Effects of Dietary Threonine Levels on Intestinal Immunity and Antioxidant Capacity Based on Cecal Metabolites and Transcription Sequencing of Broiler
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Sample Collection and Preparations
2.3. Sample Analysis
2.4. Data Analysis
3. Results
3.1. Searching for Differential Metabolites
3.2. Sequencing of the Jejunal Mucosal Transcriptome
3.3. An Analysis of the Cross-Section of the Study Groups
3.4. Identification of the Differentially Expressed Genes by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kidd, M.; Kerr, B.J. L-threonine for poultry: A review. J. Appl. Poult. Res. 1996, 5, 358–367. [Google Scholar] [CrossRef]
- Ballèvre, O.; Cadenhead, A.; Calder, A.G.; Rees, W.D.; Lobley, G.E.; Fuller, M.F.; Garlick, P.J. Quantitative partition of threonine oxidation in pigs: effect of dietary threonine. Am. J. Physiol. Metab. 1990, 259, E483–E491. [Google Scholar] [CrossRef] [PubMed]
- Applegate, T.J.; Angel, R. Nutrient requirements of poultry publication: History and need for an update. J. Appl. Poult. Res. 2014, 23, 567–575. [Google Scholar] [CrossRef]
- Kidd, M.T.; Corzo, A.; Dozier, W.A. Dietary Amino Acid Responses of Broiler Chickens. J. Appl. Poult. Res. 2008, 17, 157–167. [Google Scholar]
- Qaisrani, S.N.; Ahmed, I.; Azam, F.; Bibi, F.; Pasha, T.N.; Azam, F. Saima Threonine in broiler diets: An updated review. Ann. Anim. Sci. 2018, 18, 659–674. [Google Scholar] [CrossRef]
- Shirisha, R.; Umesh, B.U.; Prashanth, K. Effect of L-Threonine supplementation on broiler chicken: A review. J. Pharm. Innov. 2018, 7, 490–493. [Google Scholar]
- Liu, S.G.; Qu, Z.X.; Meng, G.H.; Gao, Y.P.; Min, Y.N. Effects of dietary threonine levels on growth performance, antioxidant capacities and immune function of broiler chickens. Acta Agric. Boreali Occident. Sin. 2017, 26, 1429–1437. [Google Scholar]
- Chen, Y.P.; Cheng, Y.F.; Li, X.H.; Yang, W.L.; Wen, C.; Zhuang, S.; Zhou, Y.M. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult. Sci. 2016, 96, 405–413. [Google Scholar] [CrossRef]
- Azzam, M.; Dong, X.; Xie, P.; Zou, X.; Azzam, M. Influence of L–threonine supplementation on goblet cell numbers, histological structure and antioxidant enzyme activities of laying hens reared in a hot and humid climate. Br. Poult. Sci. 2012, 53, 640–645. [Google Scholar] [CrossRef]
- Debnath, B.C. Effects of Supplemental Threonine on Antioxidant Enzyme Activities and Haemato-biochemical Profile of Commercial Broilers in Sub-Tropics. J. Anim. Res. 2019, 9, 125–133. [Google Scholar] [CrossRef]
- Min, Y.N.; Liu, S.G.; Qu, Z.X.; Meng, G.H.; Gao, Y.P. Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poult. Sci. 2017, 96, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Habte-Tsion, H.M.; Ge, X.; Liu, B.; Xie, J.; Ren, M.; Zhou, Q.; Miao, L.; Pan, L.; Chen, R. A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2015, 42, 439–446. [Google Scholar] [CrossRef]
- Filho, A.L.D.; Oliveira, C.J.B.; Neto, O.C.F.; De Leon, C.M.; Saraiva, M.M.S.; Andrade, M.F.S.; White, B.; Givisiez, P.E.N. Intra-Amnionic Threonine Administered to Chicken Embryos Reduces Salmonella Enteritidis Cecal Counts and Improves Posthatch Intestinal Development. J. Immunol. Res. 2018, 2018, 9795829. [Google Scholar]
- Wils-Plotz, E.L.; Jenkins, M.C.; Dilger, R.N. Modulation of the intestinal environment, innate immune response, and barrier function by dietary threonine and purified fiber during a coccidiosis challenge in broiler chicks. Poult. Sci. 2013, 92, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Specific Amino Acids Increase Mucin Synthesis and Microbiota in Dextran Sulfate Sodium–Treated Rats. J. Nutr. 2006, 136, 1558–1564. [CrossRef]
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Veter. Sci. 2019, 5, 348. [Google Scholar] [CrossRef]
- Dong, X.Y.; Azzam, M.M.M.; Zou, X.T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017, 96, 3654–3663. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Hicks, J.A.; Porter, T.E.; Sunny, N.E.; Liu, H.C. Delayed Feeding Alters Transcriptional and Post-Transcriptional Regulation of Hepatic Metabolic Pathways in Peri-Hatch Broiler Chicks. Genes 2019, 10, 272. [Google Scholar] [CrossRef]
- Yuan, C.; Song, H.H.; Jiang, Y.J.; Azzam, M.; Zhu, S.; Zou, X.T. Effects of lead contamination in feed on laying performance, lead retention of organs and eggs, protein metabolism, and hormone levels of laying hens. J. Appl. Poult. Res. 2013, 22, 878–884. [Google Scholar] [CrossRef]
- Gao, Y.J.; Yang, H.J.; Liu, Y.J.; Chen, S.J.; Guo, D.Q.; Yu, Y.Y.; Tian, L.X. Effects of graded levels of threonine on growth performance, biochemical parameters and intestine morphology of juvenile grass carp Ctenopharyngodon idella. Aquaculture 2014, 424, 113–119. [Google Scholar] [CrossRef]
- Mu, H.; Wang, N.; Zhao, L. Methylation of PLCDl and adenovirus- mediated PLCD1 overexpression elicits a gene therapy effect on human breast cancer. Exp. Cell Res. 2015, 332, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Carvou, N.; Norden, A.G.; Unwin, R.J.; Cockcroft, S. Signalling through phospholipase C interferes with clathrin-nediated endocytosis. J. Cell Signal 2007, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jiang, Z. Phospholipase Cδ1 (PLCD1) inhibits the proliferation, invasion and migration of CAPAN-1 and BXPC-3 pancreatic cancer cells. J. Cell Mol. Immun. 2016, 32, 739–745. [Google Scholar]
- Hu, X.T.; Zhang, F.B.; Fan, Y.C.; Shu, X.S.; Wong, A.H.Y.; Zhou, W.; Shi, Q.L.; Tang, H.M.; Fu, L.; Guan, X.Y.; et al. Phospholipase C delta 1 is a novel 3p22.3 tumor suppressor involved in cytoskeleton organization, with its epigenetic silencing correlated with high-stage gastric cancer. Oncogene 2009, 28, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.B.; Song, J.J.; Liu, Q.; Li, Y.; Yang, Z.S.; Yang, L.; Xiang, T.X.; Ren, G.S. Epigenetic inactivation of PLCD1 in chronic myeloid leukemia. Int. J. Mol. Med. 2012, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.X.; Li, L.L.; Fan, Y.C.; Jiang, Y.Y.; Ying, Y.; Putti, T.C.; Tao, Q.; Ren, G.S. PLCDl is a functional tumor suppressor inducing G2/M arrest and frequently methylated in breast cancer. J. Cancer Biol. Ther. 2010, 10, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Roseman, D.S.; Khan, T.; Rajas, F.; Jun, L.S.; Asrani, K.H.; Isaacs, C.; Farelli, J.D.; Subramanian, R.R. G6PC mRNA Therapy Positively Regulates Fasting Blood Glucose and Decreases Liver Abnormalities in a Mouse Model of Glycogen Storage Disease 1a. Mol. Ther. 2018, 26, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Das, C. Managing the sugar factory: New feather in the cap for nuclear factor Y. J. Biol. Chem. 2018, 293, 9651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Geng, T.; Wang, Z.; Zhang, R.; Cao, T.; Camporez, J.P.; Cai, S.Y.; Liu, Y.; Dandolo, L.; Shulman, G.I.; et al. Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight 2018, 3, 120304. [Google Scholar] [CrossRef] [PubMed]
- Satomura, K.; Tobiume, S.; Tokuyama, R.; Yamasaki, Y.; Kudoh, K.; Maeda, E.; Nagayama, M. Melatonin at phar-macological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. J. Pineal Res. 2007, 42, 231–239. [Google Scholar] [CrossRef]
- Tomas, C.; Montes, A.C. A proposed mechanism to explain the stimulatory effect of melatonin on antioxidantive en-zymes. J. Pineal Res. 2005, 39, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Yang, P.; Li, Z. Pyruvate kinase M2: A multifarious enzyme in non-canonical localization to promote cancer progression. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1871, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, W.; Melo, J.D.; Tang, D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int. J. Cell. Biol. 2013, 2013, 242513. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.K.; Han, Y.M.; Kim, J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int. J. Biochem. Cell Boil. 2008, 40, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Hirst, J.A.; Fujii, H. Regulation of Cell Proliferation by Interleukin-3-induced Nuclear Translocation of Pyruvate Kinase. J. Biol. Chem. 2007, 282, 17706–17711. [Google Scholar] [CrossRef] [PubMed]
- Stetak, A.; Veress, R.; Ovádi, J.; Csermely, P.; Ullrich, A.; Kéri, G. Nuclear Translocation of the Tumor Marker Pyruvate Kinase M2 Induces Programmed Cell Death. Cancer Res. 2007, 67, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Ding, M.; Wang, B.; Lu, Z.; Zhao, Q.; Shaw, K. Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget 2014, 5, 8202–8210. [Google Scholar] [CrossRef]
- Yang, W.; Xia, Y.; Ji, H.; Zheng, Y.; Liang, J.; Huang, W.; Gao, X.; Aldape, K.; Lu, Z. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 2011, 480, 118–122. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chien, M.H.; Hsiao, M. Glucose limited enhance the cancer stem cell population through PKM2/AMPK-dependent signaling. FASEB J. 2018, 32, 41–45. [Google Scholar]
- Wu, S.F. Dual roles of PKM2 in cancer metabolism. Acta Bioch. Bioph. Sin. 2013, 45, 27–35. [Google Scholar] [CrossRef]
- Palmieri, D.; Fitzgerald, D.; Shreeve, S.M.; Hua, E.; Bronder, J.L.; Weil, R.J.; Davis, S.; Stark, A.M.; Merino, M.J.; Kurek, R.; et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 2009, 7, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Shen, Z.; Xiang, P.; Hunag, T.; Xuan, Q. Expression of hk2 gene in prostate cancer and its effect on malignant phenotype of prostate cancer cells. Int. J. Clin. Exp. Pathol. 2017, 33, 149–157. [Google Scholar]
- Gareth, W.; Anthony, D.J.; Robert, E.M.; Jiang, W.G. TBXAS1 and the thromboxane A2 receptor, TBXA2R, in human breast cancer. BioMed Cent. 2005, 2, 23. [Google Scholar]
- Borths, E.L.; Locher, K.P.; Lee, A.T.; Rees, D.C. The structure of Escherichia coli BtuF and binding to its cognate ATP binding cassette transporter. Proc. Natl. Acad. Sci. USA 2002, 99, 16642–16647. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L.; et al. Hexokinase 2 Is Required for Tumor Initiation and Maintenance and Its Systemic Deletion Is Therapeutic in Mouse Models of Cancer. Cancer Cell 2013, 24, 399. [Google Scholar] [CrossRef]
- Baker, D.H.; Hill, T.M.; Kleiss, A.J. Nutritional Evidence Concerning Formation of Glycine from Threonine in the Chick. J. Anim. Sci. 1972, 34, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
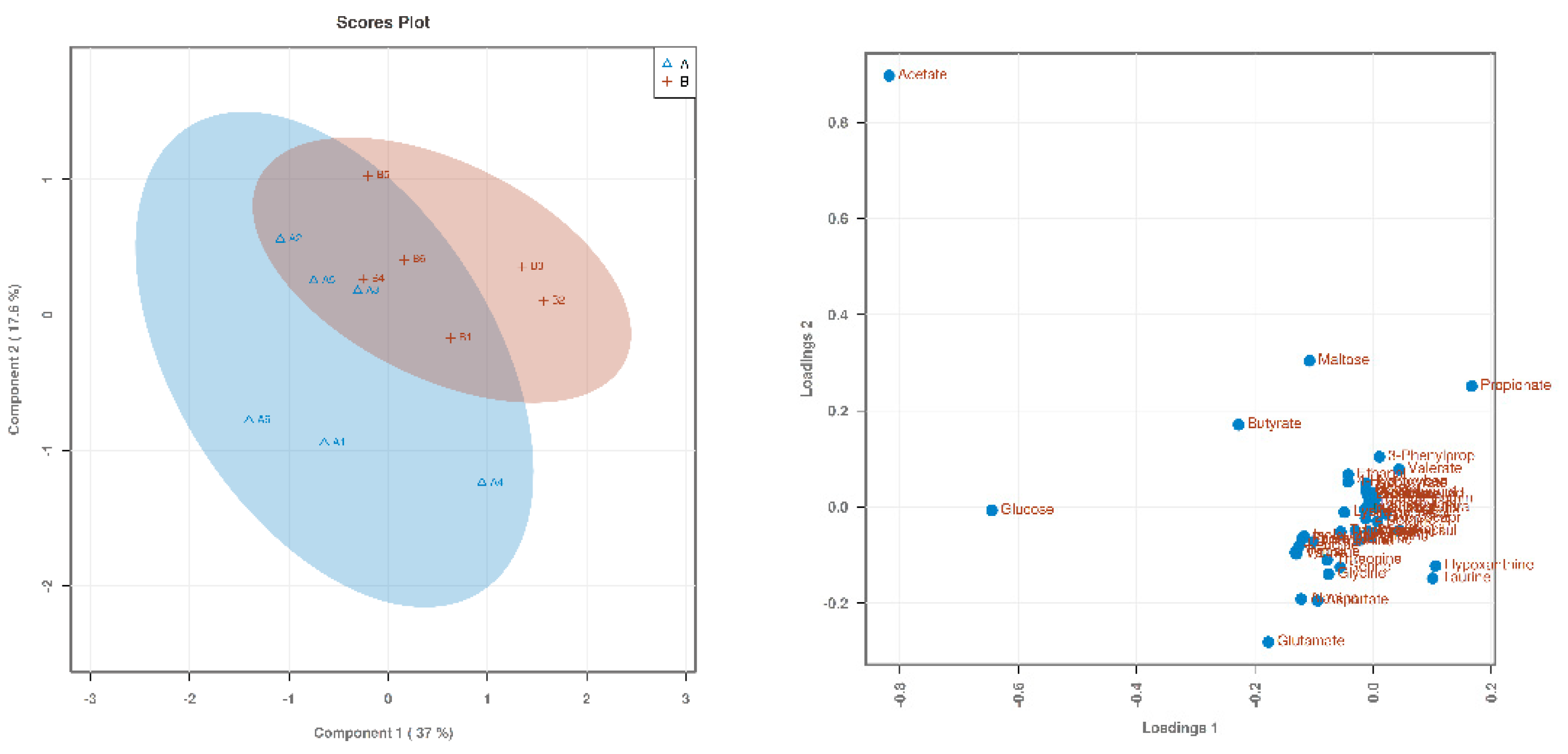
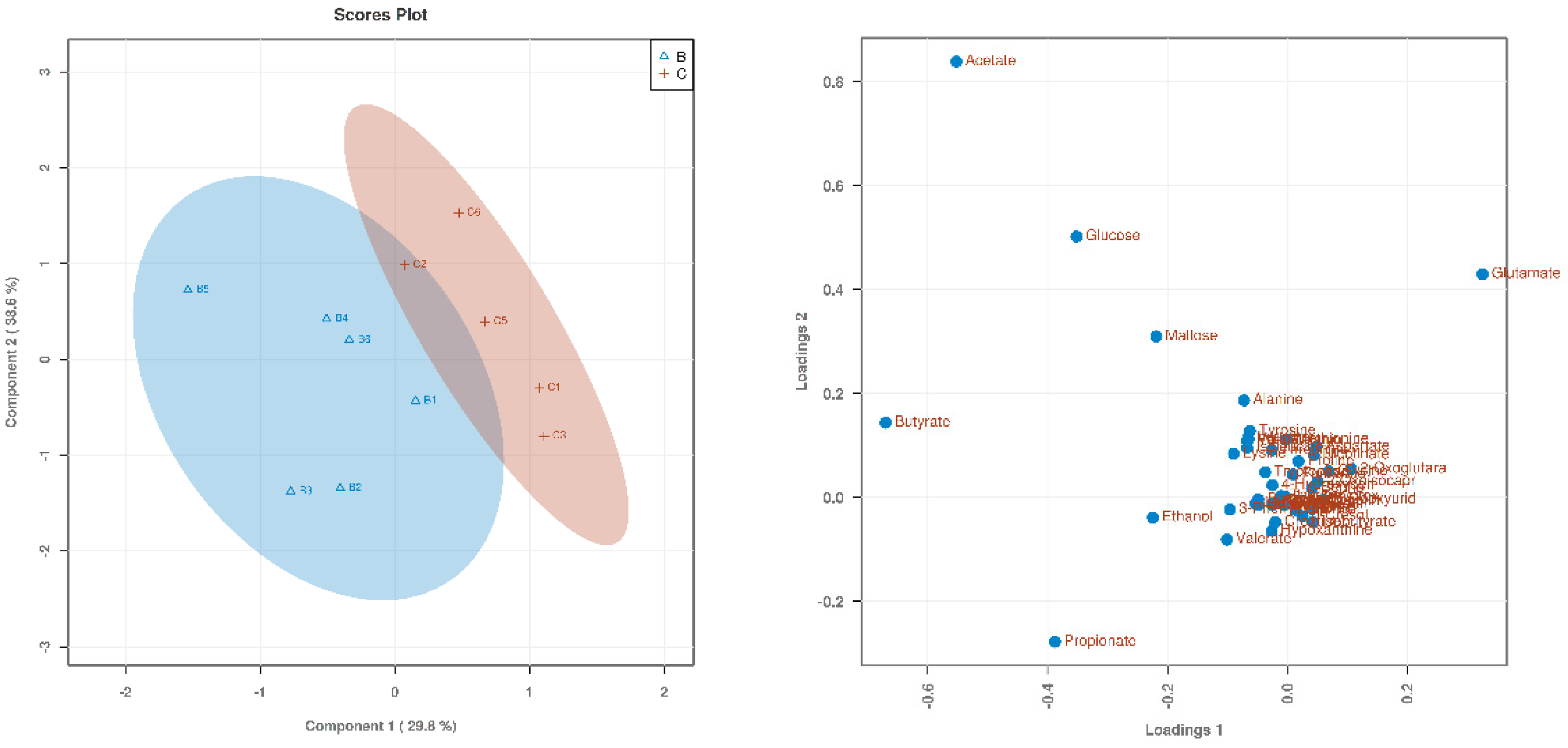
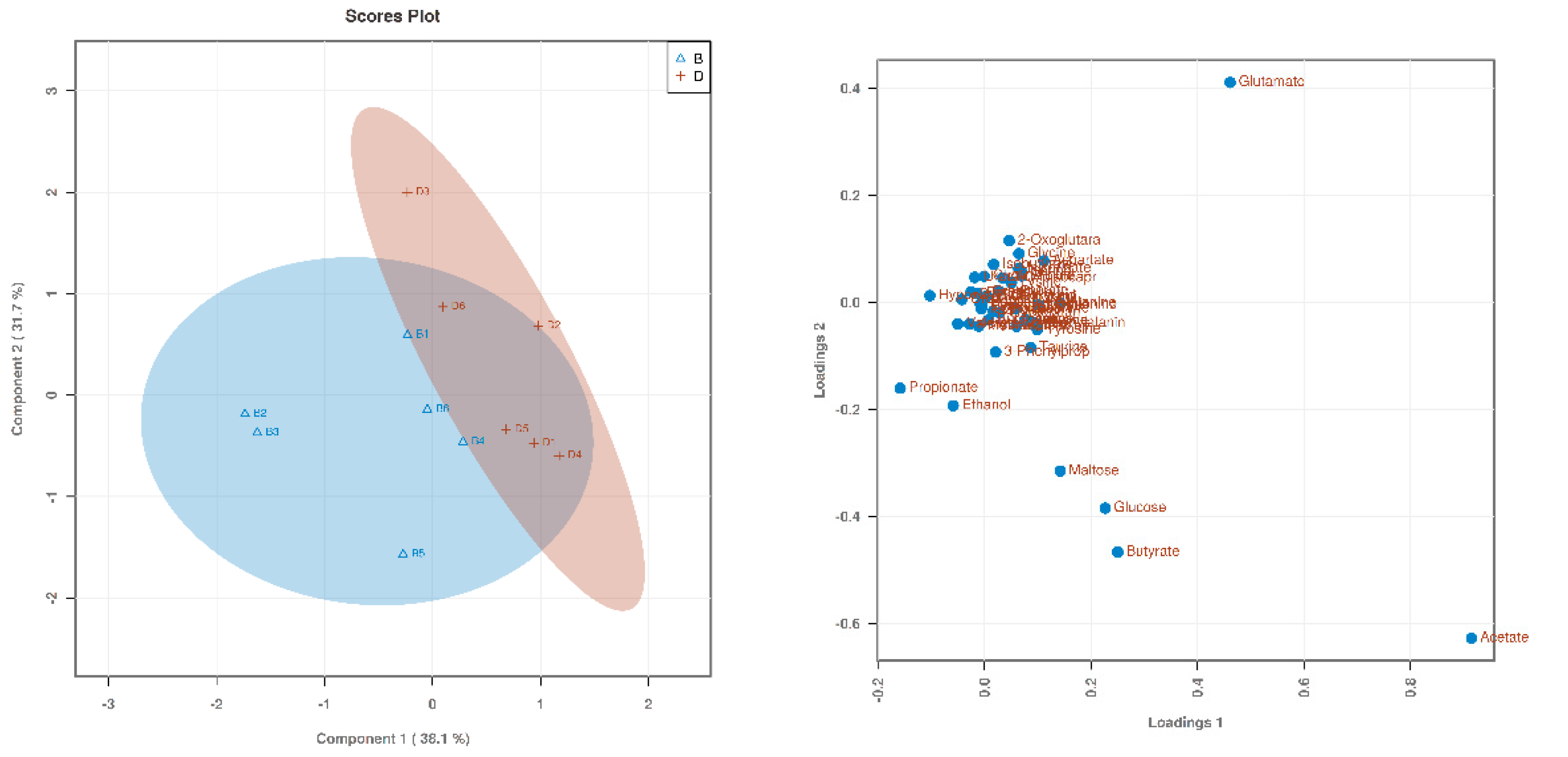

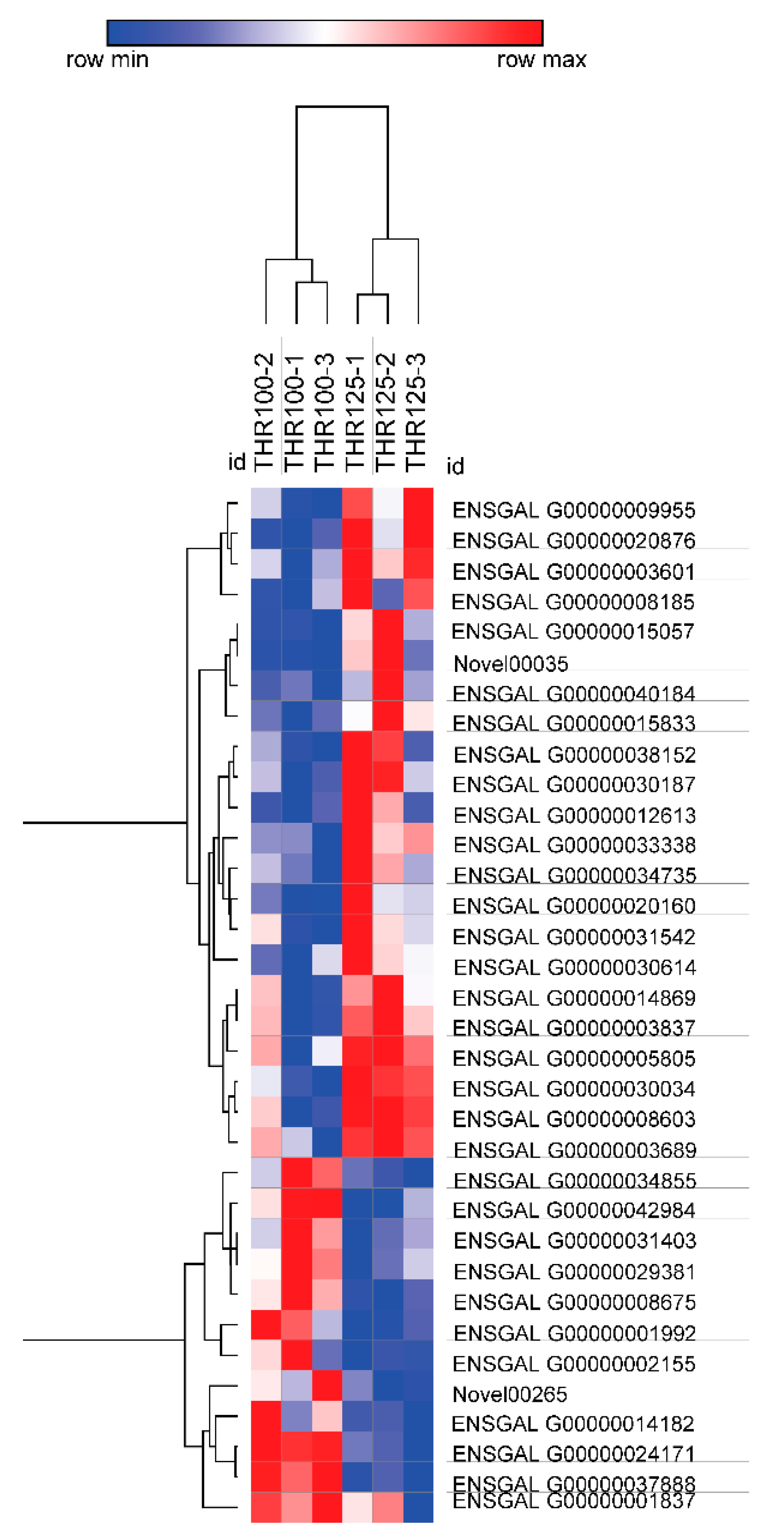
| Items (%) | Starter (0 to 21 d) | Finisher (22 to 42 d) |
|---|---|---|
| Ingredients | ||
| Corn | 31.92 | 32.54 |
| Soybean meal | 26.25 | 18.40 |
| Wheat | 25.00 | 30.00 |
| Peanut meal 1 | 5.00 | 5.00 |
| Soy bean oil | 4.20 | 4.80 |
| Distillers dried grains with solubles | 3.00 | 5.00 |
| Dicalcium phosphate | 1.65 | 1.35 |
| Limestone | 1.10 | 1.30 |
| Premix 2 | 1.00 | 1.00 |
| L-lysine | 0.32 | 0.28 |
| Salt | 0.25 | 0.25 |
| DL-methionine | 0.18 | 0.13 |
| Choline chloride | 0.10 | 0.10 |
| Antioxidant | 0.03 | 0.03 |
| L-threonine | 0.00 | 0.00 |
| Total | 100.00 | 100.00 |
| Calculated composition | ||
| Metabolizable energy (kcal/kg) | 3000.00 | 3100.00 |
| Total phosphorus | 0.68 | 0.62 |
| Available phosphorous | 0.45 | 0.40 |
| Calcium | 0.90 | 0.90 |
| Sodium chloride | 0.30 | 0.30 |
| Analyzed composition | ||
| Crude protein | 20.87 | 19.10 |
| Lysine | 1.24 | 1.11 |
| Methionine | 0.50 | 0.42 |
| Isoleucine | 0.67 | 0.58 |
| Threonine 3 | 0.69 | 0.62 |
| Tryptophan | 0.23 | 0.20 |
| Valine | 0.85 | 0.74 |
| Arginine | 1.32 | 1.14 |
| Gene Symbol | Ensemble Accession No. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size/bp |
|---|---|---|---|---|
| ABCC2 | ENSGALG00000007395 | TGTCCTTACCATTGCCCACC | CCACTTACATCCGCTCCACC | 197 |
| CAT | ENSGALG00000030187 | GAACGCCGCATAGTAAGA | GAGGGTCACGAACAGTAT | 414 |
| PKM2 | ENSGALG00000001992 | ATGTCGAAGCCCCATAGTGAA | TGGGTGGTGAATCAATGTCCA | 118 |
| G6PC | ENSGALG00000030034 | GCGTCTGGTATGTAATGG | AGAATAACTTGATGAGGGA | 181 |
| PLCD1 | ENSGALG00000005805 | CCAGTGAACGAGCCAAGAAG | TTCCACACAGCGGCAACCTT | 245 |
| GAPDH | ENSAPLG00000013511 | TCTTCACCACCATGGAGAAG | CAGGACGCATTGCTGACAAT | 154 |
| Metabolite | VIP Scores |
|---|---|
| Propionate | 3.82660 |
| Butyrate | 3.58300 |
| Glutamate | 2.89960 |
| Acetate | 1.40370 |
| Ethanol | 1.28460 |
| Valerate | 1.05500 |
| Aspartate | 0.78458 |
| Glucose | 0.77806 |
| Lysine | 0.69175 |
| 2-Oxoglutarate | 0.62168 |
| Hypoxanthine | 0.55743 |
| 3-Phenylpropionate | 0.52454 |
| Glycine | 0.49846 |
| Uridine | 0.47948 |
| Methionine | 0.45836 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Qi, X.; Ma, S.; Liu, X.; Min, Y. Effects of Dietary Threonine Levels on Intestinal Immunity and Antioxidant Capacity Based on Cecal Metabolites and Transcription Sequencing of Broiler. Animals 2019, 9, 739. https://doi.org/10.3390/ani9100739
Ji S, Qi X, Ma S, Liu X, Min Y. Effects of Dietary Threonine Levels on Intestinal Immunity and Antioxidant Capacity Based on Cecal Metabolites and Transcription Sequencing of Broiler. Animals. 2019; 9(10):739. https://doi.org/10.3390/ani9100739
Chicago/Turabian StyleJi, Shuyun, Xi Qi, Shuxue Ma, Xing Liu, and Yuna Min. 2019. "Effects of Dietary Threonine Levels on Intestinal Immunity and Antioxidant Capacity Based on Cecal Metabolites and Transcription Sequencing of Broiler" Animals 9, no. 10: 739. https://doi.org/10.3390/ani9100739
APA StyleJi, S., Qi, X., Ma, S., Liu, X., & Min, Y. (2019). Effects of Dietary Threonine Levels on Intestinal Immunity and Antioxidant Capacity Based on Cecal Metabolites and Transcription Sequencing of Broiler. Animals, 9(10), 739. https://doi.org/10.3390/ani9100739




