Nitroethanol in Comparison with Monensin Exhibits Greater Feed Efficiency Through Inhibiting Rumen Methanogenesis More Efficiently and Persistently in Feedlotting Lambs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals, Diet Treatment and Sample Collection
2.3. Analytics of CH4 Producing Activity
2.4. Chemical Analyses
2.5. Calculation
2.6. Statistical Analysis
3. Results
3.1. Effect of Monensin and NEOH on Nutrient Digestibility
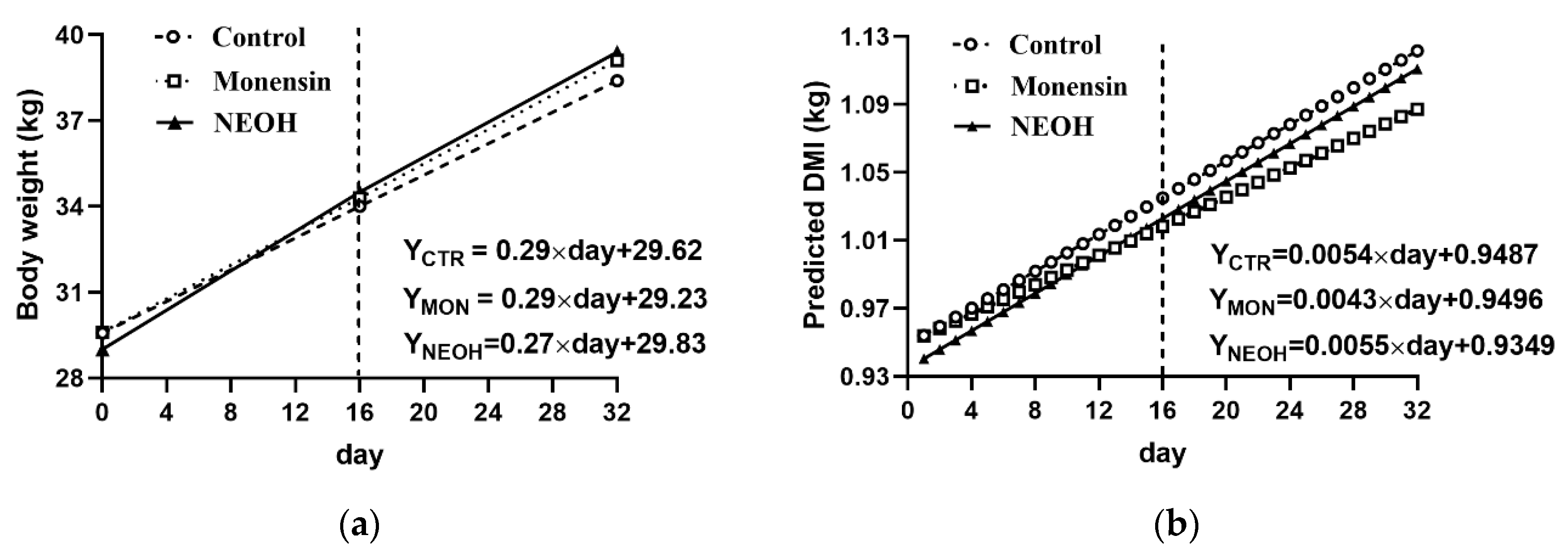
3.2. Effect of Monensin and NEOH on Feed Intake and Growth Performance
3.3. Effect of Monensin and NEOH on Ruminal Fermentation
3.4. Effect of Monensin and NEOH on CH4 Emissions and In Vitro CH4 Producing Activity
4. Discussion
4.1. Effect of NEOH in Comparison with Monensin on Feed Intake, Growth Performance and Diet Digestibility
4.2. Effect of Monensin and NEOH on Ruminal Fermentation Profiles
4.3. Effect of Monensin and NEOH on CH4 Emissions
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Rasmussen, M.A. Use of a novel nitrotoxin–metabolizing bacterium to reduce ruminal methane production. Bioresour. Technol. 1998, 64, 89–95. [Google Scholar] [CrossRef]
- Anderson, R.C.; Callaway, T.R.; Kessel, J.A.S.V.; Yong, S.J.; Edrington, T.S.; Nisbet, D.J. Effect of select nitrocompounds on ruminal fermentation; an initial look at their potential to reduce economic and environmental costs associated with ruminal methanogenesis. Bioresour. Technol. 2003, 90, 59–63. [Google Scholar] [CrossRef]
- Anderson, R.C.; Krueger, N.A.; Stanton, T.B.; Callaway, T.R.; Edrington, T.S.; Harvey, R.B.; Jung, Y.S.; Nisbet, D.J. Effects of select nitrocompounds on in vitro ruminal fermentation during conditions of limiting or excess added reductant. Bioresour. Technol. 2008, 99, 8655–8661. [Google Scholar] [CrossRef] [PubMed]
- Božic, A.K.; Anderson, R.C.; Carstens, G.E.; Ricke, S.C.; Callaway, T.R.; Yokoyama, M.T.; Wang, J.K.; Nisbet, D.J. Effects of the methane–inhibitors nitrate, nitroethane, lauric acid, Lauricidin and the Hawaiian marine algae. Bioresour. Technol. 2009, 99, 4017–4025. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Bañuelos, H.; Anderson, R.C.; Carstens, G.E.; Tedeschi, L.O.; Pinchak, W.E.; Elisa, C.D.; Krueger, N.A.; Callaway, T.R.; Nisbet, D.J. Effects of nitroethane and monensin on ruminal fluid fermentation characteristics and nitrocompound–metabolizing bacterial populations. J. Agric. Food Chem. 2008, 56, 4650–4658. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Carstens, G.E.; Miller, R.K.; Callaway, T.R.; Schultz, C.L.; Edrington, T.S.; Harvey, R.B.; Nisbet, D.J. Effect of oral nitroethane and 2–nitropropanol administration on methane–producing activity and volatile fatty acid production in the ovine rumen. Bioresour. Technol. 2006, 97, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez–Bañuelos, H.; Anderson, R.C.; Carstens, G.E.; Slay, L.J.; Ramlachan, N.; Horrocks, S.M.; Callaway, T.R.; Edrington, T.S.; Nisbet, D.J. Zoonotic bacterial populations, gut fermentation characteristics and methane production in feedlot steers during oral nitroethane treatment and after the feeding of an experimental chlorate product. Anaerobe 2007, 13, 21–31. [Google Scholar]
- Zhang, Z.W.; Wang, Y.L.; Wang, W.K.; Cao, Z.J.; Li, S.L.; Yang, H.J. The inhibitory action mode of nitrocompounds on in vitro rumen methanogenesis: A comparison of nitroethane, 2–nitroethanl and 2–nitro–1–propanol. J. Agric. Sci. 2019, in press. [Google Scholar]
- Duffield, T.F.; Rabiee, A.R.; Lean, I.J. A Meta–Analysis of the Impact of Monensin in Lactating Dairy Cattle. Part 2. Production Effects. J. Dairy Sci. 2008, 91, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F.; Merrill, J.K.; Bagg, R.N. Meta–analysis of the effects of monensin in beef cattle on feed efficiency, body weight gain, and dry matter intake. J. Anim. Sci. 2012, 90, 4583–4592. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G.; Bates, D.B. Ionophores: Their effect on production efficiency and mode of action. J. Anim. Sci 1984, 58, 1465–1483. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T.F.; Rabiee, A.R.; Lean, I.J. A meta–analysis of the impact of monensin in lactating dairy cattle. Part 3. Health and reproduction. J. Dairy Sci. 2008, 91, 2328–2341. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B.; Strobel, H.J. Effect of ionophores on ruminal fermentation. Appl. Environ. Microbiol. 1989, 55, 1–6. [Google Scholar] [PubMed]
- Huntington, G.B. Energy metabolism in the digestive tract and liver of cattle: Influence of physiological state and nutrition. Rep. Nutr. Dev. 1990, 30, 35–47. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.O.; O’Mara, F.; Mcallister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Hashem, N.M.; Morsy, A.S.; El–Azrak, K.M.; Sallam, S.M. Comparative effects of Moringa oleifera root bark and monensin supplementations on ruminal fermentation, nutrient digestibility and growth performance of growing lambs. Anim. Feed Sci. Technol. 2018, 235, 189–201. [Google Scholar] [CrossRef]
- Verdouw, H.; Echteld, C.J.A.V.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Makkar, H.P.; Sharma, O.P.; Dawra, R.K.; Negi, S.S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Yang, H.J.; Zhuang, H.; Meng, X.K.; Zhang, D.F.; Cao, B.H. Effect of melamine on in vitro rumen microbial growth, methane production and fermentation of Chinese wild rye hay and maize meal in binary mixtures. J. Agric. Sci. 2014, 152, 686–696. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained by chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 47–55. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; Mcallan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Soest, P.J.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Keulen, J.V.; Young, B.A. Evaluation of acid–insoluble ash as natural marker in ruminant digestibility. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J.; Agabriel, J.; Givens, I. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Patra, A.K.; Lalhriatpuii, M.; Debnath, B.C. Predicting enteric methane emission in sheep using linear and non-linear statistical models from dietary variables. Anim. Prod. Sci. 2016, 56, 574–584. [Google Scholar] [CrossRef]
- Benaouda, M.; Martin, C.; Li, X.; Kebreab, E.; Hristov, A.N.; Yu, Z.T.; Yáñez-Ruiz, D.R.; Reynolds, C.K.; Crompton, L.A.; Dijkstra, J.; et al. Evaluation of the performance of existing mathematical models predicting enteric methane emissions from ruminants: Animal categories and dietary mitigation strategies. Anim. Feed Sci. Technol. 2019, 255, 1–20. [Google Scholar] [CrossRef]
- Capelari, M.; Johnson, K.A.; Latack, B.; Roth, J.; Powers, W. The effect of encapsulated nitrate and monensin on ruminal fermentation using a semi–continuous culture system. J. Anim. Sci. 2018, 96, 3446–3459. [Google Scholar] [CrossRef]
- Gupta, S.; Mohini, M.; Malla, B.A.; Mondal, G.; Pandita, S. Effects of monensin feeding on performance, nutrient utilisation and enteric methane production in growing buffalo heifers. Trop. Anim. Health Prod. 2019, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aderinboye, R.Y.; Onwuka, C.F.; Arigbede, O.M.; Oduguwa, O.O.; Aina, A.B. Effect of dietary monensin inclusion on performance, nutrient utilisation, rumen volatile fatty acid concentration and blood status of West African dwarf bucks fed with basal diets of forages. Trop. Anim. Health Prod. 2012, 44, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Duynisveld, J.L.; Charmley, E. Effects of monensin and increasing dose levels of a mixture of essential oil compounds on intake, digestion and growth performance of beef cattle. Can. J. Anim. Sci. 2006, 86, 91–96. [Google Scholar]
- Owens, F.N. Ionophore effects on utilization and metabolism of nutrientes. In Proceedings of the Georgia Nutrition Conference for the Feed Industry, Athens, GA, USA, 13–15 February 1980; p. 17. [Google Scholar]
- Deswysen, A.G.; Ellis, W.C.; Pond, K.R.; Jenkins, W.L.; Connelly, J. Effects of monensin on voluntary intake, eating and ruminating behavior and ruminal motility in heifers fed corn silage. J. Anim. Sci. 1987, 64, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.E.; Chester–Jones, H.; Ziegler, D.; Clapper, J.A.; Erickson, P.S. Effects of cinnamaldehyde or monensin on performance of weaned Holstein dairy heifers. J. Dairy Sci. 2017, 100, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, J.O.P.I.; Paulino, P.V.R.; Edenio, D.; Sebasti O Campos, V.F.; Valadares, R.D.F.; Márcio Souza, D. Effects of Saccharomyces cerevisiae and monensin on digestion, ruminal parameters, and balance of nitrogenous compounds of beef cattle fed diets with different starch concentrations. Trop. Anim. Health Prod. 2013, 45, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Mcguffey, R.K.; Richardson, L.F.; Wilkinson, J.I.D. Ionophores for Dairy Cattle: Current Status and Future Outlook. J. Dairy Sci. 2001, 84, 194–203. [Google Scholar] [CrossRef]
- Thomas, M.; Webb, M.; Ghimire, S.; Blair, A.; Olson, K.; Fenske, G.J.; Fonder, A.T.; Christopherhennings, J.; Brake, D.; Scaria, J. Metagenomic characterization of the effect of feed additives on the gut microbiome and antibiotic resistome of feedlot cattle. Sci. Rep. 2017, 7, 12257. [Google Scholar] [CrossRef] [PubMed]
- Schären, M.; Drong, C.; Kiri, K.; Riede, S.; Gardener, M.; Meyer, U.; Hummel, J.; Urich, T.; Breves, G.; Dänicke, S. Differential effects of monensin and a blend of essential oils on rumen microbiota composition of transition dairy cows. J. Dairy Sci. 2017, 100, 2765–2783. [Google Scholar] [CrossRef]
- Benchaar, C. Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows: An international journal of animal bioscience. Anim. Int. J. Anim. Biosci. 2016, 10, 418–425. [Google Scholar] [CrossRef]
- Hemphill, C.N.; Wickersham, T.A.; Sawyer, J.E.; Brown–Brandl, T.M.; Freetly, H.C.; Hales, K.E. Effects of feeding monensin to bred heifers fed in a drylot on nutrient and energy balance. J. Anim. Sci. 2018, 96, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Martin, A.; Duffield, T.; Bagg, R.; Dick, P.; Mcbride, B.W. Effect of a Prepartum Administration of Monensin in a Controlled–Release Capsule on Apparent Digestibilities and Nitrogen Utilization in Transition Dairy Cows. J. Dairy Sci. 2000, 83, 2918–2925. [Google Scholar] [CrossRef]
- Ipharraguerre, I.R.; Clark, J.H. Usefulness of ionophores for lactating dairy cows: A review. Anim. Feed Sci. Technol. 2003, 106, 39–57. [Google Scholar] [CrossRef]
- Cochran, R.C.; Vanzant, E.S.; Riley, J.G.; Owensby, C.E. Influence of intraruminal monensin administration on performance and forage use in beef cattle grazing early–summer bluestem range. J. Prod. Agric. 1990, 3, 88–92. [Google Scholar] [CrossRef]
- Fredrickson, E.L.; Galyean, M.L.; Branine, M.E.; Sowell, B.; Wallace, J.D. Influence of Ruminally Dispensed Monensin and Forage Maturity on Intake and Digestion. J. Range Manag. 1993, 46, 214–220. [Google Scholar] [CrossRef]
- Rogers, M.; Jouany, J.P.; Thivend, P.; Fontenot, J.P. The effects of short–term and long–term monensin supplementation, and its subsequent withdrawal on digestion in sheep. Anim. Feed Sci. Technol. 1997, 65, 113–127. [Google Scholar] [CrossRef]
- Salles, M.S.V.; Zanetti, M.A.; Salles, F.A. Effect of monensin on mineral balance in growing ruminants reared under different environmental temperatures. Anim. Feed Sci. Technol. 2008, 141, 233–245. [Google Scholar] [CrossRef]
- Khorrami, B.; Vakili, A.R.; Mesgaran, M.D.; Klevenhusen, F. Thyme and cinnamon essential oils: Potential alternatives for monensin as a rumen modifier in beef production systems. Anim. Feed Sci. Technol. 2015, 200, 8–16. [Google Scholar] [CrossRef]
- Lemos, B.J.; Castro, F.G.; Santos, L.S.; Mendonça, B.P.; Couto, V.R.; Fernandes, J.J. Monensin, virginiamycin, and flavomycin in a no–roughage finishing diet fed to zebu cattle. J. Anim. Sci. 2016, 94, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Ren, H.; Liu, S.M.; Cai, C.J.; Han, J.T.; Li, F.; Yao, J.H. Dynamics of methanogenesis, ruminal fermentation, and alfalfa degradation during adaptation to monensin supplementation in goats. J. Dairy Sci. 2018, 101, 1048–1059. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; Mcsweeney, C.S.; Elliott, R. Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Anim. Feed Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- Wang, Z.B.; Xin, H.S.; Bao, J.; Duan, C.Y.; Chen, Y.; Qu, Y.L. Effects of hainanmycin or monensin supplementation on ruminal protein metabolism and populations of proteolytic bacteria in Holstein heifers. Anim. Feed Sci. Technol. 2015, 201, 99–103. [Google Scholar] [CrossRef]
- Russell, J.B.; Houlihan, A.J. Ionophore resistance of ruminal bacteria and its potential impact on human health. FEMS Microbiol. Rev. 2003, 27, 65–74. [Google Scholar] [CrossRef]
- Saengkerdsub, S.; Kim, W.K.; Anderson, R.C.; Nisbet, D.J.; Ricke, S.C. Effects of nitrocompounds and feedstuffs on in vitro methane production in chicken cecal contents and rumen fluid. Anaerobe 2006, 12, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J. Dairy Sci. 1998, 81, 3222–3230. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Shifts in metabolic hydrogen sinks in the methanogenesis–inhibited ruminal fermentation: A meta–analysis. Front. Microbiol. 2015, 6, 538. [Google Scholar] [CrossRef]
- Guan, H.; Wittenberg, K.M.; Ominski, K.H.; Krause, D.O. Efficacy of ionophores in cattle diets for mitigation of enteric methane. J. Anim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef]
- Eckard, R.J.; Grainger, C.; Klein, C.A.M.D. Options for the abatement of methane and nitrous oxide from ruminant production: A review. Livest. Sci. 2010, 130, 47–56. [Google Scholar] [CrossRef]
| Item | Basal Diet |
|---|---|
| Ingredients (g/kg, as fed basis) | |
| Corn silage | 600 |
| Peanut vine | 100 |
| Corn meal | 109.5 |
| Wheat bran | 30 |
| Soybean meal | 150 |
| Limestone | 3 |
| Sodium bicarbonate | 1.5 |
| Salt | 3 |
| Premix 1 | 3 |
| Nutrient level (g/kg, as Dry Matter) 2 | |
| Organic matter | 944 |
| Crude protein | 163 |
| Ether extract | 22 |
| Neutral detergent fiber | 360 |
| Acid detergent fiber | 223 |
| Calcium | 5.5 |
| Phosphorus | 4.5 |
| Gross energy (MJ/kg, as Dry Matter) | 15.19 |
| Digestibility (g/kg) | Treatments | SEM | p–Value | ||||
|---|---|---|---|---|---|---|---|
| Control | Monensin | NEOH | Diet | Period | Diet × Period | ||
| Dry matter | |||||||
| Period 1 | 780 A | 768 A | 771 A | 8.0 | 0.08 | <0.01 | 0.46 |
| Period 2 | 716 B | 701 B | 688 B | ||||
| Organic matter | |||||||
| Period 1 | 800 A | 788 A | 792 A | 8.1 | 0.10 | <0.01 | 0.43 |
| Period 2 | 735 B | 723 B | 708 B | ||||
| Crude protein | |||||||
| Period 1 | 777 A | 767 A | 765 A | 8.1 | 0.02 | <0.01 | 0.25 |
| Period 2 | 714 Ba | 678 Bb | 696 Bab | ||||
| Neutral detergent fibre | |||||||
| Period 1 | 651 A | 632 A | 635 A | 17.1 | 0.14 | <0.01 | 0.33 |
| Period 2 | 583 B | 576 B | 529 B | ||||
| Acid detergent fibre | |||||||
| Period 1 | 647 A | 613 A | 629 A | 15.9 | 0.03 | <0.01 | 0.08 |
| Period 2 | 593 Ba | 575 Ba | 519 Bb | ||||
| Item | Treatments | SEM | p–Value | ||||
|---|---|---|---|---|---|---|---|
| Control | Monensin | NEOH | Diet | Period | Diet × Period | ||
| Initial BW (kg) | 29.58 | 29.61 | 29.57 | 0.73 | 0.99 | NA | NA |
| Final BW (kg) | |||||||
| Period 1 | 34.0 B | 34.3 B | 34.5 B | 0.79 | 0.66 | <0.01 | 0.88 |
| Period 2 | 38.4 A | 39.1 A | 39.4 A | ||||
| DMI (g/day) | |||||||
| Period 1 | 1012 B | 1004 B | 996 B | 9.2 | 0.08 | <0.01 | 0.13 |
| Period 2 | 1100 A | 1065 A | 1093 A | ||||
| ADG (g) | |||||||
| Period 1 | 272 c | 293 Bb | 310 a | 2.1 | <0.01 | 0.17 | 0.06 |
| Period 2 | 273 b | 301 Aa | 309 a | ||||
| FCR | |||||||
| Period 1 | 0.27 Ac | 0.29 b | 0.31 Aa | 0.003 | <0.01 | <0.01 | 0.01 |
| Period 2 | 0.25 Bb | 0.29 a | 0.29 Ba | ||||
| Item | Treatments | SEM | p–Value | ||||
|---|---|---|---|---|---|---|---|
| Control | Monensin | NEOH | Diet | Period | Diet × Period | ||
| pH | |||||||
| Period 1 | 6.05 Bb | 6.39 a | 6.26 Ba | 0.047 | <0.01 | <0.01 | <0.01 |
| Period 2 | 6.29 Ab | 6.26 b | 6.56 Aa | ||||
| NH3N, g/L | |||||||
| Period 1 | 35.5 a | 33.6 a | 20.9 Bb | 0.96 | <0.01 | 0.03 | 0.09 |
| Period 2 | 34.8 a | 34.8 a | 27.7 Ab | ||||
| MCP, mg/mL | |||||||
| Period 1 | 0.59 Ab | 0.60 Ab | 0.66 Aa | 0.024 | 0.09 | <0.01 | 0.34 |
| Period 2 | 0.43 B | 0.45 B | 0.44 B | ||||
| Total VFA, mmol/L | |||||||
| Period 1 | 121.6 a | 115.0 Ba | 100.4 Bb | 2.18 | <0.01 | <0.01 | <0.01 |
| Period 2 | 123.1 a | 125.1 Aa | 114.6 Ab | ||||
| VFA patterns (% molar) | |||||||
| Acetate | |||||||
| Period 1 | 59.7 Bb | 60.4 b | 62.7 a | 0.56 | <0.01 | 0.01 | 0.04 |
| Period 2 | 62.5 A | 61.4 | 62.6 | ||||
| Propionate | |||||||
| Period 1 | 20.1 b | 22.4 Aa | 19.5 b | 0.48 | <0.01 | <0.01 | 0.01 |
| Period 2 | 19.2 | 18.9 B | 18.4 | ||||
| Butyrate | |||||||
| Period 1 | 13.9 a | 10.8 Bb | 11.7 Bb | 0.45 | 0.04 | <0.01 | <0.01 |
| Period 2 | 13.3 | 14.4 A | 13.3 A | ||||
| BCVFA | |||||||
| Period 1 | 5.1 A | 5.2 A | 5.1 | 0.17 | 0.22 | <0.01 | 0.06 |
| Period 2 | 4.5 B | 4.6 B | 4.7 | ||||
| Acetate: Propionate ratio | |||||||
| Period 1 | 3.0 a | 2.7 Bb | 3.3 a | 0.09 | <0.01 | <0.01 | 0.08 |
| Period 2 | 3.3 | 3.3 A | 3.4 | ||||
| Item | Treatments | SEM | p–Value | ||||
|---|---|---|---|---|---|---|---|
| Control | Monensin | NEOH | Diet | Period | Diet × Period | ||
| Methane, mmol/L 1 | |||||||
| Period 1 | 25.8 a | 19.6 Bb | 19.0 Bb | 0.59 | <0.01 | <0.01 | <0.01 |
| Period 2 | 26.9 a | 26.2 Aa | 24.2 Ab | ||||
| Methane, L/day 2 | |||||||
| Period 1 | 12.3 B | 12.2 B | 12.2 B | 0.09 | 0.08 | <0.01 | 0.13 |
| Period 2 | 13.2 A | 12.8 A | 13.1 A | ||||
| Methane, L/kg ADG 2 | |||||||
| Period 1 | 45.8 Ba | 42.0 b | 39.3 Bc | 0.44 | <0.01 | <0.01 | 0.01 |
| Period 2 | 48.4 Aa | 42.6 b | 42.7 Ab | ||||
| In vitro CH4 producing activity 3 | |||||||
| Period 1 | 11.8 Aa | 10.3 b | 8.2 Bc | 0.15 | <0.01 | <0.01 | <0.01 |
| Period 2 | 11.1 Ba | 10.1 b | 9.2 Ac | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-W.; Wang, Y.-L.; Chen, Y.-Y.; Wang, W.-K.; Zhang, L.-T.; Luo, H.-L.; Yang, H.-J. Nitroethanol in Comparison with Monensin Exhibits Greater Feed Efficiency Through Inhibiting Rumen Methanogenesis More Efficiently and Persistently in Feedlotting Lambs. Animals 2019, 9, 784. https://doi.org/10.3390/ani9100784
Zhang Z-W, Wang Y-L, Chen Y-Y, Wang W-K, Zhang L-T, Luo H-L, Yang H-J. Nitroethanol in Comparison with Monensin Exhibits Greater Feed Efficiency Through Inhibiting Rumen Methanogenesis More Efficiently and Persistently in Feedlotting Lambs. Animals. 2019; 9(10):784. https://doi.org/10.3390/ani9100784
Chicago/Turabian StyleZhang, Zhen-Wei, Yan-Lu Wang, Yong-Yan Chen, Wei-Kang Wang, Luo-Tong Zhang, Hai-Ling Luo, and Hong-Jian Yang. 2019. "Nitroethanol in Comparison with Monensin Exhibits Greater Feed Efficiency Through Inhibiting Rumen Methanogenesis More Efficiently and Persistently in Feedlotting Lambs" Animals 9, no. 10: 784. https://doi.org/10.3390/ani9100784
APA StyleZhang, Z.-W., Wang, Y.-L., Chen, Y.-Y., Wang, W.-K., Zhang, L.-T., Luo, H.-L., & Yang, H.-J. (2019). Nitroethanol in Comparison with Monensin Exhibits Greater Feed Efficiency Through Inhibiting Rumen Methanogenesis More Efficiently and Persistently in Feedlotting Lambs. Animals, 9(10), 784. https://doi.org/10.3390/ani9100784






