Occurrence of Breast Meat Abnormalities and Foot Pad Dermatitis in Light-Size Broiler Chicken Hybrids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Diet
2.2. Productive Performance
2.3. Evaluation of Breast Meat Abnormalities
2.4. Evaluation of Foot Pad Dermatitis
2.5. Statistical Analysis
3. Results
3.1. Productive Performance
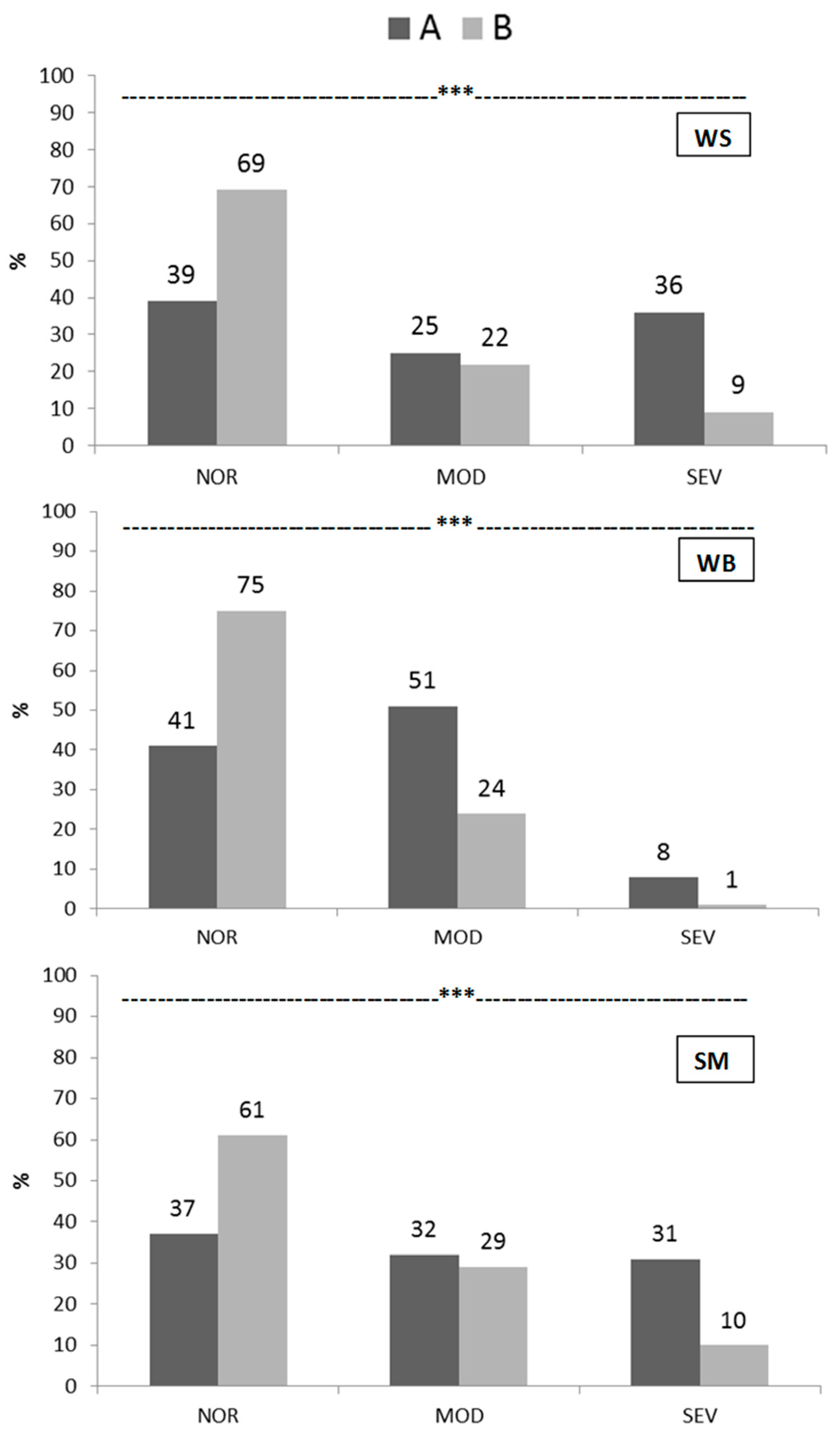
3.2. Incidence and Severity of Breast Myopathies
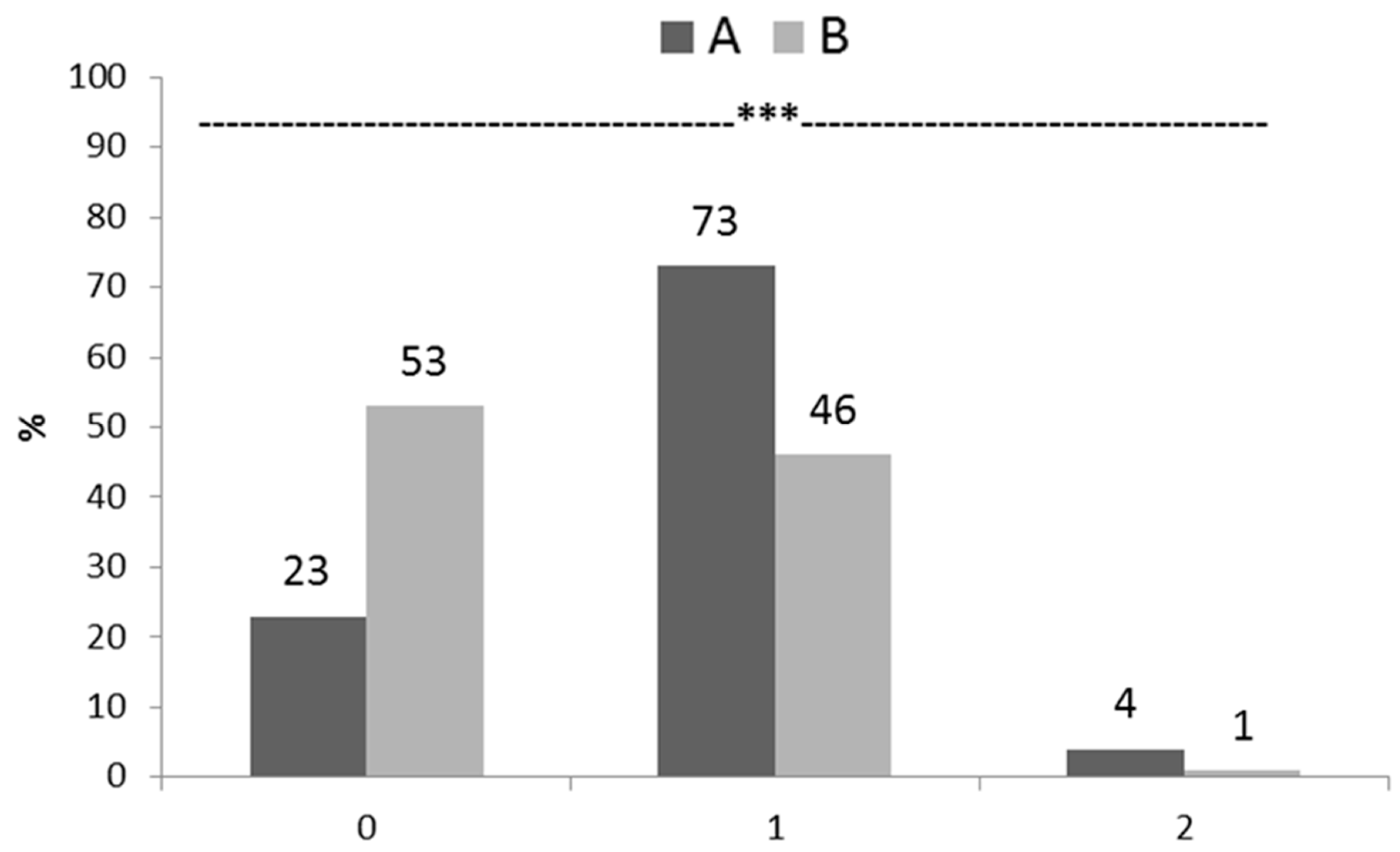
3.3. Incidence and Severity of Foot Pad Dermatitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbut, S.; Sosnicki, A.A.; Lonergan, S.M.; Knapp, T.; Ciobanu, D.C.; Gatcliffe, L.J.; Huff-Lonergan, E.; Wilson, E.W. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 2008, 79, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef] [PubMed]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Artificial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 2018, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Willems, O.W.; Miller, S.P.; Wood, B.J. Aspects of selection for feed efficiency in meat producing poultry. World Poult. Sci. J. 2013, 69, 77–88. [Google Scholar] [CrossRef]
- Dridi, S.; Anthony, N.; Kong, B.W.; Bottje, W. Feed efficiency: A key production trait and a global challenge. Adv. Food Technol. Nutr. Sci. Open. J. 2015, 1, e11–e13. [Google Scholar] [CrossRef]
- Richards, M.P. Genetic regulation of feed intake and energy balance in poultry. Poult. Sci. 2003, 82, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, A.; Greene, E.; Anthony, N.B.; Bottje, W.; Dridi, S. Crosstalk between autophagy and obesity: Potential use of avian model. Adv. Food Technol. Nutr. Sci. Open. J. 2015, 1, 32–37. [Google Scholar] [CrossRef]
- Kestin, S.C.; Gordon, S.; Su, G.; Sørensen, P. Relationships in broiler chickens between lameness, liveweight, growth rate and age. Vet. Rec. 2001, 148, 195–197. [Google Scholar] [CrossRef]
- Meluzzi, A.; Sirri, F. Welfare of broiler chickens. Ital. J. Anim. Sci. 2009, 8, 161–173. [Google Scholar] [CrossRef]
- Petracci, M.; Sirri, F.; Mazzoni, M.; Meluzzi, A. Comparison of breast muscle traits and meat quality characteristics in 2 commercial chicken hybrids. Poult. Sci. 2013, 92, 2438–2447. [Google Scholar] [CrossRef]
- Velleman, S.G. Relationship of skeletal muscle development and growth to breast muscle myopathies: A review. Avian Dis. 2015, 59, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014, 51, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Hargis, B.M.; Owens, C.M. White striping and woody breast myopathies in the modern poultry industry: A review. Poult. Sci. 2016, 95, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Baldi, G.; Soglia, F.; Mazzoni, M.; Sirri, F.; Canonico, L.; Babini, E.; Laghi, L.; Cavani, C.; Petracci, M. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal 2018, 12, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estévez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Compr. Rev. Food Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Brewer, V.B.; Clark, F.D.; McKee, S.R.; Meullenet, J.F.; Emmert, J.L.; Owens, C.M. Effect of white striping on the histological and meat quality characteristics of broiler fillets. Poult. Sci. 2009, 88, 136–137. [Google Scholar]
- Sirri, F.; Maiorano, G.; Tavaniello, S.; Chen, J.; Petracci, M.; Meluzzi, A. Effect of different levels of dietary zinc, manganese, and copper from organic or inorganic sources on performance, bacterial chondronecrosis, intramuscular collagen characteristics, and occurrence of meat quality defects of broiler chickens. Poult. Sci. 2016, 95, 1813–1824. [Google Scholar] [CrossRef]
- Soglia, F.; Mazzoni, M.; Petracci, M. Spotlight on avian pathology: Current growth-related breast meat abnormalities in broilers. Avian Pathol. 2019, 48, 1–3. [Google Scholar] [CrossRef]
- Bailey, R.A.; Watson, K.A.; Bilgili, S.F.; Avendano, S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015, 94, 2870–2879. [Google Scholar] [CrossRef]
- Alnahhas, N.; Berri, C.; Chabault, M.; Chartrin, P.; Boulay, M.; Bourin, M.C.; Le Bihan-Duval, E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genet. 2016, 17, 61. [Google Scholar] [CrossRef]
- Petracci, M.; Mudalal, S.; Bonfiglio, A.; Cavani, C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult. Sci. 2013, 92, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M.; Mudalal, S.; Cavani, C.; Petracci, M. Incidence of white striping under commercial conditions in medium and heavy broiler chickens in Italy. J. Appl. Poult. Res. 2014, 23, 754–758. [Google Scholar] [CrossRef]
- Trocino, A.; Piccirillo, A.; Birolo, M.; Radaelli, G.; Bertotto, D.; Filiou, E.; Petracci, M.; Xiccato, G. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult. Sci. 2015, 94, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Livingston, M.L.; Landon, C.; Barnes, H.J.; Brake, J. White striping and wooden breast myopathies of broiler breast muscle is affected by time-limited feeding, genetic background, and egg storage. Poult. Sci. 2018, 98, 217–226. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Council Directive 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production. Off. J. 2007, L182, 19–28. [Google Scholar]
- Mayne, R.K. A review of the aetiology and possible causative factors of foot pad dermatitis in growing turkeys and broilers. World Poult. Sci. J. 2005, 61, 256–267. [Google Scholar] [CrossRef]
- Shepherd, E.M.; Fairchild, B.D. Footpad dermatitis in poultry. Poult. Sci. 2010, 89, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Martland, M.F. Wet litter as a cause of plantar pododermatitis, leading to foot ulceration and lameness in fattening turkeys. Avian Pathol. 1984, 13, 241–252. [Google Scholar] [CrossRef]
- Martland, M.F. Ulcerative dermatitis in broiler chickens: The effects of wet litter. Avian Pathol. 1985, 14, 353–364. [Google Scholar] [CrossRef]
- Kestin, S.C.; Su, G.; Sørensen, P. Different commercial broiler crosses have different susceptibilities to leg weakness. Poult. Sci. 1999, 78, 1085–1090. [Google Scholar] [CrossRef]
- Sanotra, G.S.; Berg, C.; Damkjer Lund, J. A comparison between leg problems in Danish and Swedish broiler production. Anim. Welf. 2003, 12, 677–683. [Google Scholar]
- Bilgili, S.F.; Alley, M.A.; Hess, J.B.; Nagaraj, M. Influence of age and sex on footpad quality and yield in broiler chickens reared on low and high density diets. J. Appl. Poult. Res. 2006, 15, 433–441. [Google Scholar] [CrossRef]
- European Commission. Council Regulation (EC) No 1099/2009 of 24 Sept. 2009 on the protection of animals at the time of killing. Off. J. 2009, L303, 1–30. [Google Scholar]
- European Commission. Council Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. 2010, L276, 33–79. [Google Scholar]
- Sirri, F.; Petracci, M.; Zampiga, M.; Meluzzi, A. Effect of EU electrical stunning conditions on breast meat quality of broiler chickens. Poult. Sci. 2017, 96, 3000–3004. [Google Scholar] [PubMed]
- Zampiga, M.; Laghi, L.; Petracci, M.; Zhu, C.; Meluzzi, A.; Dridi, S.; Sirri, F. Effect of dietary arginine to lysine ratios on productive performance, meat quality, plasma and muscle metabolomics profile in fast-growing broiler chickens. J. Anim. Sci. Biotechnol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Zampiga, M.; Soglia, F.; Petracci, M.; Meluzzi, A.; Sirri, F. Effect of different arginine-to-lysine ratios in broiler chicken diets on the occurrence of breast myopathies and meat quality attributes. Poult. Sci. 2019, 98, 2691–2697. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Lee, Y.; Erf, G.F.; Meullenet, J.F.; Owens, C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012, 91, 1240–1247. [Google Scholar] [CrossRef]
- Ekstrand, C.; Algers, B.; Svedberg, J. Rearing conditions and foot-pad dermatitis in Swedish broiler chickens. Prev. Vet. Med. 1997, 31, 167–174. [Google Scholar] [CrossRef]
- Zampiga, M.; Meluzzi, A.; Sirri, F. Effect of dietary supplementation of lysophospholipids on productive performance, nutrient digestibility and carcass quality traits of broiler chickens. Ital. J. Anim. Sci. 2016, 15, 521–528. [Google Scholar] [CrossRef]
- SAS. SAS/STAT Guide for Personal Computers; V. 6.03 ed.; SAS Institute Inc.: Cary, NC, USA, 1988. [Google Scholar]
- US Poultry & Egg Export Council. US Chicken Feet Kicked Out of China. Available online: http://www.thepoultrysite.com/poultrynews/18142/us-chicken-feet-kicked-out-of-china (accessed on 29 July 2019).
- Zampiga, M.; Bertocchi, M.; Bosi, P.; Trevisi, P.; Meluzzi, A.; Sirri, F. Differences in productive performance and intestinal transcriptomic profile in two modern fast-growing chicken hybrids. J. Anim. Physiol. Anim. Nutr. 2019, 103, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, L.J.; Morey, A.U.; Moran, E.T.; Singh, M.; Owens, C.M.; McKee, S.R. Occurrence of WS in chicken breast fillets in relation to broiler size. Poult. Sci. 2009, 88, 104. [Google Scholar]
- Zampiga, M.; Tavaniello, S.; Soglia, F.; Petracci, M.; Mazzoni, M.; Maiorano, G.; Meluzzi, A.; Clavenzani, P.; Sirri, F. Comparison of 2 commercial turkey hybrids: Productivity, occurrence of breast myopathies, and meat quality properties. Poult. Sci. 2019, 98, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, J.B.; Su, G.; Nielsen, B.L.; Sørensen, P. Foot pad dermatitis and hock burn in broiler chickens and degree of inheritance. Poult. Sci. 2006, 85, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
| Starter 0–9 d | Grower 10–21 d | Finisher 22–35 d | |
|---|---|---|---|
| Ingredients, g/100 g | |||
| Corn | 33.4 | 36.7 | 34.2 |
| Wheat | 20.0 | 20.0 | 25.0 |
| Vegetable oil | 2.45 | 2.68 | 3.61 |
| Soybean meal 48% | 18.2 | 20.2 | 14.2 |
| Full-fat soybean | 10.0 | 10.0 | 15.0 |
| High-protein soybean meal | 5.00 | 0.00 | 0.00 |
| Sunflower | 2.00 | 2.00 | 2.00 |
| Pea | 3.00 | 3.00 | 3.00 |
| Corn gluten | 2.00 | 2.00 | 0.00 |
| Lysine | 0.54 | 0.53 | 0.46 |
| DL-Methionine | 0.29 | 0.32 | 0.33 |
| L-Threonine | 0.12 | 0.11 | 0.10 |
| Choline chloride | 0.10 | 0.10 | 0.05 |
| Calcium carbonate | 0.53 | 0.52 | 0.60 |
| Dicalcium phosphate | 1.29 | 0.80 | 0.47 |
| Sodium chloride | 0.29 | 0.30 | 0.23 |
| Sodium bicarbonate | 0.05 | 0.05 | 0.15 |
| Vit.-min premix 1 | 0.54 | 0.46 | 0.38 |
| Phytase | 0.05 | 0.05 | 0.05 |
| Xylanase | 0.05 | 0.05 | 0.05 |
| Emulsifier | 0.08 | 0.08 | 0.08 |
| Calculated chemical composition | |||
| Dry matter *, % | 88.8 | 88.2 | 88.5 |
| Crude protein *, % | 22.7 | 21.0 | 19.1 |
| Total lipid *, % | 6.25 | 6.51 | 8.29 |
| Crude fiber *, % | 2.96 | 2.92 | 2.99 |
| Ash, % | 5.24 | 4.60 | 4.29 |
| Lysine (total), % | 1.42 | 1.31 | 1.20 |
| Met. + Cyst. (total), % | 0.99 | 0.92 | 0.85 |
| Arginine (total), % | 1.46 | 1.34 | 1.25 |
| Threonine (total), % | 0.94 | 0.87 | 0.79 |
| Ca (total), % | 0.77 | 0.62 | 0.55 |
| P (total), % | 0.61 | 0.51 | 0.44 |
| AME, kcal/kg | 3100 | 3150 | 3275 |
| Variables | A | B | SEM | p-Value |
|---|---|---|---|---|
| n. replicates | 12 | 12 | ||
| 0–9 d | ||||
| Chick body weight (g/bird) | 44.1 A | 41.0 B | 0.38 | <0.01 |
| Body weight (g/bird) | 227 A | 216 B | 2.32 | <0.01 |
| Daily weight gain (g/bird/d) * | 20.3 a | 19.4 b | 0.26 | <0.05 |
| Daily feed intake (g/bird/d) * | 25.1 | 24.0 | 0.53 | ns |
| Feed conversion ratio * | 1.235 | 1.237 | 0.02 | ns |
| Mortality (%) | 0.12 | 0.00 | 0.01 | ns |
| 10–21 d | ||||
| Body weight (g/bird) | 751 B | 775 A | 5.77 | <0.01 |
| Daily weight gain (g/bird/d) * | 43.5 B | 46.6 A | 0.57 | <0.01 |
| Daily feed intake (g/bird/d) * | 71.0 | 70.3 | 1.50 | ns |
| Feed conversion ratio * | 1.632 a | 1.511 b | 0.04 | <0.05 |
| Mortality (%) | 0.31 | 1.15 | 0.02 | ns |
| 22–35 d | ||||
| Body weight (g/bird) | 1929 | 1962 | 13.8 | ns |
| Daily weight gain (g/bird/d) * | 84.1 | 84.8 | 0.88 | ns |
| Daily feed intake (g/bird/d) * | 138.1 b | 142.5 a | 1.20 | <0.05 |
| Feed conversion ratio * | 1.643 | 1.681 | 0.02 | ns |
| Mortality (%) | 0.31 | 0.10 | 0.01 | ns |
| 0–35 d | ||||
| Body weight (g/bird) | 1929 | 1962 | 13.8 | ns |
| Daily weight gain (g/bird/d) * | 53.8 | 54.9 | 0.39 | ns |
| Daily feed intake (g/bird/d) * | 86.0 | 87.0 | 0.68 | ns |
| Feed conversion ratio * | 1.600 | 1.590 | 0.01 | ns |
| Mortality (%) | 0.85 | 1.25 | 0.02 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampiga, M.; Meluzzi, A.; Pignata, S.; Sirri, F. Occurrence of Breast Meat Abnormalities and Foot Pad Dermatitis in Light-Size Broiler Chicken Hybrids. Animals 2019, 9, 706. https://doi.org/10.3390/ani9100706
Zampiga M, Meluzzi A, Pignata S, Sirri F. Occurrence of Breast Meat Abnormalities and Foot Pad Dermatitis in Light-Size Broiler Chicken Hybrids. Animals. 2019; 9(10):706. https://doi.org/10.3390/ani9100706
Chicago/Turabian StyleZampiga, Marco, Adele Meluzzi, Stefano Pignata, and Federico Sirri. 2019. "Occurrence of Breast Meat Abnormalities and Foot Pad Dermatitis in Light-Size Broiler Chicken Hybrids" Animals 9, no. 10: 706. https://doi.org/10.3390/ani9100706
APA StyleZampiga, M., Meluzzi, A., Pignata, S., & Sirri, F. (2019). Occurrence of Breast Meat Abnormalities and Foot Pad Dermatitis in Light-Size Broiler Chicken Hybrids. Animals, 9(10), 706. https://doi.org/10.3390/ani9100706





