Leptin Receptor Mediates Bmal1 Regulation of Estrogen Synthesis in Granulosa Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Welfare Assurance, and Management
2.2. Granulosa Cell Culture and Treatment
2.3. Transfection with siRNA
2.4. RNA Extraction and Quantitative Real-Time PCR Measurement
2.5. Western Blotting
2.6. Estradiol Assay
2.7. Statistical Analysis
3. Results
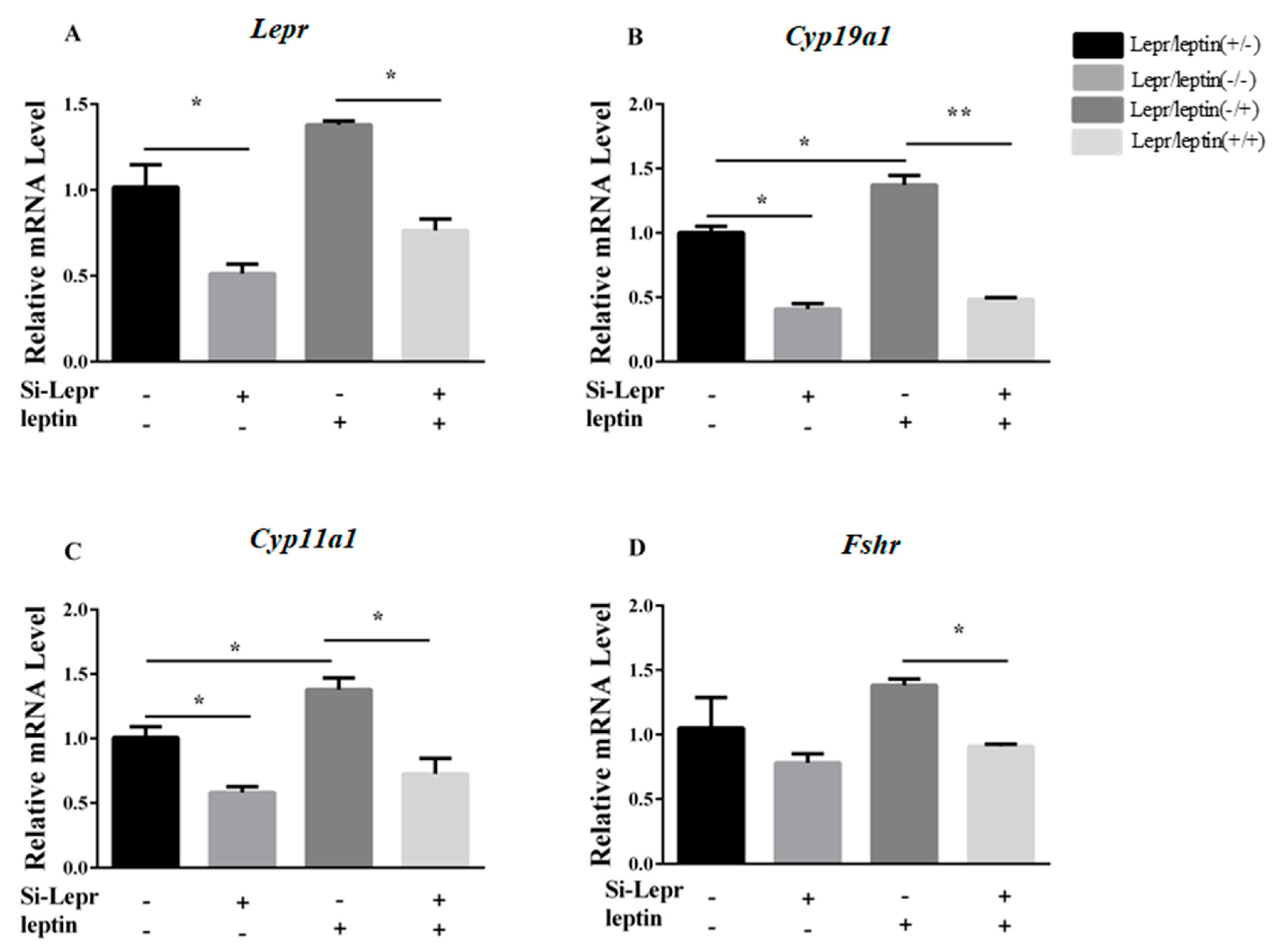
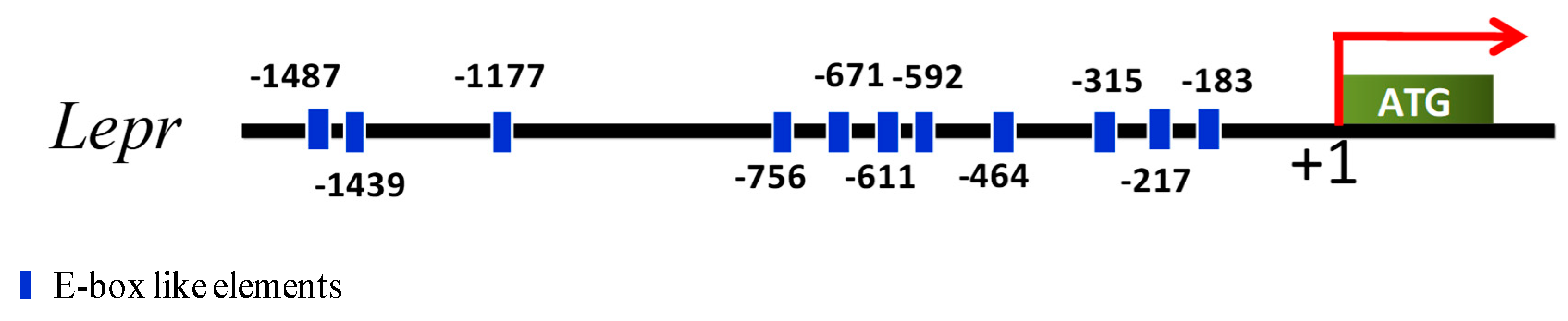
3.1. Leptin Function in Steroidogenesis-Related Genes
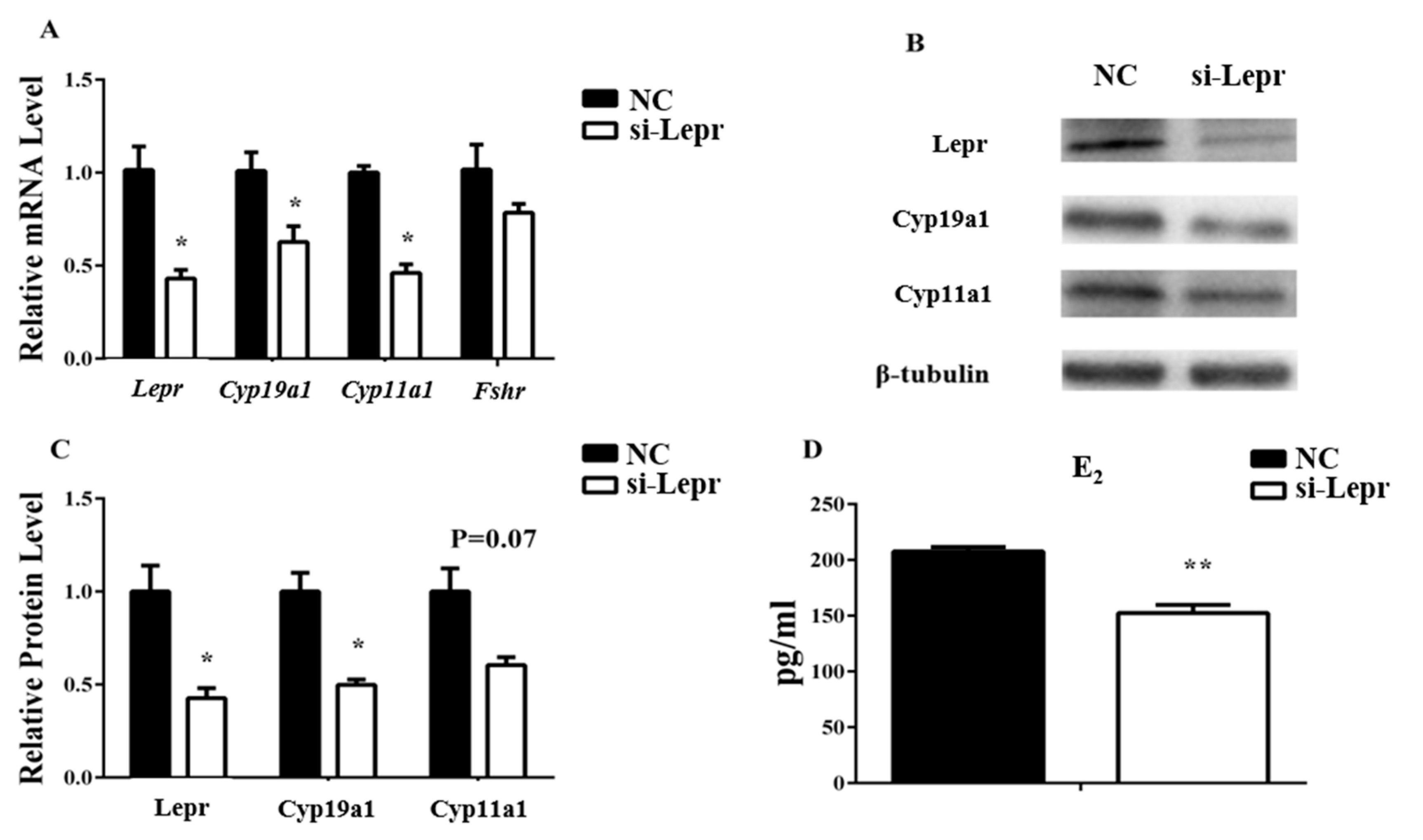
3.2. Lepr is Necessary for E2 Synthesis
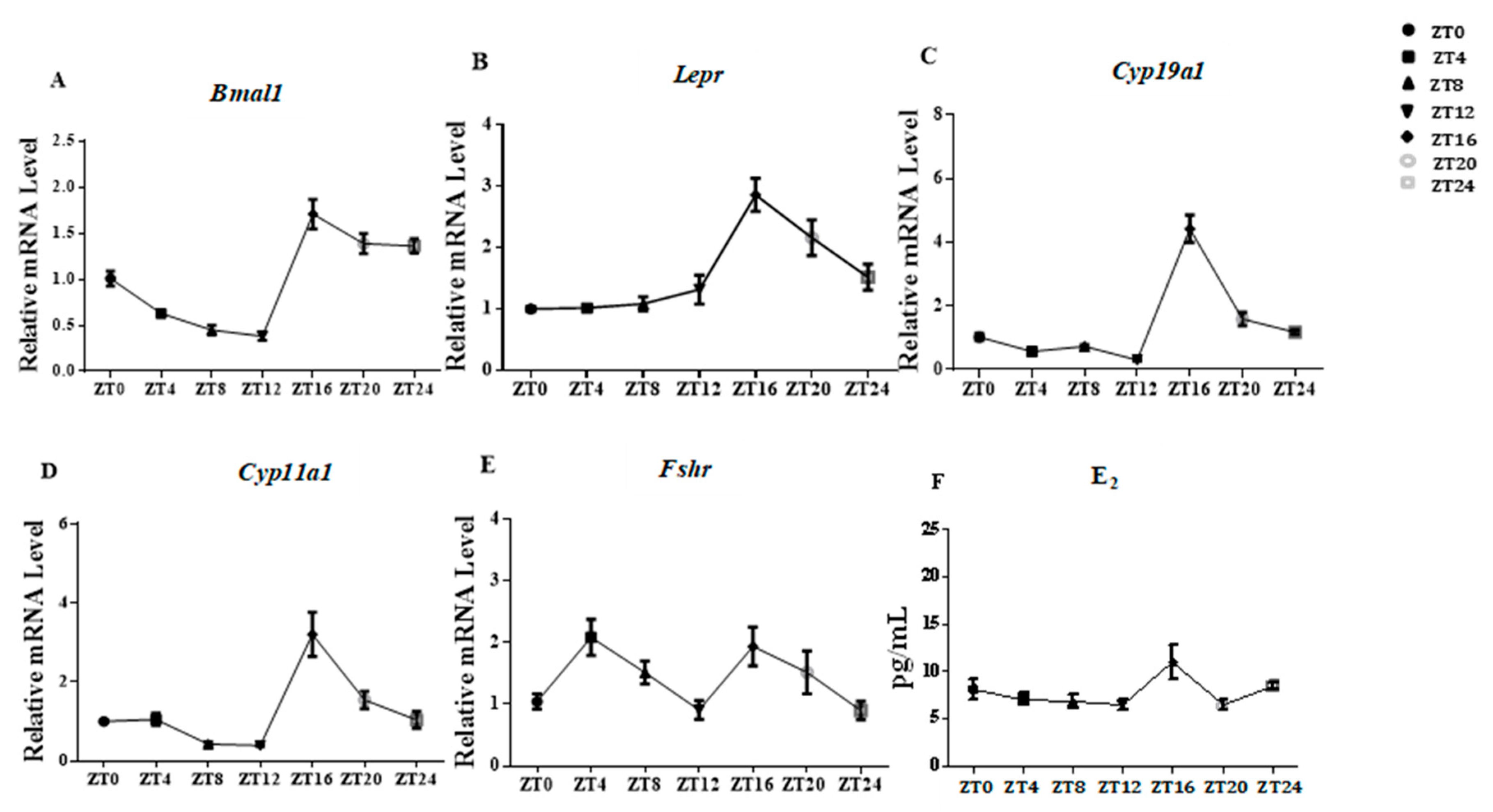
3.3. E2 Concentration in Serum and Lepr Expression in Ovaries Presented Evident Rhythm
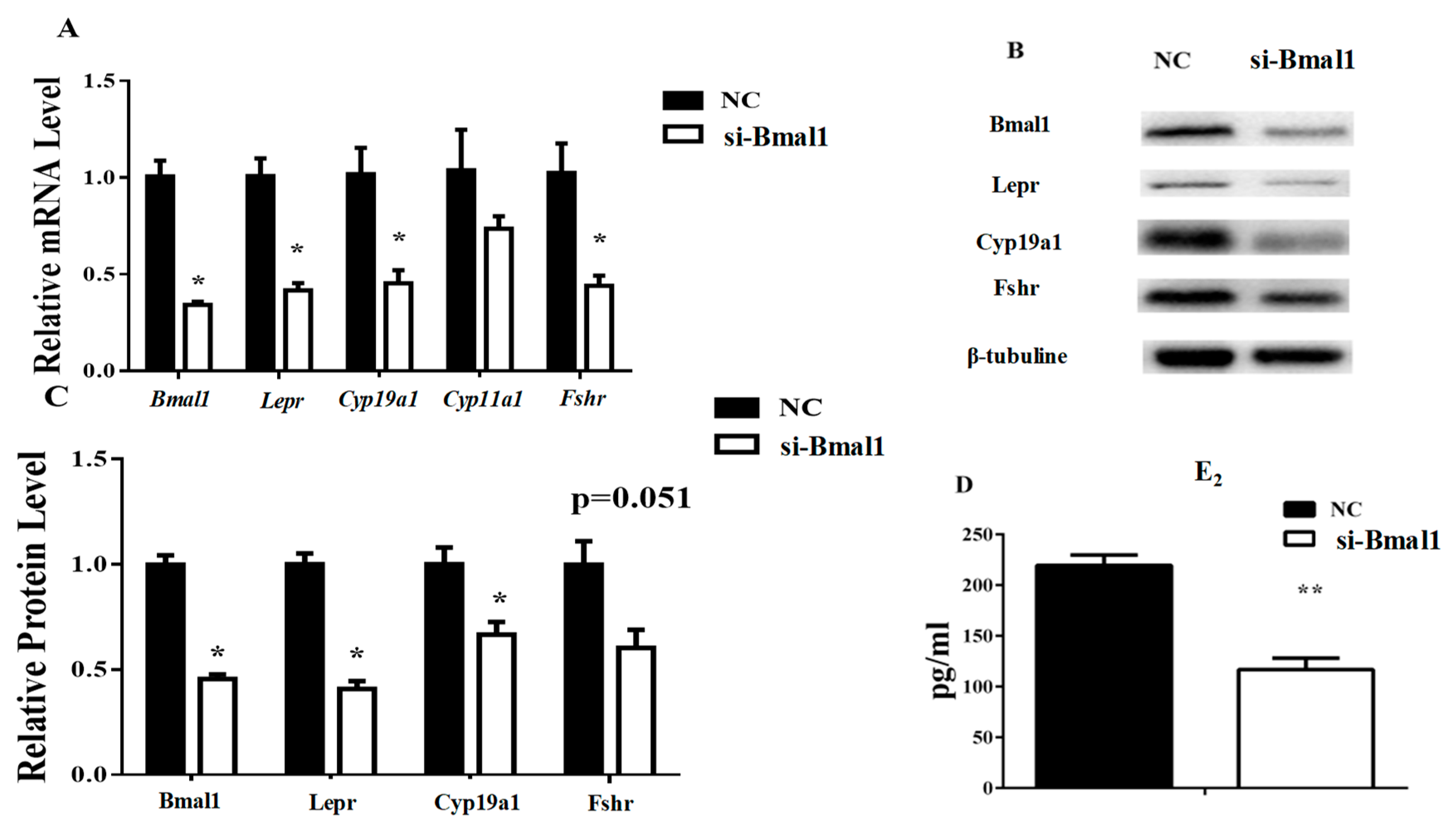
3.4. Bmal1 siRNA Repressed Leptin-Induced E2 Synthesis-Related Genes
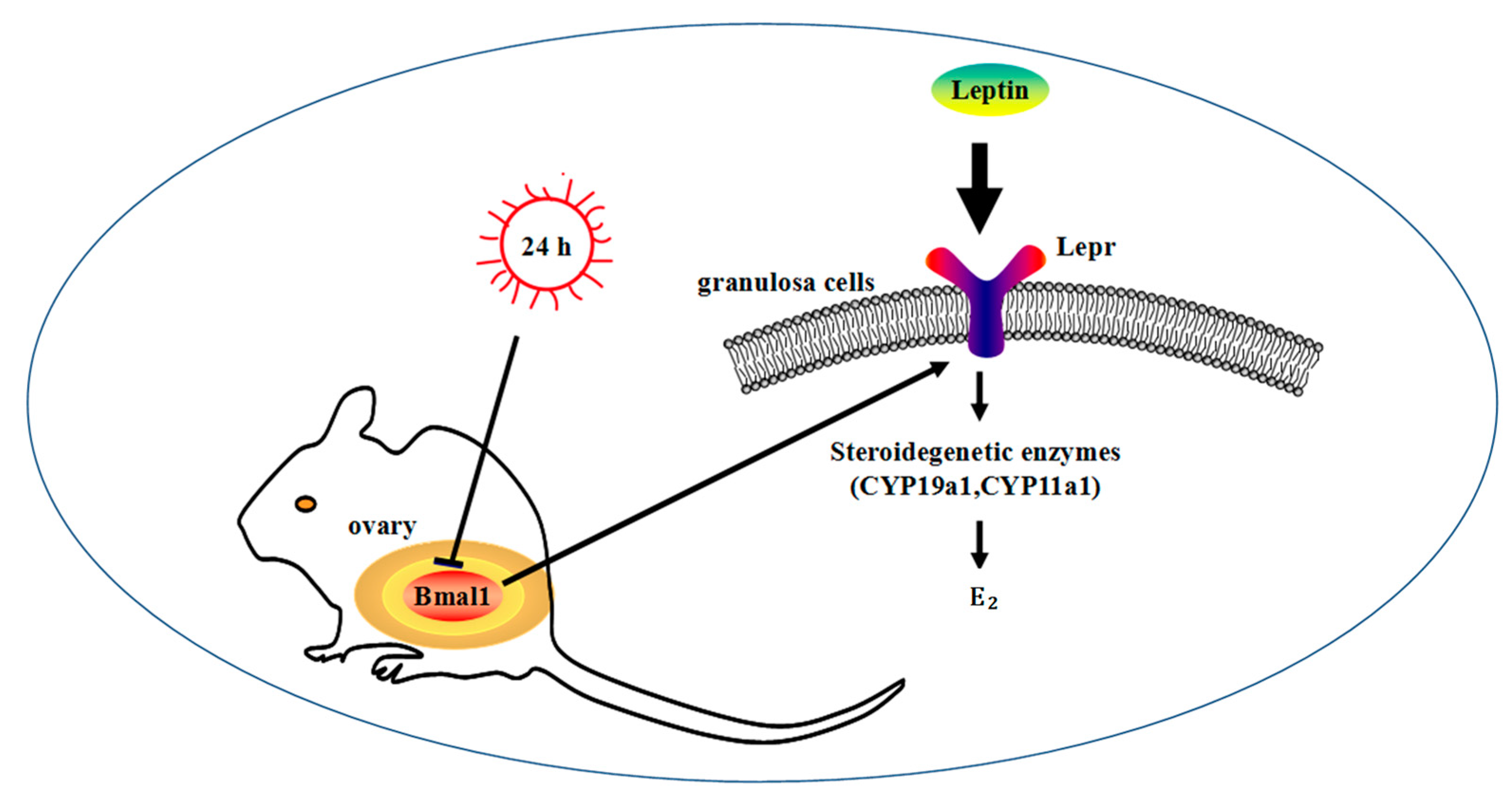
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sen, A.; Sellix, M.T. The Circadian Timing System and Environmental Circadian Disruption: From Follicles to Fertility. Endocrinology 2016, 157, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.G.; Castrucci, A.M.; Cipolla-Neto, J.; Poletini, M.O.; Mendez, N.; Richter, H.G.; Sellix, M.T. Environmental control of biological rhythms: Effects on development, fertility and metabolism. J. Neuroendocrinol. 2014, 26, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Hermann-Luibl, C.; Helfrich-Forster, C. Circadian light-input pathways in Drosophila. Commun. Integr. Biol. 2016, 9, e1102805. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.; Zhu, L.; Blum, I.D.; Mai, O.; Leliavski, A.; Fahrenkrug, J.; Oster, H.; Boehm, U.; Storch, K.F. Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology 2013, 154, 2924–2935. [Google Scholar] [CrossRef]
- Beymer, M.; Henningsen, J.; Bahougne, T.; Simonneaux, V. The role of kisspeptin and RFRP in the circadian control of female reproduction. Mol. Cell. Endocrinol. 2016, 438, 89–99. [Google Scholar] [CrossRef]
- Cardinet, G.H. Skeletal Muscle Function. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 407–440. [Google Scholar]
- Reinberg, A.; Bicakova-Rocher, A.; Nouguier, J.; Gorceix, A.; Mechkouri, M.; Touitou, Y.; Ashkenazi, I. Circadian rhythm period in reaction time to light signals: Difference between right- and left-hand side. Brain Res. Cogn. Brain Res. 1997, 6, 135–140. [Google Scholar] [CrossRef]
- Shub, Y.; Ashkenazi, I.E.; Reinberg, A. Differences between left-and right-hand reaction time rhythms: Indications of shifts in strategies of human brain activity. Cogn. Brain Res. 1997, 6, 141–146. [Google Scholar] [CrossRef]
- De Placido, G.; Alviggi, C.; Clarizia, R.; Mollo, A.; Alviggi, E.; Strina, I.; Fiore, E.; Wilding, M.; Pagano, T.; Matarese, G. Intra-follicular leptin concentration as a predictive factor for in vitro oocyte fertilization in assisted reproductive techniques. J. Endocrinol. Investig. 2006, 29, 719–726. [Google Scholar] [CrossRef]
- Elias, C.F.; Purohit, D. Leptin signaling and circuits in puberty and fertility. Cell. Mol. Life Sci. 2013, 70, 841–862. [Google Scholar] [CrossRef]
- Finn, P.D.; Cunningham, M.J.; Pau, K.Y.; Spies, H.G.; Clifton, D.K.; Steiner, R.A. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 1998, 139, 4652–4662. [Google Scholar] [CrossRef]
- Friedman, J.M. The function of leptin in nutrition, weight, and physiology. Nutr. Rev. 2002, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Hu, S.; Xiao, Q.; Han, C.; Gan, C.; Gou, H.; Liu, H.; Li, L.; Xu, H.; He, H.; et al. Leptin exerts proliferative and anti-apoptotic effects on goose granulosa cells through the PI3K/Akt/mTOR signaling pathway. J. Steroid Biochem. Mol. Biol. 2015, 149, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [PubMed]
- Mounzih, K.; Lu, R.; Chehab, F.F. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology 1997, 138, 1190–1193. [Google Scholar] [CrossRef]
- Craig, J.; Zhu, H.; Dyce, P.W.; Petrik, J.; Li, J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway. Endocrinology 2004, 145, 5355–5363. [Google Scholar] [CrossRef]
- Batista, A.M.; Silva, D.M.; Rego, M.J.; Silva, F.L.; Silva, E.C.; Beltrao, E.I.; Gomes Filho, M.A.; Wischral, A.; Guerra, M.M. The expression and localization of leptin and its receptor in goat ovarian follicles. Anim. Reprod. Sci. 2013, 141, 142–147. [Google Scholar] [CrossRef]
- Dupuis, L.; Schuermann, Y.; Cohen, T.; Siddappa, D.; Kalaiselvanraja, A.; Pansera, M.; Bordignon, V.; Duggavathi, R. Role of leptin receptors in granulosa cells during ovulation. Reprod. Camb. Engl. 2014, 147, 221–229. [Google Scholar] [CrossRef]
- Bjorbaek, C.; Uotani, S.; da Silva, B.; Flier, J.S. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J. Biol. Chem. 1997, 272, 32686–32695. [Google Scholar] [CrossRef]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Harrath, A.H.; Ostrup, O.; Rafay, J.; Konickova Florkovicova, I.; Laurincik, J.; Sirotkin, A.V. Metabolic state defines the response of rabbit ovarian cells to leptin. Reprod. Biol. 2017, 17, 19–24. [Google Scholar] [CrossRef]
- Hu, S.; Gan, C.; Wen, R.; Xiao, Q.; Gou, H.; Liu, H.; Zhang, Y.; Li, L.; Wang, J. Role of leptin in the regulation of sterol/steroid biosynthesis in goose granulosa cells. Theriogenology 2014, 82, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Chu, G.; Kito, G.; Yamauchi, N.; Shigeyoshi, Y.; Hashimoto, S.; Hattori, M.A. FSH induces the development of circadian clockwork in rat granulosa cells via a gap junction protein Cx43-dependent pathway. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yin, L.; Bai, L.; Ma, G.; Zhao, C.; Xiang, A.; Pang, W.; Yang, G.; Chu, G. Bmal1 interference impairs hormone synthesis and promotes apoptosis in porcine granulosa cells. Theriogenology 2017, 99, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mereness, A.L.; Murphy, Z.C.; Forrestel, A.C.; Butler, S.; Ko, C.; Richards, J.S.; Sellix, M.T. Conditional Deletion of Bmal1 in Ovarian Theca Cells Disrupts Ovulation in Female Mice. Endocrinology 2016, 157, 913–927. [Google Scholar] [CrossRef]
- Zachow, R.J.; Magoffin, D.A. Direct intraovarian effects of leptin: Impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 beta production by rat ovarian granulosa cells. Endocrinology 1997, 138, 847–850. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, B.P.; Shen, A.L.; Wallisser, J.A.; Krentz, K.J.; Moran, S.M.; Sullivan, R.; Glover, E.; Parlow, A.F.; Drinkwater, N.R.; et al. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl. Acad. Sci. USA 2014, 111, 14295–14300. [Google Scholar] [CrossRef]


| Gene | Accession No. | Primer Sequence 5′-3′ | Length | Tm/°C |
|---|---|---|---|---|
| Bmal1 | NM_001357070.1 | F: ACAGTCAGATTGAAAAGAGGCG R: GCCATCCTTAGCACGGTGAG | 124 | 60 |
| Lepr | NM_010704.2 | F: ACCTGGCATATCCAATCTCTCC R: TTCAAAGCCGAGGCATTGTTT | 115 | 60 |
| Cyp11a1 | NM_001346787.1 | F: GGGCAGTTTGGAGTCAGTTTAC R: TTTAGGACGATTCGGTCTTTCTT | 186 | 60 |
| Cyp19a1 | NM_001348171.1 | F: AACCCCATGCAGTATAATGTCAC R: AGGACCTGGTATTGAAGACGAG | 132 | 60 |
| Fshr | NM_013523.3 | F: TGCTCTAACAGGGTCTTCCTC R: TCTCAGTTCAATGGCGTTCCG | 84 | 60 |
| Gapdh | NM_017321385.1 | F: TGCTGAGTATGTCGTGGAGTCT R: ATGCATTGCTGACAATCTTGAG | 179 | 60 |
| Gene | Accession No. | Primer Sequence 5′-3′ | Length | Tm/°C |
|---|---|---|---|---|
| Bmal1 | NM_024362.2 | F: ACAGTCAGATTGAAAAGAGGCG R: GCCATCCTTAGCACGGTGAG | 124 | 60 |
| Lepr | NM_012596.1 | F: CCCACAATGGGACATGGTCA R: GCACCGATGGAATTGATGGC | 109 | 60 |
| Cyp11a1 | NM_017286.3 | F: CAGACGCATCAAGCAGCAAA R: GGTCCACGATCTCCTCCAAC | 134 | 60 |
| Cyp19a1 | NM_017085.2 | F: AGAGACGTGGAGACCTGACA R: CCTCCGGATACTCTGCGATG | 126 | 60 |
| Fshr | NM_199237.1 | F: ATTCTTGGGCACGGGATCTG R: CGGTCGGAATCTCTGTCACC | 98 | 60 |
| Gapdh | NM_017008.4 | F: AAGGTCGGTGTGAACGGATT R: CTTTGTCACAAGAGAAGGCAGC | 70 | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, G.; Ma, G.; Sun, J.; Zhu, Y.; Xiang, A.; Yang, G.; Sun, S. Leptin Receptor Mediates Bmal1 Regulation of Estrogen Synthesis in Granulosa Cells. Animals 2019, 9, 899. https://doi.org/10.3390/ani9110899
Chu G, Ma G, Sun J, Zhu Y, Xiang A, Yang G, Sun S. Leptin Receptor Mediates Bmal1 Regulation of Estrogen Synthesis in Granulosa Cells. Animals. 2019; 9(11):899. https://doi.org/10.3390/ani9110899
Chicago/Turabian StyleChu, Guiyan, Guangjun Ma, Jingchun Sun, Youbo Zhu, Aoqi Xiang, Gongshe Yang, and Shiduo Sun. 2019. "Leptin Receptor Mediates Bmal1 Regulation of Estrogen Synthesis in Granulosa Cells" Animals 9, no. 11: 899. https://doi.org/10.3390/ani9110899
APA StyleChu, G., Ma, G., Sun, J., Zhu, Y., Xiang, A., Yang, G., & Sun, S. (2019). Leptin Receptor Mediates Bmal1 Regulation of Estrogen Synthesis in Granulosa Cells. Animals, 9(11), 899. https://doi.org/10.3390/ani9110899





