Simple Summary
Pig keeping is an important source of income but the high cost of fishmeal (FM), which is the main protein source in animal feeds, has hindered the sector from realizing its full potential. As an alternative, we investigated the suitability of a meal derived from black soldier fly larvae (BSFLM) as a protein source. Pigs were fed different diet types: Control (no BSFLM: 0% (T0)), 25% (T25), 50% (T50), 75% (T75) and 100% (T100) replacement of FM by BSFLM. Average daily feed intake, body weight gain and feed conversion ratio were not affected by the replacement of FM by BSFLM. Red or white blood cell parameters did not differ among diets, except for neutrophil counts, which were higher at T75 and T100 compared to T0. At T25, T75 and T100, pigs had lower platelet counts compared to pigs fed T0 and T50. Dietary BSFLM inclusion did not influence blood cholesterol levels. The cost–benefit ratio and return on investment were similar across diets. Our study shows that BSFLM is a suitable and cost-effective alternative to FM in pig feeds.
Abstract
Pig production is one of the fastest growing livestock sectors. Development of this sector is hampered by rapidly increasing costs of fishmeal (FM), which is a common protein source in animal feeds. Here, we explored the potential of substituting FM with black soldier fly larval meal (BSFLM) on growth and blood parameters of pigs as well as economic aspects. At weaning, 40 hybrid pigs, i.e., crossbreeds of purebred Large White and Landrace were randomly assigned to five iso-nitrogenous and iso-energetic dietary treatments: Control (0% BSFLM and 100% FM (T0)), and FM replaced at 25% (T25), 50% (T50), 75% (T75) and 100% (T100) with BSFLM. Average daily feed intake (ADFI), average daily gain (ADG), body weight gain (BWG) and feed conversion ratio (FCR) were calculated for the whole trial. Hematological and serum biochemical parameters, the cost–benefit ratio (CBR) and return on investment (RoI) were evaluated. No significant effect of diet type was observed on feed intake and daily weight gain. Red or white blood cell indices did not differ among diets. Pigs fed T25, T75 and T100, had lower platelet counts compared to T0 and T50. Dietary inclusion of BSFLM did not affect blood total cholesterol, triglycerides, low-density lipoprotein and high-density lipoprotein. CBR and RoI were similar for the various diets. In conclusion, BSFLM is a suitable and cost-effective alternative to fishmeal in feed for growing pigs.
1. Introduction
Pig production is one of the fastest growing livestock sectors globally, with most of the growth occurring in the developing countries [1]. Pigs are of socio-economic value to smallholder farmers and provide a safety net in times of financial crisis [1]. A short breeding cycle, high fecundity, high feed conversion efficiency and increasing demand are major drivers of growth in this sector. In communities currently experiencing a shift from ruminant to non-ruminant livestock production, pig farming is becoming relevant [1,2]. However, expansion and profitability are constrained by increasing feed costs, especially the protein ingredients [1]. Feed costs represent 60%–70% of total costs in intensive pig production, which is especially due to costs of protein. In East Africa, major protein ingredients such as fishmeal (FM) and soybean meal are increasingly unavailable and expensive for smallholder farmers [3]. Consequently, farmers resort to alternative feed sources considered to be cheaper without knowledge of their influence on the physiological response and animal growth [2,4,5,6]. Considering the importance of pig production in livelihoods of smallholder farmers and the growing scarcity of protein ingredients as well as the environmental implications of producing these resources [7,8], dependence on FM and soybean meal is not sustainable [9].
Insects have high protein and fat content and have been considered promising high-quality feed components [10,11,12]. Insects could replace 25% to 100% of FM or soybean meal in feeds for livestock and aquaculture, depending on the insect, livestock and fish species [13]. The black soldier fly (BSF) Hermetia illucens L. (Diptera: Stratiomyidae) is distributed worldwide in the tropics and warm temperate regions [14]. BSF larvae feed on organic resources such as fruit remains, animal manure, vegetables and brewers’ spent grains [14,15,16,17,18,19,20,21,22] and convert these resources into high-quality insect protein and fat. In contrast to other dipteran species such as the house fly Musca domestica, BSF is not considered a pest and its larvae can reduce populations of harmful bacteria [23,24]. On a dry matter basis, BSF larvae contain 37% to 63% crude protein, 7% to 39% fat [11,25], 8% calcium, 1% to 2% phosphorus, 0.1% to 0.3% sodium and 0.4% to 1% magnesium [25,26,27,28]. BSF larval meal has been used as an ingredient in feed for fish [29,30,31,32,33,34,35,36], poultry [3,37,38] and pigs [39,40,41,42], with promising results. However, there is no exhaustive information on the influence of BSF larval meal (BSFLM) on performance and health response of growing pigs. The few studies on the inclusion of BSFLM in pig feeds have focused on piglets, replacing either FM or soybean meal with low (less than 10%) levels of BSFLM over short (10–40 days) periods of feeding [39,40,41,42]. The short experimental periods do not allow for a complete growth phase under the dietary supplementation and, therefore, do not reflect the common practice of pig feeding. In addition, higher levels of BSFLM inclusion in pig feed have not been assessed. In the present study, we subjected growing pigs to feeds with higher (25%–100%) dietary replacement levels of FM with BSFLM over an extended period (>60 days) of feeding, covering the complete pig grower phase and measured the effect of diet on performance and economics of pig feeding.
Hematological and biochemical parameters are affected by several factors, including diet [4]. Nutritional deficiencies and diseases influence clinical health status of animals [43]. In the case of nutritional deficiencies, blood profiling can provide an indication of the clinical health status as well as the extent to which dietary deficiencies impact physiological status of the animal, which allows farmers to adjust the diets of the animals to ensure that they receive adequate feed ingredients for optimal production [4,44,45]. BSF larvae represent a novel protein source in animal feed. Studies have reported unaffected growth performance, blood parameters, nutrient digestibility, gut morphology and histological features of piglets as well as gut antimicrobial potential of the inclusion of full-fat or partially defatted BSFLM in piglet feed at the rate of 4%–10% to replace soybean meal or FM [40,41,42], but there is little information on the effect of complete replacement of FM by full-fat BSFLM in pig feed. Utilization of insect meal as an animal feed ingredient is attracting interest from researchers because conventional feed ingredients are increasingly becoming unaffordable to resource-poor farmers due to rapidly rising costs. This requires us to search for alternative protein ingredients that can economically supplement conventional feed ingredients used in feed formulation without adverse effects on the health and performance of the animals. The inclusion of ingredients in feed formulation does not only aim at a balanced nutrient content for optimal growth performance, but also considers profitability of the production process [46]. Therefore, the present study aimed at evaluating the effect of substituting FM with BSFLM on (a) growth performance, (b) hematological and serum biochemical indices and (c) economic implications of BSFLM inclusion in growing pig diets.
2. Materials and Methods
The present study was conducted at the pig rearing facility of the Non-ruminant Research Institute (NRI) of the Kenya Agricultural and Livestock Research Organization (KALRO), Naivasha, Kenya. The general care and management of the animals followed accepted guidelines as described by the Federation of Animal Science Societies [47].
2.1. BSF Larval Meal (BSFLM) and Experimental Diets
Diets were formulated to meet growing pig requirements [48]. Isonitrogenous and isoenergetic diets were prepared by replacing the FM content of a control diet (T0) at 25%, 50%, 75% and 100% (T25, T50, T75 and T100, respectively) with BSFLM, as experimental diets (Table 1). Maize meal, wheat pollard and rice polishing were included as energy sources. BSFLM and FM served as major protein sources. Vitamin and mineral premix, salt, limestone and bone meal served as vitamin and mineral sources (Table 1). FM, maize meal, rice polishing, salt, limestone, vitamin and mineral premix, lysine, methionine and bone meal were purchased from commercial animal feed retailers. BSF larvae were obtained from the International Centre of Insect Physiology and Ecology (icipe), reared following the BSF rearing procedure of the Animal Rearing and Containment Unit (ARCU), icipe. BSF larvae were reared on a mixture of brewers’ spent grains (BSGs) obtained from Kenya Breweries Limited (KBL). After harvest, larvae were sterilized by washing in warm water (84 °C) for 10 minutes and then oven-dried using a commercial stainless-steel fruit/vegetable/meat/fish drying machine model (CT-C-III Series hot air circulating drying oven, Henan Forchen Machinery Co., Ltd., Henan, China). The machine can dry 360 kg/batch of fresh insects for 2.5 h at 120 °C. Dried larvae were ground into larval meal using a hammer mill [39].

Table 1.
Ingredients, composition of black soldier fly larval meal and experimental diets.
2.2. Proximate, Amino Acids and Mineral Composition of Experimental Diets
The dry matter content of formulated feed samples was gravimetrically determined after the loss of water. The samples were heated to 103 ± 2 °C for 3 h to constant weight. Ash content was determined by ignition of samples at 550 °C in a muffle furnace. Dried and ground samples were exposed to an electromagnetic scan in the absorbance mode using near infrared (NIR) spectroscopy (CROPNUTS, Nairobi, Kenya). The crude protein, fat, starch, oil, acid detergent fiber, neutral detergent fiber, sugar and digestibility (NCGD) values were determined following standard laboratory procedures and energy values were calculated [49,50].
Essential and non-essential amino acid contents of BSF larvae and experimental diets (Table 1) were analyzed by AMINOLab® (Evonik Industries, Hanau, Germany) using an amino acid analyzer (Biochrom 30 plus, Biochrom Ltd. Cambridge, UK) [51,52,53,54,55]. Feed samples were homogenously ground with an Ultra Centrifugal Mill RETSCH-ZM 200 to pass through a 0.5 mm sieve. Finely ground samples were weighed using an analytical balance, display accuracy ±0.01 mg into 50 mL laboratory bottles, with thread of DURAN glass (Schott, Mainz, Germany), red polybutylene terephthalate (PBTP) caps with silicone/Teflon seal. Then, 25 mL of hydrochloric-phenol reagent was added to the sample in the bottles and the mixture was introduced into a thermostatically controlled heating oven (UT 6060 AR, Thermo Electron LED, Langenselbold, Germany) at 110 °C, with loose screw tops for one hour and then with tightened screw tops for 23 h to complete the sample hydrolysis. Methionine and cystine samples were prepared through performic acid oxidation procedures followed by the acid hydrolysis–sodium metabisulfite method [52,56]. The resulting hydrolysate solutions were then introduced into the amino acid analyzer and the sample aminograms were detected at 570 nm and 440 nm. Amino acid concentrations in the samples were determined in duplicate.
The mineral content of feed samples (Table 2) was analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-OES; CROPNUTS, Nairobi, Kenya). Sample preparation involved microwave-assisted acid digestion. Aliquots of ground feed samples were transferred to a glass tube of the microwave system. Then, a mixture of nitric acid and hydrochloric acid was added to the sample and allowed to digest. The resulting solution was filtered into a volumetric flask and used for ICP-OES analysis to determine the following minerals: Boron, molybdenum, iron, copper, zinc, cobalt, manganese, sodium, sulphur, magnesium, potassium, phosphorus and calcium [57,58,59,60].

Table 2.
Mineral and proximate composition of experimental diets.
2.3. Experimental Animals and Housing
Before the commencement of the experiment, forty (20 male and 20 female) hybrid pig weaners, which consisted of a cross between purebred Large White and the Landrace with mean body weight of 18.25 ± 0.34 kg were sourced from Farmer’s Choice Limited, Nairobi, Kenya. The pigs were randomly assigned to five dietary treatments, each replicated four times per sex (four males and four females). Pigs were housed in concrete floor pens (3.65 m × 1.85 m) each containing two pigs (one male and one female). Each pen was provided with a one-sided self-feeder (1.80 m × 0.20 m × 0.18 m). Pigs were adapted to the pens for 14 days before the start of the experiment, during which they were fed a commercial starter feed. At the start of the experiment, each pen was labeled with a number and diet type while each animal was identified with a unique number by ear tattooing. A layer (~0.25 m thick) of dry wood shavings was carefully placed at one corner of the floor of each pen, which served as bedding for the pig and provided warmth. Pig pens were cleaned every day by scrubbing the floor using Teepee straight brooms (c27, Chandarana Foodplus, Nairobi, Kenya) and water. Each pen was provided with a nipple drinker fitted to the wall and the distance between the nipple and floor was adjusted as the pigs increased in height. Experimental animals were allowed ad libitum access to feed and water throughout the experiment.
2.4. Growth Performance
Individual pig body weight was recorded on a weekly basis using a 150 kg × 500 g suspended weighing scale (Salter, model 235, Bilston, England), with the sides covered with a wire mesh to prevent uncontrolled exit of the animal. The entry gate of the weighing cage was opened sideward and the animal was led into a stable, non-moving floor after which the gate was closed. Once inside the cage, the animal was allowed to settle, and the weight value read on an analogue display screen above the weighing cage. After every reading, the pig was released from the weighing cage into its pen and the scale was moved to the next pen on two wheels fitted on the front end of the cage. On the day of weighing, pigs were only provided with feed after recording their body weight. The weekly body weights were used to calculate average daily weight gain (ADG). Feed offered to the pigs and unconsumed portions were weighed daily using a digital platform weighing scale (XK3190-A12, >300 kg, Gromy Scale Co., Ltd., Hangzhou, China) to calculate average daily feed intake (ADFI). The trial lasted nine weeks. Total body weight gain and feed consumed were used to calculate feed conversion ratio (FCR) for each dietary treatment.
2.5. Blood Characteristics
At the end of the growing pig phase, three randomly selected pigs from each dietary treatment were starved for 12 h, with access to drinking water only. After this period, two blood samples (5 mL each) were drawn from the peripheral ear vein using flashback blood collection needles and 9 mL vacutainer blood collection tubes (VP4082, Sunphoria Co., Ltd., Taipei, Taiwan). One of the samples was treated with the anticoagulant ethylene diamine tetra acetic acid (K2EDTA) and the other with serum clot activator. These samples were transported immediately to the laboratory for further analysis.
2.6. Hematological and Serum Lipid Parameters
A 5-part white blood cell (WBC) differential and complete blood cell count was performed using the automated IDEXX ProCyte DxTM Hematology analyzer (IDEXX, Westbrook, ME, USA) by laser flow cytometry, optical fluorescence and laminar flow impedance. Each ethylene diamine tetra acetic acid (EDTA)-anticoagulated whole blood sample was mixed thoroughly for seven minutes on a sample rocker. Once the processing was over, the sample details were entered and the type of analysis to be carried out was indicated. Thereafter, the blood samples were loaded into the analyzer and automatically run to generate the following parameters: Red blood cells (RBC), hemoglobin concentration (Hb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet count, total white blood cell count (WBC), neutrophil percentage, lymphocyte percentage, monocyte percentage, eosinophil percentage and basophil percentage. The WBC differential counts were qualitatively verified through Romanowsky-stained thin blood smear examination using a light microscope at the oil immersion objective (100×). Clotted blood samples were centrifuged at 4000 revolutions per minute (rpm) for 10 minutes. The serum lipoprotein (HDL), triglyceride and low-density lipoprotein (LDL) levels in the samples were measured on an automatic Cobas Integra 400 plus Chemistry Analyzer (Roche, Rotkreuz, Switzerland) using enzymatic colorimetry.
2.7. Economic Analysis
Two key parameters, the cost–benefit analysis (CBA) and return on investment (RoI) [3] were used to evaluate the economic implication of replacing FM in pig diets with BSFLM. The cost–benefit ratio (CBR), as an indicator in CBA, was used to summarize the economic value of replacing FM with BSFLM in pig diets. Here, it was assumed that all other costs of production were constant for all dietary treatments, except the cost of the feed, which was considered in the CBR and RoI calculations. Feed costs were calculated from the ingredient prices based on quantities of each item incorporated in the dietary treatments. The total revenue from the pigs was estimated by considering 3.0 US $/kg of pig’s live body weight, assumed to represent all the benefits that would be received from the production. The ratio between the production revenue and the production cost represents the CBR. A CBR value greater than one suggests that the benefits of the production exceeded the production costs and vice versa. RoI is a measure of gain/loss generated from an investment relative to the money invested. The higher the RoI value the better the returns of the project under consideration [3,61].
2.8. Statistical Analysis
General linear modeling was used to assess the effect of diet on growth performance, blood parameters and economic parameters of pigs fed BSFLM-based diets and a control diet over a nine-week period. Collinearity of variables was checked to obtain independent covariates. The model for each analysis included all independent variables, which were removed one by one until the Akaike information criterion (AIC) was at a minimal level. For growth performance, diet, sex and their interaction effect were included for the analysis of ADG, body weight gain (BWG) and final body weight (FBW). For weekly body weight (BW), diet, sex, time (week) and their interaction effects were included in the model. Two pigs (female and male) per replicate were provided with feed in the same trough. Hence, ADFI analysis by sex was not possible. Three randomly selected pigs per dietary treatment were used for the blood parameter assessment, with diet as the explanatory variable. Similarly, the economic analysis was based on ADFI, hence diet was included as the explanatory variable in the model. Mean effects were considered statistically significant at p < 0.05, with a least significant difference test (LSD) as the post-hoc test. All statistical analyses of the data were implemented using R software (version 3.5.1).
2.9. Ethical Approval
Ethical approval for the study was provided by the Institutional Animal Care and Use Committee (IACUC) of Kenya Agricultural and Livestock Research Organization (KALRO)—Veterinary Science Research Institute (VSRI); approval Code No.: KALRO-VSRI/IACUC019/30082019.
3. Results
3.1. Growth Performance and Feed Conversion
Pigs readily accepted experimental diets and no mortality was recorded. Neither diet nor sex affected initial weight, final body weight (FBW) or average daily weight gain (ADG); the interaction between diet and sex was also not significant (Table 3).

Table 3.
Effects of dietary BSFLM inclusion on the growth performance of growing pigs.
Weekly body weight (BW) differed significantly among diets (p < 0.001) and sexes (p = 0.005). BW increased significantly (p < 0.001) from week 1 to week 9 for male and female pigs (Figure 1). The interaction between diet and sex on BW was significant (p < 0.001). There was no significant interaction between diet and week (p = 0.110) or between sex and week on BW (p = 0.388). Furthermore, the interaction between diet, sex and week on BW was also not significant (p = 0.345).
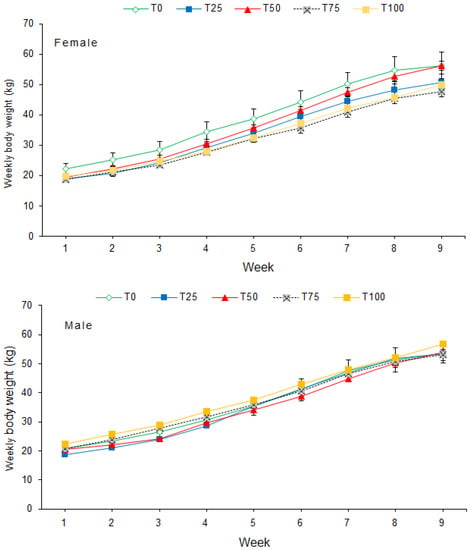
Figure 1.
Mean (±SE) weekly body weight of pigs fed BSFLM-based diets and a control diet. BSFLM = black soldier fly larval meal. T0 = 0% (control), T25 = 25%, T50 = 50%, T75 = 75% and T100 = 100% levels of replacement of fishmeal with BSFLM. For each diet, four males and four females were investigated.
Body weight gain for the entire experimental period (BWG) was neither affected by diet (p = 0.351; Figure 2) nor by sex (p = 0.486) and neither was the interaction between diet and sex (p = 0.340).
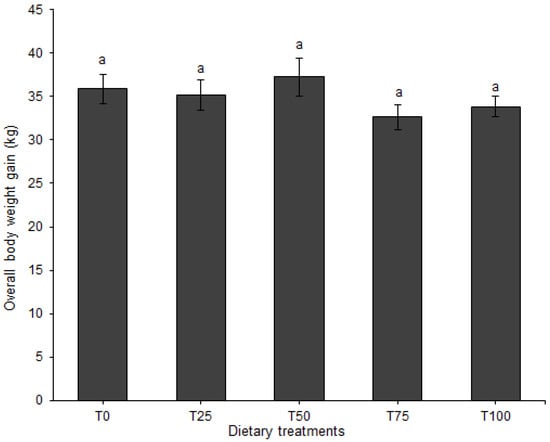
Figure 2.
Body weight gain (mean ± SE) of pigs fed BSFLM-based diets and a control diet for the whole trial. Bars followed by the same letter are not significantly different: p < 0.05, general linear model (GLM, least significant difference test (LSD)). BSFLM = black soldier fly larval meal. T0 = 0% (control), T25 = 25%, T50 = 50%, T75 = 75% and T100 = 100% levels of replacement of fishmeal with BSFLM. For each diet, four males and four females were investigated. Data for female and male pigs pooled together because there is no significant effect of sex. n = 8 per bar.
ADFI did not differ among diets (Figure 3). FCR differed significantly among diets (p = 0.011). When fed T75 or T100, FCR of the pigs was significantly higher compared to T25 (Figure 4).
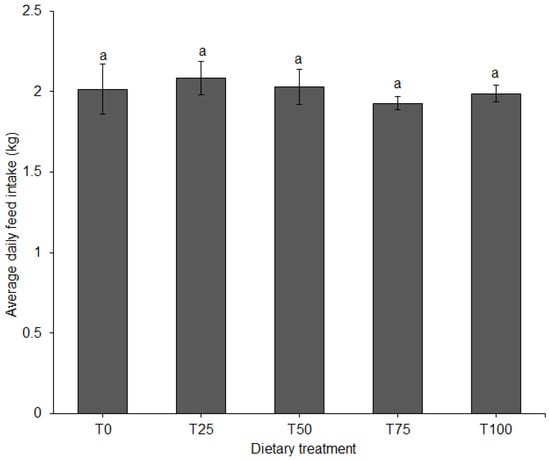
Figure 3.
Average daily feed intake (±SE) in pigs fed BSFLM-based diets and a control diet. Bars with the same letters are not significantly different: p < 0.05, general linear model (GLM), BSFLM = black soldier fly larval meal. T0 = 0% (control), T25 = 25%, T50 = 50%, T75 = 75% and T100 = 100% levels of replacement of fishmeal with BSFLM. n = 8 per bar.
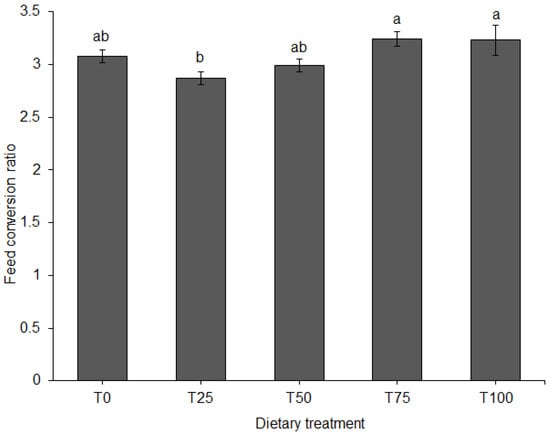
Figure 4.
Feed conversion ratio (mean ± SE) for pigs fed BSFLM-based diets and a control diet. Bars with different letters are significantly different: p < 0.05; general linear model (GLM), LSD. BSFLM = black soldier fly larval meal. T0 = 0% (control), T25 = 25%, T50 = 50%, T75 = 75% and T100 = 100% levels of replacement of fishmeal with BSFLM. n = 8 per bar.
3.2. Hematological and Serum Lipid Parameters
Red blood cell indices did not differ among dietary treatments (Table 4). Hb at T25-T100 was within the normal range, whereas at T0, Hb was slightly below the normal range. MCHC values at T25–T100 were below the normal range compared to T0. Platelet count differed significantly among diets (Table 4). At T0, platelet count was and within the normal range compared to treatments T25, T75 and T100 (Table 4).

Table 4.
Effects of dietary BSFLM inclusion on red blood cell indices and platelet count of growing pigs.
WBC count did not differ among pigs fed with different diets (p = 0.463). At T0 and T50, WBC counts were within the normal range, whereas the WBC counts of pigs from the other dietary groups were slightly above the normal range (Table 5). Diet significantly affected neutrophil counts (Table 5). At T75 and T100, neutrophil counts were significantly higher and within the normal range compared to T0 and T25 (Table 5). Lymphocyte counts did not differ among diets (Table 5). At T100, lymphocyte count was within the normal range, whereas at T0–T75 the values were above the normal range. Monocyte, eosinophil and basophil counts did not differ among diets and were all within the normal range (Table 5). Serum lipid parameters did not differ among diets. All serum lipid parameters investigated were within the normal range (Table 5).

Table 5.
Effects of dietary BSFLM inclusion on white blood cell and serum biochemical indices of growing pigs.
3.3. Economic Analyses of the Inclusion of Black Soldier Fly Larva Meal in Pig Diets
Replacing fishmeal by BSFLM in pig diet did not affect the profit accrued from the sale of pigs (Table 6). The cost–benefit ratio and return on investment did not differ among diets (Table 6).

Table 6.
Economic analyses of replacing fishmeal by BSFLM in growing pig diets.
4. Discussion
In Africa, to the best of our knowledge, the current study is the first to report the positive impact of non-defatted BSF larvae as a protein-rich ingredient in pig feeds. Protein is an important component of animal feeds required for growth and development. The source of protein is crucial because it affects availability and utilization of the essential amino acids [62]. The crude protein (CP) content of BSF larvae largely depends on the substrate used to rear the larvae and varies from 39% to 44% [63], which is comparable or superior to that of the commonly used soybean and FM [3]. BSFLM is a suitable ingredient in pig feed [13] and feeds containing BSFLM are as palatable as those containing soybean meal [39]. Our results agree with earlier studies in which partially defatted or full-fat BSF inclusion levels of only 4%–10% in partial replacement of either FM or soybean meal did not result in a significant difference in growth performance of the piglets [40,41,42], when compared to the conventional FM /soybean diets. The present study shows that much higher replacement levels, up to 100% were acceptable and well tolerated by the pigs. No significant decline in growth parameters or mortality was recorded. Acceptability and suitability of BSFLM has also been successfully recorded for fish and chicken [33,64]. Thus, our study shows that BSFLM can successfully replace FM as a sustainable protein-rich ingredient in growing pig diet as reflected in the growth performance and feed conversion rate, which were similar for all the treatment and control diet groups of pigs. Rejection of feed due to texture, palatability or inclusion of BSFLM was not observed, which is in line with the observations by Ramos-Elorduy et al. [65] for broiler chickens.
In animal production, ADG is a critical index of growth performance [66]. We did not observe a significant effect of dietary treatment on ADG of the pigs, which is a clear indication of adequate nutrient supply by the different formulated diets. Tolerance of insect-based protein-rich diets for pigs has been documented in other studies [67,68,69,70,71]. In India, silkworm meal was used to completely replace FM in the diet of growing and finishing pigs without altering carcass and meat quality and blood parameters [67,68,69]. Similarly, in Nigeria, feeding early weaned pigs with a 3:1 mixture of dried rumen content and maggot meal in the diet replacing 10% wheat offal did not have adverse effects on performance [72]. The inclusion of 10% and 15% of defatted BSFLM in the diet of growing quails (from 10 to 28 d of age) led to comparable production performances and carcass traits with those of quails fed conventional soybean meal and oil-based diets [73]. The suitability of BSFLM for the growing pigs in our experiments can be attributed to the high level of digestibility, which is consistent with other studies on pigs fed with BSF larvae [39], and broilers fed with housefly pupal meal [74].
In contrast to the current study, Newton et al. [70] reported that a complete replacement of dried plasma with BSF pre-pupal meal in the diet of early weaned pigs reduced performance of the pigs by 3%–13%. Poor performance of weaner pigs fed on pre-pupal meal might be attributed to the higher chitin levels in the pre-pupae than in larvae as used in our study, which has been reported to contribute to decreased digestibility resulting in reduced nutrient utilization and growth performance in animals when higher substitution rates are used [70,75]. Low digestibility of BSF pre-pupal protein in animal feeds is also supported by Bosch et al. [11], who attributed this to higher cuticular protein-sclerotization in the pupae. The similarity in feed intake and average daily weight gain recorded for all the treatment groups in the current study can be attributed to utilization of the 5th instar larval meal instead of the pre-pupal meal, which contributes sufficient nutrients in the diets with high level of digestibility. BSFLM has also been shown to be of good nutrient composition for reptiles [76].
In the present study, the values of the hematological parameters RBC, Hb, Hct, MCH, MCV, MCHC and RDW for pigs fed BSFLM were not significantly influenced by the replacement levels. The values for RBC, Hb, Hct, MCH and RDW fell within the physiological range for pigs, which is a clear indication of a good health status of the animals, implying that the quality of the test diets was adequate to maintain good health of the pigs. Dietary replacement of FM with BSFLM at the rates of 25%, 50%, 75% and 100% in pig diet improved RBC, Hb, Hct and RDW, which had higher values compared to the control FM diet group of pigs. The RBC counts and Hb concentration in blood increased to a level of 37% and 35.3%, respectively, at a FM replacement level of 50% with BSFLM. These results are consistent with the reports by Marono et al. [77] and Loponte et al. [78], who reported that dietary BSFLM inclusion positively affected the blood profile of laying hens and Barbary partridges, in terms of higher globulin levels. Our results may be attributed to high digestibility of insect-based protein and high levels of minerals such as iron, which is required for the formation of haemoglobin in the pigs. The higher the haemoglobin concentration, the better the oxygen circulation in the body, hence, better performance of the animal [79].
The results of the present study show that the composition of the various treatments significantly affected platelet count in pigs. The replacement of FM with BSFLM at rates of 25%, 75% and 100% in the feed was associated with significantly lower blood platelet counts out of the normal range than observed with 50% replacement of FM with BSFLM or with the control diet without BSFLM. This implies that diet composition in the present study significantly influenced the developing hematological system with some unknown factors suppressing the normal developmental increase in platelet counts in growing pigs. Low platelet concentration implies that blood clotting might be impaired, resulting in blood loss in case of injury [4]. According to Martin et al. [80], bleeding time largely depends on both platelet counts and mean platelet volume.
The largely similar WBC count obtained in this study implies that the ability of the pigs to respond to and eliminate infection was not compromised with the inclusion of BSFLM to replace FM in the diets. The normal monocyte levels may indicate that the pigs did not react to any infections during the experimental period. Furthermore, the similarity in basophil levels indicates that the pigs showed no hypersensitivity reaction to the inclusion of BSFLM in diets while the normal levels of eosinophils might indicate that the pigs did not suffer from parasitic infections during the experimental period [81,82]. However, higher (75% and 100%) levels of replacement of FM with BSFLM significantly improved the neutrophil count to the normal physiological range compared to the control FM diet. Neutrophils play an important role in immune responses, especially in wound healing through microbial sterilization and macrophage attraction [83]. BSF larval fat contains medium-chain saturated fatty acids with antimicrobial properties [41,84]. For instance, lauric acid has been identified as the most predominant medium chain saturated fatty acid found in BSF larvae. It has been shown that inclusion of coconut oil, which contains medium-chain saturated fatty acids in rabbit feeds significantly increases leucocytes and neutrophil counts [85]. The increase in neutrophil count in the present study could be an indication of the antimicrobial response in pigs fed high BSFLM-based diets. According to Ahlante et al. [85], increased mobilization of leucocytes and neutrophils in animals fed with coconut oil-based feed resulted from the stimulation of the pluripotent haemopoietic stem cells from which leucocytes are produced in the presence of a growth inducer and differentiation inducer, which are proteins. Neutropenia is a consequence of reduced neutrophil and leucocyte levels, which leads to reduced body immunity. Therefore, the inclusion of BSFLM in pig feed is highly recommended due to the valuable nutrients available to the growing pigs. The lymphocyte counts of pigs fed 100% FM diet, 25%, 50% and 75% BSFLM diets were higher and out of the normal range. This implies that these diets might have stimulated both cellular and humoral immune response systems of the pigs to protect against intracellular and extracellular pathogens such as Mycobacterium, Listeria, Brucella, Pasteurella or Salmonella, viruses and fungi [86].
The replacement of FM by BSFLM up to 100% did not affect the serum biochemical indices that are indicators of pig health. It is worth noting that although the use of hematological and biochemical indices have been considered as a fast means of assessing nutritional and health status of farmed animals, this has rarely been used in pig veterinary practices [42]. The observation that replacing FM by BSFLM does not affect serum biochemical indices in pigs supports the conclusion that BSFLM could form the basis of a valuable component in grower pig diets.
The cost–benefit analysis, which assesses whether an investment is sound and if—and by how much—profits outweigh costs, allows for comparing costs and benefits of alternative investments [87] as in the present study. The similarity in results obtained for the ‘control’ (100% FM diet) and BSFLM-based diets indicate that BSFLM is not only a valuable component of pig feed from a performance perspective but also from an economic perspective. This supports the need to further investigate the economic prospects of using BSFLM in large-scale pig feed formulation and feeding programs. A key advantage of insects as a feed ingredient, especially the BSF larvae over other conventional protein sources is their ability to convert waste into high-value biomass and closing nutrient cycles as they reduce pollution and costs of managing organic waste [88].
5. Conclusions
Dietary replacement of FM up to 100% with full-fat BSFLM did not adversely affect growth, blood characteristics or economic parameters. Although some changes in blood cell counts were observed, values were largely similar among diets. Pigs did not show visual signs of illness or abnormal behavior. Moreover, serum biochemical parameters were all within normal range for pigs. The present study indicates that a complete replacement of FM with full-fat BSFLM as an ingredient in growing pig feed is feasible, with reduced predisposition to heart diseases associated with high total cholesterol and LDL. Cost–benefit analysis results of the present study indicate that the inclusion of BSFLM in pig feed is a worthwhile investment for pig farmers. Finally, there is little evidence to suggest that adverse health effects should be expected in pigs following BSFLM consumption. Further studies are required to assess the effect of feeding BSFLM to pigs on meat sensory attributes and consumer perceptions.
Author Contributions
Conceptualization, M.D., C.M.T., J.J.A.v.L., S.E., S.Y.C., S.S. and K.K.M.F.; methodology, S.Y.C., I.M.O., D.M.M., A.O.A., C.M.T., M.G., S.E., S.S., J.J.A.v.L. and M.D.; software, S.Y.C.; validation, S.Y.C., M.D., C.M.T., J.J.A.v.L., S.E., S.S., A.O.A., I.M.O., D.M.M.; formal Analysis, S.Y.C. and D.M.M.; investigation, S.Y.C., C.M.T., J.J.A.v.L., M.D., S.S., M.G., I.M.O., D.M.M. and A.O.A.; resources, M.D., C.M.T., J.J.A.v.L., S.E., S.Y.C., S.S., A.O.A. and K.K.M.F.; data curation, S.Y.C., I.M.O., D.M.M., C.M.T., S.S., J.J.A.v.L. and M.D; writing—original draft preparation, S.Y.C.; writing—review & editing, S.Y.C., J.J.A.v.L., M.D., D.M.M., I.M.O., S.E., S.S., K.K.M.F., M.G. and A.O.A.; visualization, S.Y.C., C.M.T, J.J.A.v.L., M.D., S.E., S.S., I.M.O., A.O.A. and D.M.M.; supervision, C.M.T., S.E., J.J.A.v.L., M.D.; project administration, C.M.T, S.E., J.J.A.v.L., M.D; funding acquisition, S.E., J.J.A.v.L., M.D.
Funding
Financial support for this research was provided by the Netherlands Organization for Scientific Research, WOTRO Science for Global Development (NWO-WOTRO) (ILIPA—W 08.250.202), the Canadian International Development Research Centre (IDRC) and the Australian Centre for International Agricultural Research (ACIAR) (INSFEED—Phase 2: Cultivate Grant No: 108866-001), The Rockefeller Foundation (SiPFeed—Grant No: 2018 FOD 009) and Federal Ministry for Economic Cooperation and Development (BMZ) (ENTONUTRI—81194993) through the International Centre of Insect Physiology and Ecology (icipe). We also gratefully acknowledge the icipe core funding provided by UK Aid from the Government of the United Kingdom; Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); Federal Ministry for Economic Cooperation and Development (BMZ), Germany, and the Kenyan Government. The views expressed herein do not necessarily reflect the official opinion of the donors.
Acknowledgments
The senior author, S.Y. Chia was supported by the Netherlands Organization for Scientific Research, WOTRO Science for Global Development (NWO-WOTRO) scholarship. The authors gratefully acknowledge Mr. Ogetonto Wycliffe, Rachami, Isaiah E.; Nyamu Faith Wamurango; Ondiaka Shem, Wambua, Joshua M. and the Non-ruminant Research Institute staff for their technical support in the laboratory and for providing dried black soldier fly larvae produced at International Centre of Insect Physiology and Ecology (icipe). Also, we are grateful to the Director and all the staff of Kenya Agricultural and Livestock Research Organization (KALRO) for allowing us to access their field station and facilities throughout the experimental period.
Conflicts of Interest
Authors declare that they have no conflicts of interest.
References
- Githigia, S.M.; Okuthe, S.; Diop, B. Pig Sector Kenya. FAO Animal Production and Health Livestock Country Reviews. No. 3. FAO, Rome. 2012. Available online: http://www.fao.org/3/a-i2566e.pdf (accessed on 25 March 2019).
- Serem, J.K.; Wahome, R.G.; Gakuya, D.W.; Kiama, S.G.; Gitao, G.C.; Onyango, D.W. Growth performance, feed conversion efficiency and blood characteristics of growing pigs fed on different levels of Moringa oleifera leaf meal. J. Vet. Med. Animal Health 2017, 9, 327–333. [Google Scholar] [CrossRef]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for income generation through animal feed: Effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Etim, N.N.; Offiong, E.E.A.; Williams, M.E.; Asuquo, L.E. Influence of nutrition on blood parameters of pigs. Am. J. Biol. Life Sci. 2014, 2, 46–52. [Google Scholar]
- Etim, N.N. Hematological parameters and factors affecting their values. Agric. Sci. 2014, 2, 37–47. [Google Scholar] [CrossRef]
- Akpabio, U.; Etim, N.A.N.; Okpongete, R.O.; Offiong, E.E.A. Do diets affect hematological parameters of poultry? Br. J. Appl. Sci. Technol. 2014, 4, 1952–1965. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Masuda, T.; Goldsmith, P.D. World soybean production: Area harvested, yield, and long-term projections. Int. Food Agribus. Man. 2009, 12, 143–162. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef]
- Veldkamp, T.; Van Duinkerken, G.; Van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; Van Boekel, M.A.J.S. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets—A Feasibility Study; Report 638; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2012; Available online: http://library.wur.nl/WebQuery/wurpubs/fulltext/234247 (accessed on 24 March 2019).
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed. Sci. Tech. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Newton, G.L.; Thompson, S.A.; Savage, S. A value added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2013, 50, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Myers, H.M.; Tomberlin, J.K.; Lambert, B.D.; Kattes, D. Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environ. Entomol. 2008, 37, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.U.; Cai, M.; Xiao, X.; Zheng, L.; Wang, H.; Soomro, A.A.; Zhou, Y.; Li, W.; Yu, Z.; Zhang, J. Cellulose decomposition and larval biomass production from the co-digestion of dairy manure and chicken manure by mini-livestock (Hermetia illucens L.). J. Environ. Manag. 2017, 196, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of life cycle assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Fiaboe, K.K.; Niassy, S.; van Loon, J.J.; Dicke, M.; et al. Effects of waste stream combinations from brewing industry on performance of black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). PeerJ 2018, 6, e5885. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Giromini, C.; Ottoboni, M.; Tretola, M.; Marchis, D. Review: Insects and former foodstuffs for upgrading food waste biomasses/streams to feed ingredients for farm animals. Animal 2019, 13, 1365–1375. [Google Scholar] [CrossRef]
- Liu, Q.L.; Tomberlin, J.K.; Brady, J.A.; Sanford, M.R.; Yu, Z.N. Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environ. Entomol. 2008, 37, 1525–1530. [Google Scholar] [CrossRef]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157:H7 and Salmonella enterica serovar Enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Newton, G.L.; Sheppard, D.C.; Watson, D.W.; Burtle, G.J.; Dove, C.R.; Tomberlin, J.K.; Thelen, E.E. The black soldier fly, Hermetia illucens, as a manure management/resource recovery tool. Symp. State Sci. Anim. Manure Waste Manag. 2005. Available online: https://pdfs.semanticscholar.org/5aa5/81bf66145ee15551c0a86df6436b9183fd05.pdf (accessed on 25 March 2019).
- Dierenfeld, E.; King, J. Digestibility and mineral availability of phoenix worms, Hermetia illucens, ingested by mountain chicken frogs, Leptodactylus fallax. J. Herpetol. Med. Surg. 2008, 18, 100–105. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of feeder insects. Zoo Biol. 2013, 32, 27–36. [Google Scholar] [CrossRef]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Devic, E.; Leschen, W.; Murray, F.; Little, D.C. Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus) fed diets containing black soldier fly (Hermetia illucens) larvae meal. Aquacult. Nutr. 2018, 24, 416–423. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Bruni, L.; Randazzo, B.; Vargas, A.; Gioacchini, G.; Truzzi, C.; Annibaldi, A.; Riolo, P.; Parisi, G.; Cardinaletti, G.; et al. Partial dietary inclusion of Hermetia illucens (black soldier fly) full-fat prepupae in Zebrafish feed: Biometric, histological, biochemical, and molecular implications. Zebrafish 2018, 15, 519–532. [Google Scholar] [CrossRef]
- Cummins, V.C.; Rawles, S.D.; Thompson, K.R.; Velasquez, A.; Kobayashi, Y.; Hager, J.; Webster, C.D. Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2017, 473, 337–344. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly pre-pupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Maurer, V.; Holinger, M.; Amsler, Z.; Früh, B.; Wohlfahrt, J.; Stamer, A.; Leiber, F. Replacement of soybean cake by Hermetia illucens meal in diets for layers. J. Insects Food Feed 2015, 2, 83–90. [Google Scholar] [CrossRef]
- Newton, G.L.; Booram, C.V.; Barker, R.W.; Hale, O.M. Dried Hermetia illucens larvae meal as a supplement for Swine. J. Anim. Sci. 1977, 44, 395–400. [Google Scholar] [CrossRef]
- Driemeyer, H. Evaluation of Black Soldier Fly (Hermetia illucens) Larvae as an Alternative Protein Source in Pig Creep Diets in Relation to Production, Blood and Manure Microbiology Parameters. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, December 2016. [Google Scholar]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) pre-pupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- Biasato, I.; Renna, M.; Gai, F.; Dabbou, S.; Meneguz, M.; Perona, G.; Martinez, S.; Lajusticia, A.C.; Bergagna, S.; Sardi, L.; et al. Partially defatted black soldier fly larva meal inclusion in piglet diets: Effects on the growth performance, nutrient digestibility, blood profile, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2019, 10, 12. [Google Scholar] [CrossRef]
- Wilson, G.D.A.; Harvey, D.G.; Snook, C.R. A review of factors affecting blood biochemistry in the pig. Br. Vet. J. 1972, 128, 596–610. [Google Scholar] [CrossRef]
- Ameen, S.A.; Adedeji, O.S.; Akingbade, A.A.; Olayemi, T.B.; Oyedapo, L.O.; Aderinola, A. The effect of different feeding regimes on hematological parameters and immune status of commercial broilers in derived Savannah Zone of Nigeria. In Proceedings of the 32nd Annual Conference Nigerian Society Animal Production (NSAP), Calabar, Nigeria, 18–21 March 2007; pp. 176–178. [Google Scholar]
- Shanmugam, A.A.; Muliya, S.K.; Deshmukh, A.; Suresh, S.; Nath, A.; Kalaignan, P.; Venkatramappa, M.; Jose, L. Baseline hematology and serum biochemistry results for Indian leopards (Panthera pardus fusca). Vet. World 2017, 10, 818–824. [Google Scholar] [CrossRef]
- Spring, P. The challenge of cost effective poultry and animal nutrition: Optimizing existing and applying novel concepts. Lohmann Inf. 2013, 48, 38–46. [Google Scholar]
- Federation of Animal Science Societies (FASS). Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; FASS: Champaign, IL, USA, 2010. [Google Scholar]
- National Research Council. Nutrient Requirements of Swine: Eighth Revised Edition; National Academies Press: Washington, DC, USA, 1979. [Google Scholar] [CrossRef]
- Núñez-Sánchez, N.; Marín, A.L.; Hernández, M.P.; Carrion, D.; Castro, G.G.; Alba, L.M. Faecal near infrared spectroscopy (NIRS) as a tool to assess rabbit’s feed digestibility. Livest. Sci. 2012, 150, 386–390. [Google Scholar] [CrossRef]
- Rosales, A.; Galicia, L.; Oviedo, E.; Islas, C.; Palacios-Rojas, N. Near-infrared reflectance spectroscopy (NIRS) for protein, tryptophan, and lysine evaluation in quality protein maize (QPM) breeding programs. J. Agric. Food Chem. 2011, 59, 10781–10786. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.D.; Chowdhury, M.K.; Bureau, D.P. Assessing the bioavailability of L-methionine and a methionine hydroxy analogue (MHA-Ca) compared to DL-methionine in rainbow trout (Oncorhynchus mykiss). Aquac. Res. 2017, 48, 332–346. [Google Scholar] [CrossRef]
- Windham, W.R. AOAC official method 994.12, amino acids in feeds, alternative III, acid hydrolysis method. In Official methods of analysis of AOAC Intemational, 16th ed.; Cunniff, P., Ed.; AOAC International: Rockville, MD, USA, 1995; Volume 1. [Google Scholar]
- Llames, C.R.; Fontaine, J. Determination of amino acids in feeds: Collaborative study. J. Assoc. Off. Anal. Chem. 1994, 77, 1362–1402. [Google Scholar]
- Zampiga, M.; Laghi, L.; Petracci, M.; Zhu, C.; Meluzzi, A.; Dridi, S.; Sirri, F. Effect of dietary arginine to lysine ratios on productive performance, meat quality, plasma and muscle metabolomics profile in fast-growing broiler chickens. J. Anim. Sci. Biotechnol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Al-Sagan, A.A.; Khalil, S.; Smith, M.P. The benefit of L-threonine supplementation on growth performance, carcass characteristics, intestinal morphology and litter quality of broilers. Braz. J. Poult. Sci. 2018, 20, 753–758. [Google Scholar] [CrossRef]
- Slump, P.; Bos, K.D. Determination of methionine in feed concentrates. Poult. Sci. 1985, 64, 705–707. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Frankowski, M.; Simeonov, V.; Polkowska, Ż.; Namieśnik, J. Determination of metals content in wine samples by inductively coupled plasma-mass spectrometry. Molecules 2018, 23, 2886. [Google Scholar] [CrossRef]
- Sreenivasulu, V.; Kumar, N.S.; Dharmendra, V.; Asif, M.; Balaram, V.; Zhengxu, H.; Zhen, Z. Determination of boron, phosphorus, and molybdenum content in biosludge samples by microwave plasma atomic emission spectrometry (MP-AES). Appl. Sci. 2017, 7, 264. [Google Scholar] [CrossRef]
- Santos, É.J.; Baika, L.M.; Herrmann, A.B.; Kulik, S.; Sato, C.S.; Santos, A.B.; Curtius, A.J. Fast assessment of mineral constituents in grass by inductively coupled plasma optical emission spectrometry. Braz. Arch. Biol. Technol. 2012, 55, 457–464. [Google Scholar] [CrossRef]
- Barałkiewicz, D.; Kanecka-Hanc, A.; Gramowska, H. ICP slurry introduction for simple and rapid determination of Pb, Mg and Ca in plant roots. Cent. Eur. J. Chem. 2007, 5, 1148–1157. [Google Scholar] [CrossRef]
- Aok, Y.M.D. Effect of Feed Enzymes and Energy Level on Broiler Chicken (Gallus domesticus) Performance in Kenya. Master’s Thesis, University of Nairobi, Nairobi, Kenya, July 2012. [Google Scholar]
- Wallace, P.A.; Nyameasem, J.K.; Adu-Aboagye, G.A.; Affedzie-Obresi, S.; Nkegbe, E.K.; Karbo, N.; Murray, F.; Leschen, W.; Maquart, P. Impact of black soldier fly larval meal on growth performance, apparent digestibility, hematological and blood chemistry indices of guinea fowl starter keets under tropical conditions. Trop. Anim. Health Prod. 2017, 49, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Pieterse, E.; Erasmus, S.W.; Uushona, T.; Hoffman, L.C. Black soldier fly (Hermetia illucens) pre-pupae meal as a dietary protein source for broiler production ensures a tasty chicken with standard meat quality for every pot. J. Sci. Food Agric. 2018, 99, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xie, C.; Long, C.; Li, J.; Zhou, X.; Fan, Z.; Blachier, F.; Yin, Y. Effects of a daily three-meal pattern with different dietary protein contents on pig growth performance, carcass and muscle quality traits. J. Sci. Food Agric. 2018, 98, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Medhi, D. Effects of enzyme supplemented diet on finishing crossbred pigs at different levels of silk worm pupae meal in diet. Indian J. Field Vet. 2011, 7, 24–26. [Google Scholar]
- Medhi, D.; Math, N.C.; Sharma, D.N. Effect of silk worm pupae meal and enzyme supplementation on blood constituents in pigs. Indian Vet. J. 2009, 86, 433–434. [Google Scholar]
- Medhi, D.; Nath, N.C.; Gohain, A.K.; Bhuyan, R. Effect of silk worm pupae meal on carcass characteristics and composition of meat in pigs. Indian Vet. J. 2009, 86, 816–818. [Google Scholar]
- Newton, L.A.; Sheppard, C.R.; Watson, D.W.; Burtle, G.A.; Dove, R.O. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Coll, J.F.; Crespi, M.P.; Itagiba, M.G.; Souza, J.C.; Gomes, A.V.; Donatti, F.C. Utilization of silkworm pupae meal (Bombyx mori L.) as a source of protein in the diet of growing-finishing pigs. Rev. Bras. Zootec. 1992, 21, 378–383. [Google Scholar]
- Adeniji, A.A. The feeding value of rumen content-maggot meal mixture in the diets of early weaned piglets. Asian J. Anim. Vet. Adv. 2008, 3, 115–119. [Google Scholar] [CrossRef][Green Version]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Zotte, A.D. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, Q. The Evaluation of Larvae of Musca domestica (Common House Fly) as Protein Source for Broiler Production. Master’s Thesis, Stellenbosch University, Matieland, South Africa, March 2011. [Google Scholar]
- Alegbeleye, W.O.; Obasa, S.O.; Olude, O.O.; Otubu, K.; Jimoh, W. Preliminary evaluation of the nutritive value of the variegated grasshopper (Zonocerus variegatus L.) for African catfish Clarias gariepinus (Burchell. 1822) fingerlings. Aquacult. Res. 2012, 43, 412–420. [Google Scholar] [CrossRef]
- Klaphake, E. A fresh look at metabolic bone diseases in reptiles and amphibians. Vet. Clin. North Am. Exot. Anim. Pract. 2010, 13, 375–392. [Google Scholar] [CrossRef]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Loponte, R.; Nizza, S.; Bovera, F.; De Riu, N.; Fliegerova, K.; Lombardi, P.; Vassalotti, G.; Mastellone, V.; Nizza, A.; Moniello, G. Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 2017, 115, 183–188. [Google Scholar] [CrossRef]
- Olugbemi, T.S.; Mutayoba, S.K.; Lekule, F.P. Effect of Moringa (Moringa oleifera) inclusion in cassava based diets fed to broiler chickens. Int. J. Poul. Sci. 2010, 9, 363–367. [Google Scholar] [CrossRef]
- Martin, J.F.; Trowbridge, E.A.; Salmon, G.; Plumb, J. The biological significance of platelet volume: Its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb. Res. 1983, 32, 443–460. [Google Scholar] [CrossRef]
- American Association for Clinical Chemistry. White Blood Cell (WBC) Differential. 2019. Available online: https://labtestsonline.org/tests/white-blood-cell-wbc-differential (accessed on 11 July 2019).
- Konlan, S.P.; Karikari, P.K.; Ansah, T. Productive and blood indices of dwarf rams fed a mixture of rice straw and groundnut haulms alone or supplemented with concentrates containing different levels of shea nut cake. Pak. J. Nutr. 2012, 11, 566–571. [Google Scholar]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Skrivanova, E.; Marounek, M.; Benda, V.; Brezina, P. Susceptibility of Escherichia coli, Salmonella sp. and Clostridium perfringens to organic acids and monolaurin. Vet. Med. 2006, 51, 81–88. [Google Scholar] [CrossRef]
- Ahlante, B.O.; Afiukwa, J.N.; Ajay, O.I. The dietary effects of coconut oil on the leucocytes and neutrophils count in rabbits. J. Ecophysiol. Occup. Health 2010, 10, 143–148. [Google Scholar]
- Dudek, K.; Sliwa, E.; Tatara, M.R. Changes in blood leukocyte pattern in piglets from sows treated with garlic preparations. Bull. Vet. Inst. Pulawy 2006, 50, 263–267. [Google Scholar]
- David, R.; Ngulube, P.; Dube, A. A cost–benefit analysis of document management strategies used at a financial institution in Zimbabwe: A case study. J. Inf. Manag. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).