Supplementing Dairy Ewes Grazing Low Quality Pastures with Plant-Derived and Rumen-Protected Oils Containing Eicosapentaenoic Acid and Docosahexaenoic Acid Pellets Increases Body Condition Score and Milk, Fat, and Protein Yields
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animal Management and Experimental Design
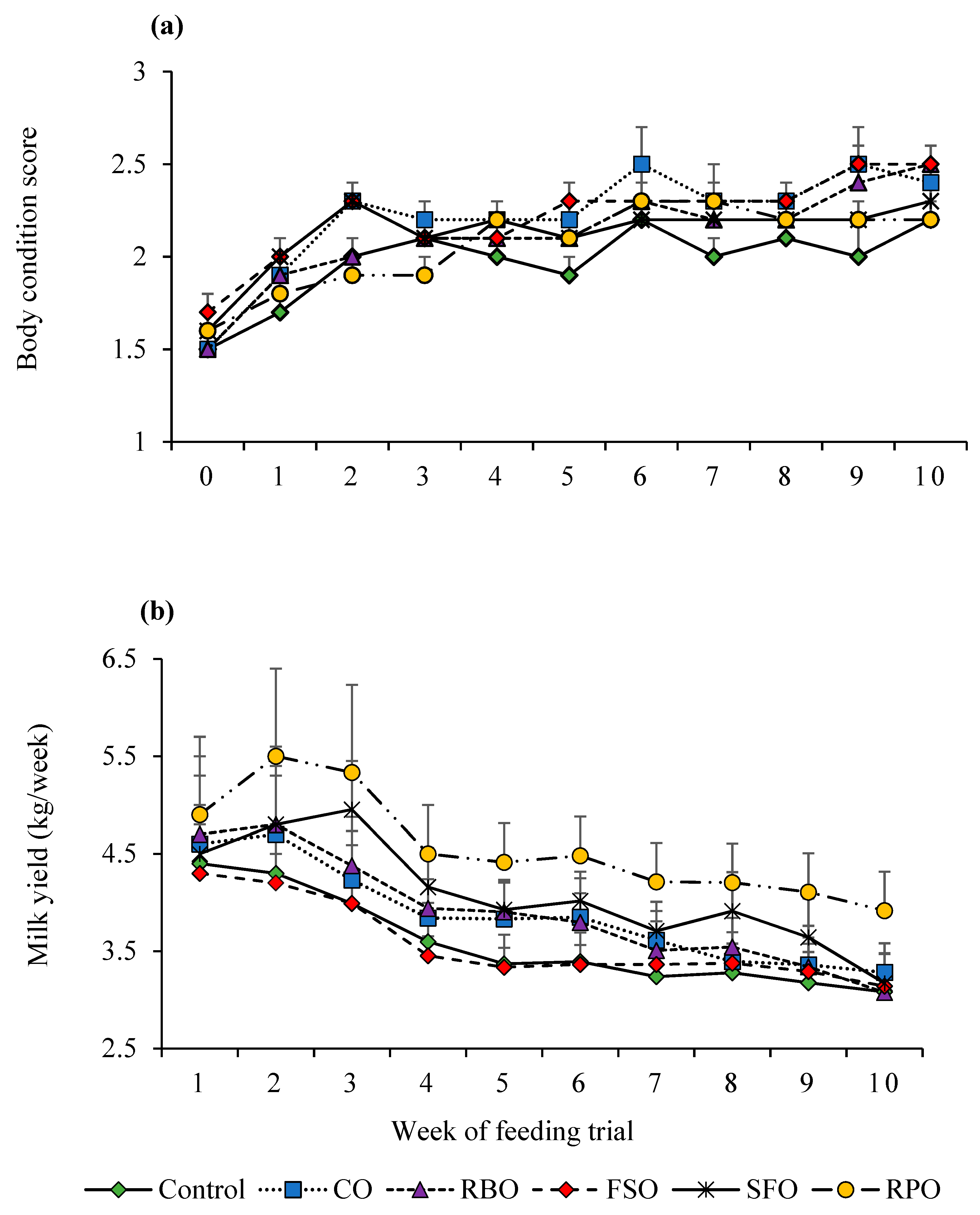
2.3. Feed Intake and Body Condition Score
2.4. Milk Sample Analyses
2.5. Chemical Analysis of Experimental and Basal Diets
2.6. Data and Statistical Analysis
3. Results
4. Discussion
4.1. Effect of Dietary Supplements on Dry Matter Intake and Body Condition Score
4.2. Effect of Dietary Supplements on Milk Yield and Milk Composition
4.3. Effect of Breed on Animal Performance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, Y.W.; Juarez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U. The interrelationships between lactose intolerance and the modern dairy industry: Global perspectives in evolutional and historical backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef] [PubMed]
- AgriFutures Australia. Dairy Sheep. 2013. Available online: https://www.agrifutures.com.au/farm-diversity/dairy-sheep/ (accessed on 31 April 2018).
- Dairy Australia. Milk. 2018. Available online: https://www.dairyaustralia.com.au/industry/production-and-sales/milk (accessed on 31 April 2018).
- Abd Allah, M.; Abass, S.; Allam, F.M. Factors affecting the milk yield and composition of Rahmani and Chios sheep. Int. J. Livest. Prod. 2011, 2, 24–30. [Google Scholar]
- Ayadi, M.; Matar, A.; Aljumaah, R.; Alshaikh, M.; Abouheif, M. Factors affecting milk yield, composition and udder health of Najdi ewes. Int. J. Anim. Vet. Adv. 2014, 6, 28–33. [Google Scholar]
- Caja, G.; Bocquier, F. Effects of nutrition on the composition of sheep’s milk. Cah. Opt. Mediterr. 2000, 55, 59–74. [Google Scholar]
- Hristov, A.N.; Price, W.J.; Shafii, B. A meta-analysis examining the relationship among dietary factors, dry matter intake, and milk and milk protein yield in dairy cows. J. Dairy Sci. 2004, 87, 2184–2196. [Google Scholar] [CrossRef]
- Kennelly, J.J.; Bell, J.A.; Keating, A.F.; Doepel, L. Nutrition as a tool to alter milk composition. Adv. Dairy Technol. 2005, 17, 255–275. [Google Scholar]
- Chilliard, Y.; Ferlay, A.; Rouel, J.; Lamberet, G. A review of nutritional and physiological factors affecting goat milk lipid synthesis and lipolysis. Livest. Prod. Sci. 2003, 86, 1751–1770. [Google Scholar] [CrossRef]
- Pulina, G.; Nudda, A.; Battacone, G.; Cannas, A. Effects of nutrition on the contents of fat, protein, somatic cells, aromatic compounds, and undesirable substances in sheep milk. Anim. Feed Sci. Technol. 2006, 131, 255–291. [Google Scholar] [CrossRef]
- McGuire, M.A.; McGuire, M.K. Conjugated linoleic acid (CLA): A ruminant fatty acid with beneficial effects on human health. J. Anim. Sci. 2000, 77, 1–8. [Google Scholar] [CrossRef]
- Calder, P.C. Long-chain fatty acids and inflammation. Proc. Nutr. Soc. 2012, 71, 284–289. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Belury, M.A. Inhibition of carcinogenesis by conjugated linoleic acid: Potential mechanisms of action. J. Nutr. 2002, 132, 2995–2998. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Cole, G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: Evidence from animal studies. Prostag. Leukotr. Ess. 2007, 77, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Santos, G.; O’Donnell, A.M.; Vicini, J.L.; Hartnell, G.F.; Bauman, D.E. Hot topic: Enhancing omega-3 fatty acids in milk fat of dairy cows by using stearidonic acid-enriched soybean oil from genetically modified soybeans. J. Dairy Sci. 2010, 93, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Castro, T.; Manso, T.; Jimeno, V.; Del Alamo, M.; Mantecon, A.R. Effects of dietary sources of vegetable fats on performance of dairy ewes and conjugated linoleic acid (CLA) in milk. Small Rumin. Res. 2009, 84, 47–53. [Google Scholar] [CrossRef]
- Otto, J.R.; Nish, P.; Balogun, R.O.; Freeman, M.J.; Malau-Aduli, B.S.; Lane, P.A.; Malau-Aduli, A.E.O. Effect of dietary supplementation of pasture-based primiparous Holstein-Friesian cows with degummed crude canola oil on body condition score, liveweight, milk yield and composition. J. Appl. Anim. Res. 2016, 44, 194–200. [Google Scholar] [CrossRef]
- Pirondini, M.; Colombini, S.; Mele, M.; Malagutti, L.; Rapetti, L.; Galassi, G.; Crovetto, G.M. Effect of dietary starch concentration and fish oil supplementation on milk yield and composition, diet digestibility, and methane emissions in lactating dairy cows. J. Dairy Sci. 2015, 98, 357–372. [Google Scholar] [CrossRef]
- Pulina, G.; Macciotta, N.; Nudda, A. Milk composition and feeding in the Italian dairy sheep. Ital. J. Anim. Sci. 2005, 4, 5–14. [Google Scholar] [CrossRef]
- Akbaridoust, G.; Plozza, T.; Trenerry, V.C.; Wales, W.J.; Auldist, M.J.; Dunshea, F.R.; Ajlouni, S. Influence of different systems for feeding supplements to grazing dairy cows on milk fatty acid composition. J. Dairy Res. 2014, 81, 156–163. [Google Scholar] [CrossRef]
- Bell, J.A.; Griinari, J.M.; Kennelly, J.J. Effect of safflower oil, flaxseed oil, monensin, and vitamin E on concentration of conjugated linoleic acid in bovine milk fat. J. Dairy Sci. 2006, 89, 733–748. [Google Scholar] [CrossRef]
- Lunsin, R.; Wanapat, M.; Rowlinson, P. Effect of cassava hay and rice bran oil supplementation on rumen fermentation, milk yield and milk composition in lactating dairy cows. Asian-Australas J. Anim. Sci. 2012, 25, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Kwon, E.G.; Kim, C.H. Effects of increasing supplementation levels of rice bran on milk production and fatty acid composition of milk in Saanen dairy goats. Anim. Prod. Sci. 2013, 53, 413–418. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Bruno, A.; Fedele, V.; Santillo, A.; Sevi, A. Effect of solar radiation and flaxseed supplementation on milk production and fatty acid profile of lactating ewes under high ambient temperature. J. Dairy Sci. 2011, 94, 3856–3867. [Google Scholar] [CrossRef] [PubMed]
- Caroprese, M.; Ciliberti, M.G.; Marino, R.; Santillo, A.; Sevi, A.; Albenzio, M. Polyunsaturated fatty acid supplementation: Effects of seaweed Ascophyllum nodosum and flaxseed on milk production and fatty acid profile of lactating ewes during summer. J. Dairy Res. 2016, 83, 289–297. [Google Scholar] [CrossRef]
- Mughetti, L.; Sinesio, F.; Acuti, G.; Antonini, C.; Moneta, E.; Peparaio, M.; Trabalza-Marinucci, M. Integration of extruded linseed into dairy sheep diets: Effects on milk composition and quality and sensorial properties of Pecorino cheese. Anim. Feed Sci. Technol. 2012, 178, 27–39. [Google Scholar] [CrossRef]
- Kenyon, P.R.; Maloney, S.K.; Blache, D. Review of sheep body condition score in relation to production characteristics. N. Z. J. Agric. Res. 2014, 57, 38–64. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Mavrogenis, A.P.; Papachristoforou, C. Estimation of the energy value of milk and prediction of fat-corrected milk yield in sheep. Small Rumin. Res. 1988, 1, 229–236. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis System, Version 9.2; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Hervas, G.; Luna, P.; Mantecon, A.R.; Castanares, N.; de la Fuente, M.A.; Juarez, M.; Frutos, P. Effect of diet supplementation with sunflower oil on milk production, fatty acid profile and ruminal fermentation in lactating dairy ewes. J. Dairy Res. 2008, 75, 399–405. [Google Scholar] [CrossRef]
- Ammah, A.A.; Benchaar, C.; Bissonnette, N.; Gevry, N.; Ibeagha-Awemu, E.M. Treatment and post-treatment effects of dietary supplementation with safflower oil and linseed oil on milk components and blood metabolites of Canadian Holstein cows. J. Appl. Anim. Res. 2018, 46, 898–906. [Google Scholar] [CrossRef]
- Mapato, C.; Wanapat, M.; Cherdthong, A. Effects of urea treatment of straw and dietary level of vegetable oil on lactating dairy cows. Trop. Anim. Health Prod. 2010, 42, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.J.; Reynolds, C.K.; Hervas, G.; Griinari, J.M.; Grandison, A.S.; Beever, D.E. Examination of the persistency of milk fatty acid composition responses to fish oil and sunflower oil in the diet of dairy cows. J. Dairy Sci. 2006, 89, 714–732. [Google Scholar] [CrossRef]
- Illius, A.W.; Jessop, N.S. Metabolic constraints on voluntary intake in ruminants. J. Anim. Sci. 1996, 74, 3052–3062. [Google Scholar] [CrossRef]
- Petit, H.V.; Ivan, M.; Mir, P.S. Effects of flaxseed on protein requirements and N excretion of dairy cows fed diets with two protein concentrations. J. Dairy Sci. 2005, 88, 1755–1764. [Google Scholar] [CrossRef]
- Gonthier, C.; Mustafa, A.F.; Berthiaume, R.; Petit, H.V.; Martineau, R.; Ouellet, D.R. Effects of feeding micronized and extruded flaxseed on ruminal fermentation and nutrient utilization by dairy cows. J. Dairy Sci. 2004, 87, 1854–1863. [Google Scholar] [CrossRef]
- Doreau, M.; Chilliard, Y. Digestion and metabolism of dietary fat in farm animals. Br. J. Nutr. 1997, 78, S15–S35. [Google Scholar] [CrossRef] [PubMed]
- Malau-Aduli, A.E.O.; Anlade, Y.R. Comparative study of milk compositions of cattle, sheep and goats in Nigeria. Anim. Sci. J. 2002, 73, 541–544. [Google Scholar] [CrossRef]
- Morgan-Davies, C.; Waterhouse, A.; Pollock, M.L.; Milner, J.M. Body condition score as an indicator of ewe survival under extensive conditions. Anim. Welfare 2008, 17, 71–77. [Google Scholar]
- Phythian, C.J.; Michalopoulou, E.; Jones, P.H.; Winter, A.C.; Clarkson, M.J.; Stubbings, L.A.; Grove-White, D.; Cripps, P.J.; Duncan, J.S. Validating indicators of sheep welfare through a consensus of expert opinion. Animal 2011, 5, 943–952. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef]
- Abdel-Mageed, I. Body condition scoring of local Ossimi ewes at mating and its impact on fertility and prolificacy. Egypt. J. Sheep Goat Sci. 2009, 4, 37–44. [Google Scholar]
- Kenyon, P.R.; Morel, P.C.H.; Morris, S.T. The effect of individual liveweight and condition scores of ewes at mating on reproductive and scanning performance. N. Zeal. Vet. J. 2004, 52, 230–235. [Google Scholar] [CrossRef]
- Yilmaz, M.; Altin, T.; Karaca, O.; Cemal, I.; Bardakcioglu, H.E.; Yilmaz, O.; Taskin, T. Effect of body condition score at mating on the reproductive performance of Kivircik sheep under an extensive production system. Trop. Anim. Health Prod. 2011, 43, 1555–1560. [Google Scholar] [CrossRef]
- Komaragiri, M.V.S.; Casper, D.P.; Erdman, R.A. Factors affecting body tissue mobilization in early lactation dairy cows. 2. Effect of dietary fat on mobilization of body fat and protein. J. Dairy Sci. 1998, 81, 169–175. [Google Scholar] [CrossRef]
- Ricegrowers’ Association of Australia (RAG). Overview of the Australian Rice Industry. 2013. Available online: http://www.rga.org.au/f.ashx/overview.pdf (accessed on 18 December 2018).
- Seymour, M.; Kirkegaard, J.A.; Peoples, M.B.; White, P.F.; French, R.J. Break-crop benefits to wheat in Western Australia—Insights from over three decades of research. Crop Pasture Sci. 2012, 63, 1–16. [Google Scholar] [CrossRef]
- Nudda, A.; Battacone, G.; Neto, O.B.; Cannas, A.; Francesconi, A.H.D.; Atzori, A.S.; Pulina, G. Feeding strategies to design the fatty acid profile of sheep milk and cheese. R. Bras. Zootec. 2014, 43, 445–456. [Google Scholar] [CrossRef]
- Antonacci, L.E.; Bussetti, M.; Rodriguez, M.A.; Cano, A.V.; Gagliostro, G.A. Effect of diet supplementation with combinations of soybean and linseed oils on milk production and fatty acid profile in lactating dairy ewes. Agric. Sci. 2018, 9, 200–220. [Google Scholar]
- Gomez-Cortes, P.; Gallardo, B.; Mantecon, A.R.; Juarez, M.; de la Fuente, M.A.; Manso, T. Effects of different sources of fat (calcium soap of palm oil vs. extruded linseed) in lactating ewes’ diet on the fatty acid profile of their suckling lambs. Meat Sci. 2014, 96, 1304–1312. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Marzano, A.; Battacone, G.; Nicolussi, P.; Bonelli, P.; Pulina, G. Effects of diets containing grape seed, linseed, or both on milk production traits, liver and kidney activities, and immunity of lactating dairy ewes. J. Dairy Sci. 2015, 98, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Brossillon, V.; Reis, S.F.; Moura, D.C.; Galvao, J.G.B.; Oliveira, A.S.; Cortes, C.; Brito, A.F. Production, milk and plasma fatty acid profile, and nutrient utilization in Jersey cows fed flaxseed oil and corn grain with different particle size. J. Dairy Sci. 2018, 101, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Beaudoin, F.; Ammah, A.A.; Bissonnette, N.; Benchaar, C.; Zhao, X.; Lei, C.Z.; Ibeagha-Awemu, E.M. Deep sequencing shows microRNA involvement in bovine mammary gland adaptation to diets supplemented with linseed oil or safflower oil. BMC Genomics 2015, 16, 884. [Google Scholar] [CrossRef] [PubMed]
- Nudda, A.; Battacone, G.; Atzori, A.S.; Dimauro, C.; Rassu, S.P.G.; Nicolussi, P.; Bonelli, P.; Pulina, G. Effect of extruded linseed supplementation on blood metabolic profile and milk performance of Saanen goats. Animal 2013, 7, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Glibert, J.S.; Porter, T. International Safflower Production-An Overview. In Proceedings of the 7th International Safflower Conference, Wagga Wagga, NSW, Australia, 3–6 November 2008; pp. 1–7. [Google Scholar]
- Alizadeh, A.R.; Alikhani, M.; Ghorbani, G.R.; Rahmani, H.R.; Rashidi, L.; Loor, J.J. Effects of feeding roasted safflower seeds (variety IL-111) and fish oil on dry matter intake, performance and milk fatty acid profiles in dairy cattle. J. Anim. Physiol. Anim. Nutr. 2012, 96, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Shingfield, K.J.; Bonnet, M.; Scollan, N.D. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Ahmadpour, A.; Aliarabi, H.; Khan, M.G.; Patton, R.A.; Bruckmaier, R.M. Temporal changes in milk fatty acid distribution due to feeding different levels of rolled safflower seeds to lactating Holstein cows. J. Dairy Sci. 2017, 100, 4484–4499. [Google Scholar] [CrossRef] [PubMed]
- Dschaak, C.M.; Noviandi, C.T.; Eun, J.S.; Fellner, V.; Young, A.J.; ZoBell, D.R.; Israelsen, C.E. Ruminal fermentation, milk fatty acid profiles, and productive performance of Holstein dairy cows fed 2 different safflower seeds. J. Dairy Sci. 2011, 94, 5138–5150. [Google Scholar] [CrossRef] [PubMed]
- Oguz, M.N.; Oguz, F.K.; Buyukoglu, T.I. Effect of different concentrations of dietary safflower seed on milk yield and some rumen and blood parameters at the end stage of lactation in dairy cows. R. Bras. Zootec. 2014, 43, 207–211. [Google Scholar] [CrossRef]
- Shi, H.P.; Luo, J.; Zhang, W.; Sheng, H.J. Using safflower supplementation to improve the fatty acid profile in milk of dairy goat. Small Rumin. Res. 2015, 127, 68–73. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef]
- Naik, P.K. Bypass Fat in Dairy Ration—A Review. Anim. Nutr. Feed Technol. 2013, 13, 147–163. [Google Scholar]
- Appeddu, L.A.; Ely, D.G.; Aaron, D.K.; Deweese, W.P.; Fink, E. Effects of supplementing with calcium salts of palm oil fatty acids or hydrogenated tallow on ewe milk production and twin lamb growth. J. Anim. Sci. 2004, 82, 2780–2789. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.D.; Hernandez, M.P.; Cantalapiedra, G.; Salas, J.M.; Merino, J.A. Bypassing the rumen in dairy ewes: The reticular groove reflex vs. calcium soap of olive fatty acids. J. Dairy Sci. 2005, 88, 741–747. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Peake, D.; Bencini, R.; Williams, A.J. Fish oil metabolism in ruminants. Anim. Feed Sci. Technol. 2003, 108, 1–14. [Google Scholar] [CrossRef]
- Rotunno, T.; Sevi, A.; Di Caterina, R.; Muscio, A. Effects of graded levels of dietary rumen-protected fat on milk characteristics of Comisana ewes. Small Rumin. Res. 1998, 30, 137–145. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. About the evolution of goat and sheep milk production. Small Rumin. Res. 2007, 68, 3–6. [Google Scholar] [CrossRef]
- Gootwine, E.; Goot, H. Lamb and milk production of Awassi and East-Friesian sheep and their crosses under Mediterranean environment. Small Rumin. Res. 1996, 20, 255–260. [Google Scholar] [CrossRef]
- Konečná, L.; Kuchtík, J.; Králíčková, Š.; Pokorná, M.; Šustová, K.; Filipčík, R.; Lužová, T. Effect of different crossbreeds of Lacaune and East Friesian breeds on milk yield and basic milk parameters. Acta Univ. Agric. Silvic. Meldel. Brun. 2013, 61, 93–98. [Google Scholar] [CrossRef]
- Thomas, D.L.; Berger, Y.M.; McKusick, B.C. Milk and lamb production of East Friesian-cross ewes in northwestern Wisconsin. In Proceedings of the 4th Great Lakes Dairy Sheep Symposium, Spooner, WI, USA, 25–27 June 1998; pp. 11–17. [Google Scholar]
- Galal, S.; Gursoy, O.; Shaat, I. Awassi sheep as a genetic resource and efforts for their genetic improvement-A review. Small Rumin. Res. 2008, 79, 99–108. [Google Scholar] [CrossRef]
- Stubbs, A.; Abud, G.; Bencini, R. Dairy Sheep Manual: Farm Management Guidelines; Rural Industries research and Development Corporation: Wagga Wagga, NSW, Australia, 2009. [Google Scholar]
- Clement, P.; Agboola, S.O.; Bencini, R. A study of polymorphism in milk proteins from local and imported dairy sheep in Australia by capillary electrophoresis. LWT-Food Sci. Technol. 2006, 39, 63–69. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- West, J.W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]

| Items | Control | CO | RBO | FSO | SFO | RPO |
|---|---|---|---|---|---|---|
| Ingredient, g/kg | ||||||
| Wheat | 585 | 545 | 535 | 465 | 535 | 530 |
| Paddy rice | 210 | 210 | 220 | 280 | 210 | 215 |
| Lupins | 148 | 148 | 148 | 148 | 148 | 148 |
| Canola oil, ml/kg | - | 50 | - | - | - | - |
| Flaxseed oil, ml/kg | - | - | - | 50 | - | - |
| Safflower oil, ml/kg | - | - | - | - | 50 | - |
| Rice bran oil, ml/kg | - | - | 50 | - | - | - |
| EPA + DHA, ml/kg | - | - | - | - | - | 50 |
| Ammonium sulphate | 12.6 | 12.6 | 12.6 | 12.6 | 12.6 | 12.6 |
| Salt | 10 | 10 | 10 | 10 | 10 | 10 |
| Limestone | 20.9 | 20.9 | 20.9 | 20.9 | 20.9 | 20.9 |
| Sheep premix | 1 | 1 | 1 | 1 | 1 | 1 |
| Acid buff | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| Sodium bicarbonate | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| Component (% DM) | Pasture | Hay | Control | CO | RBO | FSO | SFO | RPO |
|---|---|---|---|---|---|---|---|---|
| DM | 96.5 | 95.5 | 91.5 | 93.0 | 91.6 | 90.0 | 91.7 | 91.6 |
| OM | 90.5 | 97.3 | 92.2 | 93.3 | 92.7 | 91.0 | 91.8 | 92.0 |
| Ash | 9.5 | 2.7 | 7.8 | 6.7 | 7.3 | 9.0 | 8.2 | 8.0 |
| ADF | 45.5 | 37.6 | 10.6 | 7.1 | 8.1 | 9.7 | 9.0 | 8.5 |
| NDF | 69.9 | 68.3 | 30.0 | 21.8 | 19.4 | 23.3 | 23.9 | 22.0 |
| EE | 1.4 | 1.2 | 3.3 | 5.7 | 5.2 | 5.4 | 5.0 | 5.1 |
| CP | 4.7 | 4.3 | 14.6 | 14.0 | 14.7 | 14.6 | 14.5 | 15.6 |
| TDN | 48.5 | 54.1 | 73.4 | 75.9 | 75.2 | 74.1 | 74.5 | 74.9 |
| ME, MJ/kg DM | 7.1 | 8.1 | 11.7 | 12.2 | 12.0 | 11.8 | 11.9 | 12.0 |
| Items | Feed Intake | DMI | OM | ADF | NDF | EE | CP |
|---|---|---|---|---|---|---|---|
| Treatment b (T) | |||||||
| Control | 885.5 a | 810.3 a | 741.4 a | 85.9 a | 243.1 a | 26.7 e | 118.3 a |
| CO | 751.3 c | 698.7 c | 651.9 b | 49.6 e | 152.3 d | 39.8 b | 97.8 e |
| RBO | 860.4 b | 788.0 b | 730.5 a | 63.8 c | 152.9 d | 40.9 a | 115.8 b |
| FSO | 754.3 c | 678.9 d | 617.8 d | 65.9 b | 158.2 c | 36.7 c | 99.1 e |
| SFO | 767.1 c | 703.4 c | 645.8 bc | 63.3 c | 168.1 b | 35.2 d | 102.0 d |
| RPO | 753.9 c | 690.5 cd | 635.3 c | 58.7 d | 151.9 d | 35.2 d | 107.7 c |
| Breed c | |||||||
| AW | 793.5 | 726.5 | 678.8 | 64.3 | 170.6 | 35.7 | 106.5 |
| AW × EF | 797.1 | 729.9 | 671.9 | 64.7 | 171.5 | 35.8 | 107.0 |
| SEM | 4.1 | 3.8 | 3.5 | 0.6 | 1.7 | 0.3 | 0.6 |
| p-values | |||||||
| Treatment | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Breed | 0.4483 | 0.4384 | 0.4423 | 0.3670 | 0.3492 | 0.5652 | 0.4358 |
| T × Breed | 0.7877 | 0.7982 | 0.7993 | 0.7557 | 0.6935 | 0.8934 | 0.8082 |
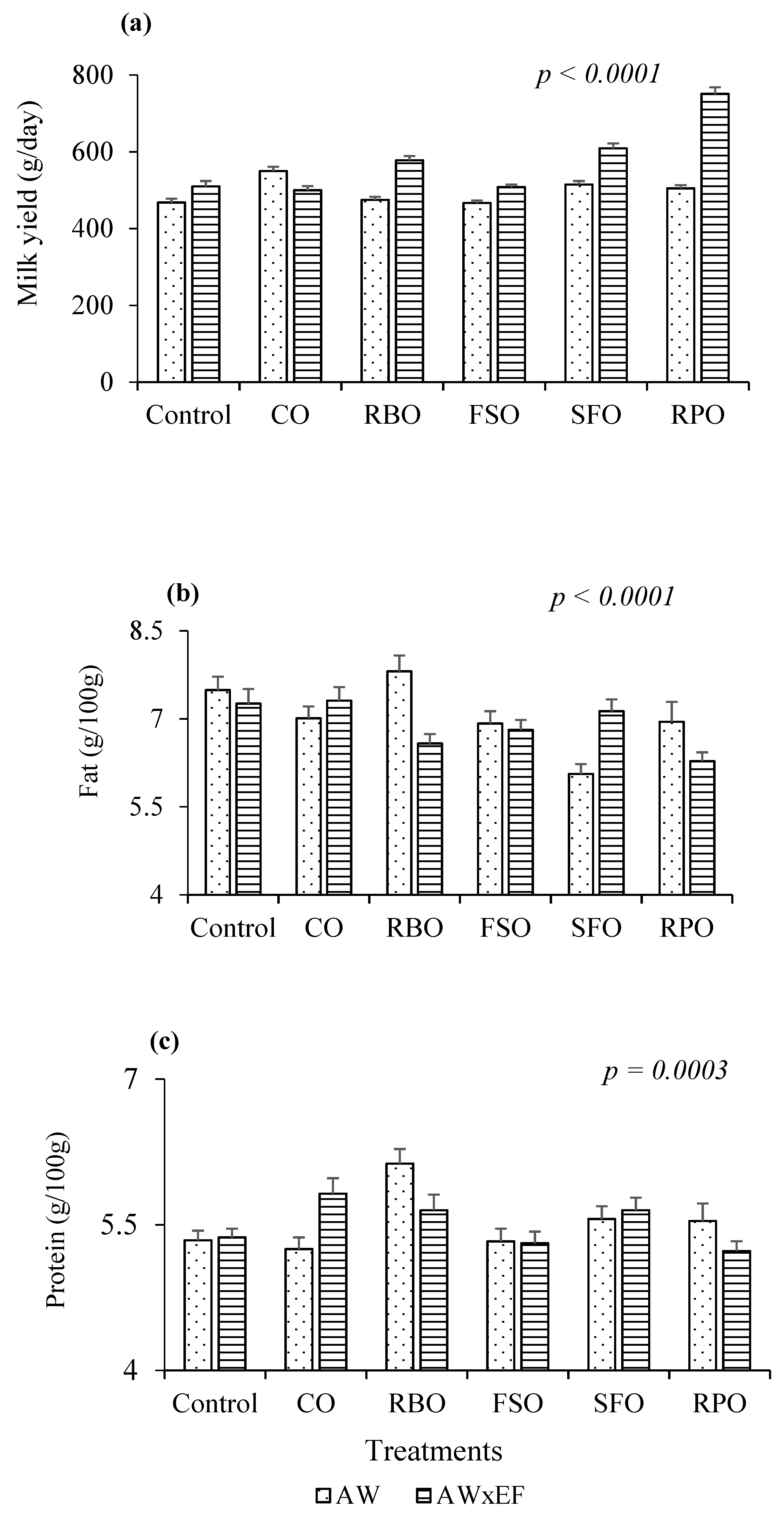
| Item | MY | FCM | Fat | FY | Protein | PY | Lacto-se | SNF | SCC | BCS |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment b (T) | ||||||||||
| Control | 484 d | 542 bc | 7.4 a | 36 bc | 5.4 c | 26 c | 4.9 | 10.9 bc | 109 a | 2.1 c |
| CO | 525 c | 573 b | 7.2 ab | 38 b | 5.5 bc | 29 b | 4.9 | 11.1 bc | 98 ab | 2.3 a |
| RBO | 527 c | 578 b | 7.2 ab | 38 b | 5.9 a | 31 b | 4.9 | 11.7 a | 73 c | 2.2 bc |
| FSO | 489 d | 523 c | 6.9 bc | 34 c | 5.4 c | 26 c | 4.8 | 10.8 c | 60 c | 2.3 a |
| SFO | 562 b | 587 b | 6.6 c | 37 b | 5.6 b | 31 ab | 4.8 | 11.2 b | 105 ab | 2.2 bc |
| RPO | 628 a | 649 a | 6.6 c | 41 a | 5.4 c | 34 a | 4.8 | 11.0 bc | 81 bc | 2.2 bc |
| Breed c (B) | ||||||||||
| AW | 496 b | 535 b | 7.1 | 35 b | 5.5 | 27 b | 4.8 b | 11.1 | 97 a | 2.2 b |
| AW × EF | 578 a | 617 a | 6.9 | 40 a | 5.5 | 32 a | 4.9 a | 11.2 | 78 b | 2.3 a |
| SEM | 3.4 | 7.8 | 0.07 | 3.6 | 0.04 | 2.9 | 0.02 | 0.05 | 3.6 | 0.0 |
| p-Values | ||||||||||
| Treatment | 0.0001 | 0.0001 | 0.0001 | 0.0021 | 0.0001 | 0.0001 | 0.1689 | 0.0001 | 0.0002 | 0.0018 |
| Breed (B) | 0.0001 | 0.0001 | 0.1765 | 0.0001 | 0.7444 | 0.0001 | 0.0006 | 0.1351 | 0.115 | 0.0030 |
| Week (W) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0257 | 0.0012 | 0.0001 |
| T × B | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0003 | 0.0001 | 0.0001 | 0.0257 | 0.0795 | 0.0002 |
| T × W | 1.0000 | 1.0000 | 0.9766 | 0.9999 | 0.8717 | 1.0000 | 0.8348 | 0.8039 | 0.3630 | 0.9999 |
| B × W | 0.9061 | 0.8724 | 0.9494 | 0.8517 | 0.9971 | 0.9380 | 0.6808 | 0.9910 | 0.9974 | 0.8640 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.V.; Le, H.V.; Nguyen, D.V.; Nish, P.; Otto, J.R.; Malau-Aduli, B.S.; Nichols, P.D.; Malau-Aduli, A.E.O. Supplementing Dairy Ewes Grazing Low Quality Pastures with Plant-Derived and Rumen-Protected Oils Containing Eicosapentaenoic Acid and Docosahexaenoic Acid Pellets Increases Body Condition Score and Milk, Fat, and Protein Yields. Animals 2018, 8, 241. https://doi.org/10.3390/ani8120241
Nguyen QV, Le HV, Nguyen DV, Nish P, Otto JR, Malau-Aduli BS, Nichols PD, Malau-Aduli AEO. Supplementing Dairy Ewes Grazing Low Quality Pastures with Plant-Derived and Rumen-Protected Oils Containing Eicosapentaenoic Acid and Docosahexaenoic Acid Pellets Increases Body Condition Score and Milk, Fat, and Protein Yields. Animals. 2018; 8(12):241. https://doi.org/10.3390/ani8120241
Chicago/Turabian StyleNguyen, Quang V., Hung V. Le, Don V. Nguyen, Peter Nish, John R. Otto, Bunmi S. Malau-Aduli, Peter D. Nichols, and Aduli E. O. Malau-Aduli. 2018. "Supplementing Dairy Ewes Grazing Low Quality Pastures with Plant-Derived and Rumen-Protected Oils Containing Eicosapentaenoic Acid and Docosahexaenoic Acid Pellets Increases Body Condition Score and Milk, Fat, and Protein Yields" Animals 8, no. 12: 241. https://doi.org/10.3390/ani8120241
APA StyleNguyen, Q. V., Le, H. V., Nguyen, D. V., Nish, P., Otto, J. R., Malau-Aduli, B. S., Nichols, P. D., & Malau-Aduli, A. E. O. (2018). Supplementing Dairy Ewes Grazing Low Quality Pastures with Plant-Derived and Rumen-Protected Oils Containing Eicosapentaenoic Acid and Docosahexaenoic Acid Pellets Increases Body Condition Score and Milk, Fat, and Protein Yields. Animals, 8(12), 241. https://doi.org/10.3390/ani8120241





