKO, and PAPPA-KO mice. Aging Cell 2017, 16, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Ahima, R.S. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef]
- Reiter, A.K.; Anthony, T.G.; Anthony, J.C.; Jefferson, L.S.; Kimball, S.R. The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int. J. Biochem. Cell Biol. 2004, 36, 2169–2179. [Google Scholar] [CrossRef]
- Jirimutu, W.Z.; Ding, G.H.; Chen, G.L.; Sun, Y.M.; Sun, Z.H. Bactrian Camels Genome, S. Genome sequences of wild and domestic bactrian camels. Nat. Commun. 2012, 3, 1202. [Google Scholar] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Fu, W.; Zhao, J.; Shen, J.; Chen, Q.; Zheng, Z.; Chen, H.; Sonstegard, T.S.; Lei, C.; Jiang, Y. The Bovine Genome Variation Database (BGVD): Integrated Web-database for Bovine Sequencing Variations and Selective Signatures. 2019. Available online: https://www.biorxiv.org/content/10.1101/802223v1 (accessed on 13 October 2019).
- Zhang, Y. Animal Genetic Resources in China – Bovines (in Chinese); China Agriculture Press: Beijing, China, 2011. [Google Scholar]
- Qiu, H.; Qing, Z.R.; Chen, Y.C.; Wang, D.A. Bovine Breeds in China; Shanghai Scientific and Technical Publishers: Shanghai, China, 1988. [Google Scholar]
- Xia, X.; Yao, Y.; Li, C.; Zhang, F.; Qu, K.; Chen, H.; Lei, C. Genetic diversity of Chinese cattle revealed by Y-SNP and Y-STR markers. Anim. Genet. 2019, 50, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Hansen, P. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 2004, 82, 349–360. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, N.; Ning, Q.; Lei, C. PRLH and SOD1 gene variations associated with heat tolerance in Chinese cattle. Anim. Genet. 2018, 49, 447–451. [Google Scholar] [CrossRef]
- Zeng, L.; Cao, Y.; Wu, Z.; Huang, M.; Zhang, G.; Lei, C. A Missense Mutation of the HSPB7 Gene Associated with Heat Tolerance in Chinese Indicine Cattle. Animals. 2019, 9, 554. [Google Scholar] [CrossRef]
- Yang, R.-C.; Yeh, F.C. Multilocus structure in Pinus-contorta Dougl. Theor. Appl. Genet. 1993, 87, 568–576. [Google Scholar] [CrossRef]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and genetic distance. Genetics 1974, 76, 379–390. [Google Scholar]
- Yeh, F.C. POPGENE (version 1.3. 1). Microsoft Window-Bases Freeware for Population Genetic Analysis. 1999. Available online: http://www.ualberta.ca/~{}fyeh/ (accessed on 19 June 2019).
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- McDowell, R.E.; Hooven, N.W.; Adcamoens, J.K. Effects of climate on performance of Holsteins in first lactation. J. Dairy Sci. 1976, 59, 965–971. [Google Scholar] [CrossRef]
- Cun, W.T.; Yu, X.; Liu, W.J.; Zhang, H.; Shi, L.; Xing, W.T.; Cheng, L.M.; Huang, X.X.; Ma, X.Y. Study on Genetic Diversity and Genetic Differentiation of 13 Sheep Populations in Xinjiang Uygur Autonomous Region. Acta Ecol. Anim. Domastici. 2011, 32, 13–19. [Google Scholar]
- Becker, J.A.; Stewart, L.K. Heat-related illness. Am. Fam. Physician 2011, 83, 1325–1330. [Google Scholar] [PubMed]
- Bouchama, A.; Knochel, J.P. Heat stroke. N. Engl. J. Med. 2002, 346, 1978–1988. [Google Scholar] [CrossRef] [PubMed]
- Epstein, Y.; Roberts, W.O. The pathopysiology of heat stroke: An integrative view of the final common pathway. Scand J. Med. Sci. Sports 2011, 21, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R.; Helwig, B.G. Role of endotoxin and cytokines in the systemic inflammatory response to heat injury. Front. Biosci. 2010, 2, 916–938. [Google Scholar] [CrossRef]
- Lei, C.Z.; Chen, H.; Zhang, H.C.; Cai, X.; Liu, R.Y.; Luo, L.Y.; Wang, C.F.; Zhang, W.; Ge, Q.L.; Zhang, R.F.; et al. Origin and phylogeographical structure of Chinese cattle. Anim. Genet. 2010, 37, 579–582. [Google Scholar] [CrossRef]
- Xia, X.; Qu, K.; Zhang, G.; Jia, Y.; Ma, Z.; Zhao, X.; Lei, C. Comprehensive analysis of the mitochondrial DNA diversity in Chinese cattle. Anim. Genet. 2019, 50, 70–73. [Google Scholar] [CrossRef]
| Breeds | p-Value | Ho | He | Ne | PIC |
|---|---|---|---|---|---|
| Kazakh (KZ) | 0.0625 | 0.8830 | 0.1170 | 1.1330 | 0.1103 |
| Yanbian (YB) | 0.0000 | 1.0000 | 0.0000 | 1.0000 | 0.0000 |
| Chaidamu (QD) | 0.2667 | 0.6089 | 0.3911 | 1.6423 | 0.3146 |
| Mongolian (MG) | 0.1143 | 0.7976 | 0.2024 | 1.2538 | 0.1820 |
| Jiaxian Red (JX) | 0.2333 | 0.6422 | 0.3578 | 1.5571 | 0.2938 |
| Nanyang (NY) | 0.2895 | 0.5886 | 0.4114 | 1.6988 | 0.3267 |
| Luxi (LX) | 0.2759 | 0.6005 | 0.3995 | 1.6653 | 0.3197 |
| Bohai Black (BH) | 0.2667 | 0.6089 | 0.3911 | 1.6423 | 0.3146 |
| Jinnan (JN) | 0.3750 | 0.5313 | 0.4688 | 1.8824 | 0.3589 |
| Qinchuan (QC) | 0.1429 | 0.7551 | 0.2449 | 1.3243 | 0.2149 |
| Ji’an (JA) | 0.3824 | 0.5277 | 0.4723 | 1.8951 | 0.3608 |
| Jinjiang (JJ) | 0.2727 | 0.6033 | 0.3967 | 1.6575 | 0.3180 |
| Wannan (WN) | 0.4167 | 0.5139 | 0.4861 | 1.9459 | 0.3680 |
| Weining (GZWN) | 0.3500 | 0.5450 | 0.4550 | 1.8349 | 0.3515 |
| Zaobei (ZB) | 0.2000 | 0.6800 | 0.3200 | 1.4706 | 0.2688 |
| Dabeishan (DBS) | 0.2167 | 0.6606 | 0.3394 | 1.5139 | 0.2818 |
| Bashan (BS) | 0.2500 | 0.6250 | 0.3750 | 1.6000 | 0.3047 |
| Leiqiong (LQ) | 0.4286 | 0.5102 | 0.4898 | 1.9600 | 0.3698 |
| Wengshan (WS) | 0.5500 | 0.5050 | 0.4950 | 1.9802 | 0.3725 |
| Dianzhong (DZ) | 0.6000 | 0.5200 | 0.4800 | 1.9231 | 0.3648 |
| Guangfeng (GF) | 0.1500 | 0.7450 | 0.2550 | 1.3423 | 0.2225 |
| Sanjiang (SJ) | 0.2500 | 0.6250 | 0.3750 | 1.6000 | 0.3047 |
| Wuling (WL) | 0.3308 | 0.5573 | 0.4427 | 1.7944 | 0.3447 |
| Guanling (GL) | 0.3500 | 0.5450 | 0.4550 | 1.8349 | 0.3515 |
| Wuchuan (WC) | 0.2586 | 0.6165 | 0.3835 | 1.6220 | 0.3099 |
| Minnan (MN) | 0.3000 | 0.5800 | 0.4200 | 1.7241 | 0.3318 |
| Longling (LL) | 0.3462 | 0.5473 | 0.4527 | 1.8270 | 0.3502 |
| Weizhou (WZ) | 0.5536 | 0.5057 | 0.4943 | 1.9773 | 0.3721 |
| Nandan (ND) | 0.3400 | 0.5512 | 0.4488 | 1.8142 | 0.3481 |
| Diqing (DQ) | 0.0833 | 0.8472 | 0.1528 | 1.1803 | 0.1411 |
| Yunnan Humped (DH) | 0.7368 | 0.6122 | 0.3878 | 1.6335 | 0.3126 |
| Zhaotong (ZT) | 0.2708 | 0.6050 | 0.3950 | 1.6528 | 0.3170 |
| Yunling (YL) | 0.4200 | 0.5128 | 0.4872 | 1.9501 | 0.3685 |
| Tibetan (TB) | 0.0000 | 1.0000 | 0.0000 | 1.0000 | 0.0000 |
| Shigatse Humped (SH) | 0.3833 | 0.5272 | 0.4728 | 1.8967 | 0.3610 |
| Burma (MD) | 0.7167 | 0.5939 | 0.4061 | 1.6838 | 0.3236 |
| Angus (AG) | 0.0000 | 1.0000 | 0.0000 | 1.0000 | 0.0000 |
| Geographical Grouping | Breeds (Codes) | MTOR (NC_037343.1:c. 2062G>C) | Mutation Frequencies | ||||
|---|---|---|---|---|---|---|---|
| Genotype Frequencies (Number) | Allele Frequencies | ||||||
| GG | GC | CC | G | C | |||
| Northern group | Kazakh (KZ) | 0.08750 (21) | 0.1250 (3) | 0.0000 (0) | 0.9375 | 0.0625 | 0.0625 |
| Yanbian (YB) | 1.0000 (30) | 0.0000 (0) | 0.0000 (0) | 1.0000 | 0.0000 | 0.0000 | |
| Chaidamu (QD) | 0.5333 (16) | 0.4000 (12) | 0.0667 (2) | 0.7333 | 0.2667 | 0.2667 | |
| Mongolian (MG) | 0.7714 (27) | 0.2286 (8) | 0.0000 (0) | 0.8857 | 0.1143 | 0.1143 | |
| 0.7899 (94) | 0.1933 (23) | 0.0168 (2) | 0.8866 | 0.1134 | 0.1134 | ||
| Central group | Jiaxian Red (JX) | 0.5667 (17) | 0.4000 (12) | 0.0333 (1) | 0.7667 | 0.2333 | 0.2333 |
| Nanyang (NY) | 0.4737 (9) | 0.4737 (9) | 0.0526 (1) | 0.7105 | 0.2895 | 0.2895 | |
| Luxi (LX) | 0.5172 (15) | 0.4138 (12) | 0.0690 (2) | 0.7241 | 0.2759 | 0.2759 | |
| Bohai Black (BH) | 0.6000 (18) | 0.2667 (8) | 0.1333 (4) | 0.7333 | 0.2667 | 0.2667 | |
| Jinnan (JN) | 0.5000 (6) | 0.2500 (3) | 0.2500 (3) | 0.6250 | 0.3750 | 0.3750 | |
| Qinchuan (QC) | 0.7500 (21) | 0.2143 (6) | 0.0357 (1) | 0.8571 | 0.1429 | 0.1429 | |
| 0.5811 (86) | 0.3378 (50) | 0.0811 (12) | 0.7500 | 0.2500 | 0.2500 | ||
| Southern group | Ji’an (JA) | 0.3529 (6) | 0.5294 (9) | 0.1176 (2) | 0.6176 | 0.3824 | 0.3824 |
| Jinjiang (JJ) | 0.4545 (5) | 0.5454 (6) | 0.0000 (0) | 0.7273 | 0.2727 | 0.2727 | |
| Wannan (WN) | 0.3333 (10) | 0.5000 (15) | 0.1667 (5) | 0.5833 | 0.4167 | 0.4167 | |
| Weining (GZWN) | 0.3667 (11) | 0.5667 (17) | 0.0667 (2) | 0.6500 | 0.3500 | 0.3500 | |
| Zaobei (ZB) | 0.6000 (6) | 0.4000 (4) | 0.0000 (0) | 0.8000 | 0.2000 | 0.2000 | |
| Dabeishan (DBS) | 0.6333 (19) | 0.3000 (9) | 0.0667 (2) | 0.7833 | 0.2167 | 0.2167 | |
| Bashan (BS) | 0.5227 (23) | 0.4545 (20) | 0.0227 (1) | 0.7500 | 0.2500 | 0.2500 | |
| Leiqiong (LQ) | 0.3061 (15) | 0.5306 (26) | 0.1633 (8) | 0.5714 | 0.4286 | 0.4286 | |
| Wengshan (WS) | 0.1000 (3) | 0.7000 (21) | 0.2000 (6) | 0.4107 | 0.5893 | 0.5893 | |
| Dianzhong (DZ) | 0.1333 (4) | 0.5333 (16) | 0.3333 (10) | 0.4000 | 0.6000 | 0.6000 | |
| Guangfeng (GF) | 0.7000 (7) | 0.3000 (3) | 0.0000 (0) | 0.8500 | 0.1500 | 0.3000 | |
| Sanjiang (SJ) | 0.5385 (14) | 0.4231 (11) | 0.0385 (1) | 0.7500 | 0.2500 | 0.2500 | |
| Wuling (WL) | 0.4923 (32) | 0.3538 (23) | 0.1538 (10) | 0.6692 | 0.3308 | 0.3308 | |
| Guanling (GL) | 0.5000 (15) | 0.3000 (9) | 0.2000 (6) | 0.6500 | 0.3500 | 0.3500 | |
| Wuchuan (WC) | 0.6207 (18) | 0.2414 (7) | 0.1379 (4) | 0.7414 | 0.2586 | 0.2586 | |
| Minnan (MN) | 0.4000 (6) | 0.6000 (9) | 0.0000 (0) | 0.7000 | 0.3000 | 0.3000 | |
| Longling (LL) | 0.3846 (5) | 0.5385 (7) | 0.0769 (1) | 0.6538 | 0.3462 | 0.3462 | |
| Weizhou (WZ) | 0.2500 (7) | 0.3929 (11) | 0.3571 (10) | 0.4464 | 0.5536 | 0.5536 | |
| Nandan (ND) | 0.4400 (11) | 0.4400 (11) | 0.1200 (3) | 0.6600 | 0.3400 | 0.3400 | |
| Diqing (DQ) | 0.8667 (26) | 0.1000 (3) | 0.0333 (1) | 0.9167 | 0.0833 | 0.0833 | |
| Yunnan Humped (DH) | 0.0000 (0) | 0.5263 (10) | 0.4737 (9) | 0.2632 | 0.7368 | 0.7368 | |
| Zhaotong (ZT) | 0.5625 (27) | 0.3333 (16) | 0.1042 (5) | 0.7292 | 0.2708 | 0.2708 | |
| Yunling (YL) | 0.2800 (7) | 0.6000 (15) | 0.1200 (3) | 0.5800 | 0.4200 | 0.4200 | |
| 0.4283 (275) | 0.4330 (278) | 0.1386 (89) | 0.6449 | 0.3551 | 0.3551 | ||
| Special | Tibetan (TB) | 1.0000 (29) | 0.0000 (0) | 0.0000 (0) | 1.0000 | 0.0000 | 0.0000 |
| Shigatse Humped (SH) | 0.40000 (12) | 0.4333 (13) | 0.1667 (5) | 0.6167 | 0.3833 | 0.3833 | |
| 0.6949 (41) | 0.2203 (13) | 0.0848 (5) | 0.8051 | 0.1949 | 0.1949 | ||
| Exotic | Over all | 0.5134 (498) | 0.3753 (364) | 0.1113 (108) | 0.7010 | 0.2990 | 0.2990 |
| Burma (MD) | 0.0667 (2) | 0.4333 (13) | 0.5000 (15) | 0.2833 | 0.7167 | 0.7167 | |
| Angus (AG) | 1.0000 (30) | 0.0000 (0) | 0.0000 (0) | 1.0000 | 0.0000 | 0.0000 | |
| SNP | Genotype (n) | T (°C) (LSM ± SE) | RH (%) (LSM ± SE) | THI (LSM ± SE) |
|---|---|---|---|---|
| MTOR :NC_037343.1:c.2062G>C | CC(108) | 16.06 A ± 0.54 | 74.43 A ± 0.99 | 60.19 A ± 0.76 |
| GC(364) | 15.12 A ± 0.29 | 72.89 A ± 0.59 | 59.07 A ± 0.41 | |
| GG(498) | 12.05 B ± 0.25 | 68.53 B ± 0.49 | 54.60 B ± 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Q.; Qu, K.; Hanif, Q.; Jia, Y.; Cheng, H.; Zhang, J.; Chen, N.; Chen, H.; Huang, B.; Lei, C. MTOR Variation Related to Heat Resistance of Chinese Cattle. Animals 2019, 9, 915. https://doi.org/10.3390/ani9110915
Ning Q, Qu K, Hanif Q, Jia Y, Cheng H, Zhang J, Chen N, Chen H, Huang B, Lei C. MTOR Variation Related to Heat Resistance of Chinese Cattle. Animals. 2019; 9(11):915. https://doi.org/10.3390/ani9110915
Chicago/Turabian StyleNing, Qingqing, Kaixing Qu, Quratulain Hanif, Yutang Jia, Haijian Cheng, Jicai Zhang, Ningbo Chen, Hong Chen, Bizhi Huang, and Chuzhao Lei. 2019. "MTOR Variation Related to Heat Resistance of Chinese Cattle" Animals 9, no. 11: 915. https://doi.org/10.3390/ani9110915
APA StyleNing, Q., Qu, K., Hanif, Q., Jia, Y., Cheng, H., Zhang, J., Chen, N., Chen, H., Huang, B., & Lei, C. (2019). MTOR Variation Related to Heat Resistance of Chinese Cattle. Animals, 9(11), 915. https://doi.org/10.3390/ani9110915





