4.1. Fermentation Parameters and Post-Fermentation Quality Changes of the Two Fresh Distiller’s Grains
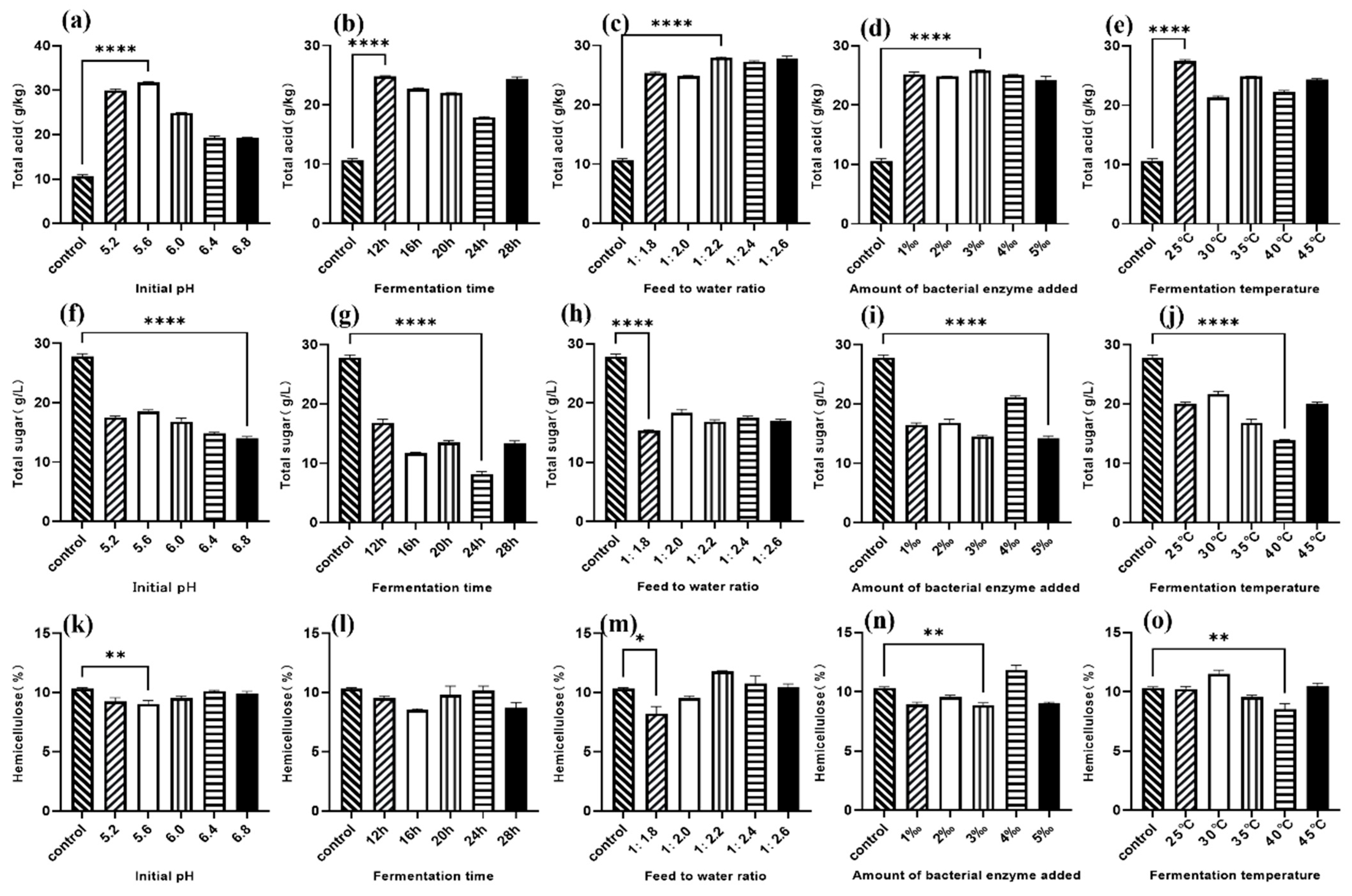
The results of this study demonstrate that liquid fermentation of fresh distiller’s grains using a composite microbial–enzyme preparation containing
Lactobacillus,
Bacillus subtilis, and
Saccharomyces cerevisiae, combined with xylanase, cellulase, and protease, effectively degraded hemicellulose and increased CP content. Research has shown that
Bacillus subtilis can degrade polysaccharides. Patel et al. reported that
Bacillus consumes glucose and xylose, converting them to lactate [
22], and xylose is a major component of hemicellulose. Another study also confirmed the polysaccharide-degrading role of
Bacillus, where it was shown to produce cellulolytic and xylanolytic enzymes, effectively reducing the crude fiber content of rapeseed meal after fermentation [
23]. The single-factor optimization results for the fermentation of fresh distiller’s grains in this study are consistent with these findings. The decrease in total sugar content is attributed not only to the action of cellulase and xylanase but also to the degradation of part of the polysaccharides by
Bacillus subtilis, which saccharifies the enzymatically hydrolyzed small molecular carbohydrates to produce lactic acid, thereby increasing the total acid content in the fermented distiller’s grains.
Lactobacillus is commonly used in feed ingredient fermentation and is itself an indispensable beneficial microbiota in the animal intestine. The most important role of
Lactobacillus in the fermentation process is its ability to effectively control fermentation conditions. Its rapid reproduction leads to the production of large amounts of lactic acid in the fermentation environment, lowering the pH, inhibiting the growth of most contaminating bacteria, and improving the hygiene of the liquid fermentation process [
24]. In a study by Anderson et al. on protein synthesis by yeast using glucose as a substrate, it was found that adding 25 mM inorganic phosphate increased protein synthesis by nearly 3.5 times [
25]. Another study showed that fermentation of Huangjiu lees using
Candida utilis and
Bacillus subtilis increased CP by 14.5% [
26]. In the current experiment, the CP content of the two types of distiller’s grains increased by 13.62% and 8.83%, respectively, consistent with the aforementioned research results. Variance analysis of the response surface optimization experiment for strong-aroma fresh distiller’s grains indicated the order of influence of the four factors on the response value as follows: fermentation temperature > water-to-material ratio > fermentation time > inoculum dosage. For soy-sauce-aroma fresh distiller’s grains, the order was as follows: water-to-material ratio > fermentation temperature > fermentation time > inoculum dosage. The greatest advantage of liquid fermentation compared to solid-state fermentation is the shorter fermentation time. Previous studies report that lactic acid bacteria in liquid fermentation show significant differences in lactic acid accumulation compared to the control group after just 8 h, and the lactic acid accumulation reached maximal levels and stabilized after 16 h [
27]. The water-to-material ratio is a key condition distinguishing liquid from solid-state fermentation. During the fermentation of distiller’s grains, the overall trend in hemicellulose content change was an increase with a higher water-to-material ratio. This might be because
Bacillus subtilis, which degrades hemicellulose, is an aerobic bacterium, and an excessively high water-to-material ratio reduces the oxygen content in the fermentation environment. Experiments fermenting corn stover with
Lactobacillus plantarum and
Bacillus showed that CF degradation rate at 95% moisture content was significantly lower than at 85% moisture content [
28].
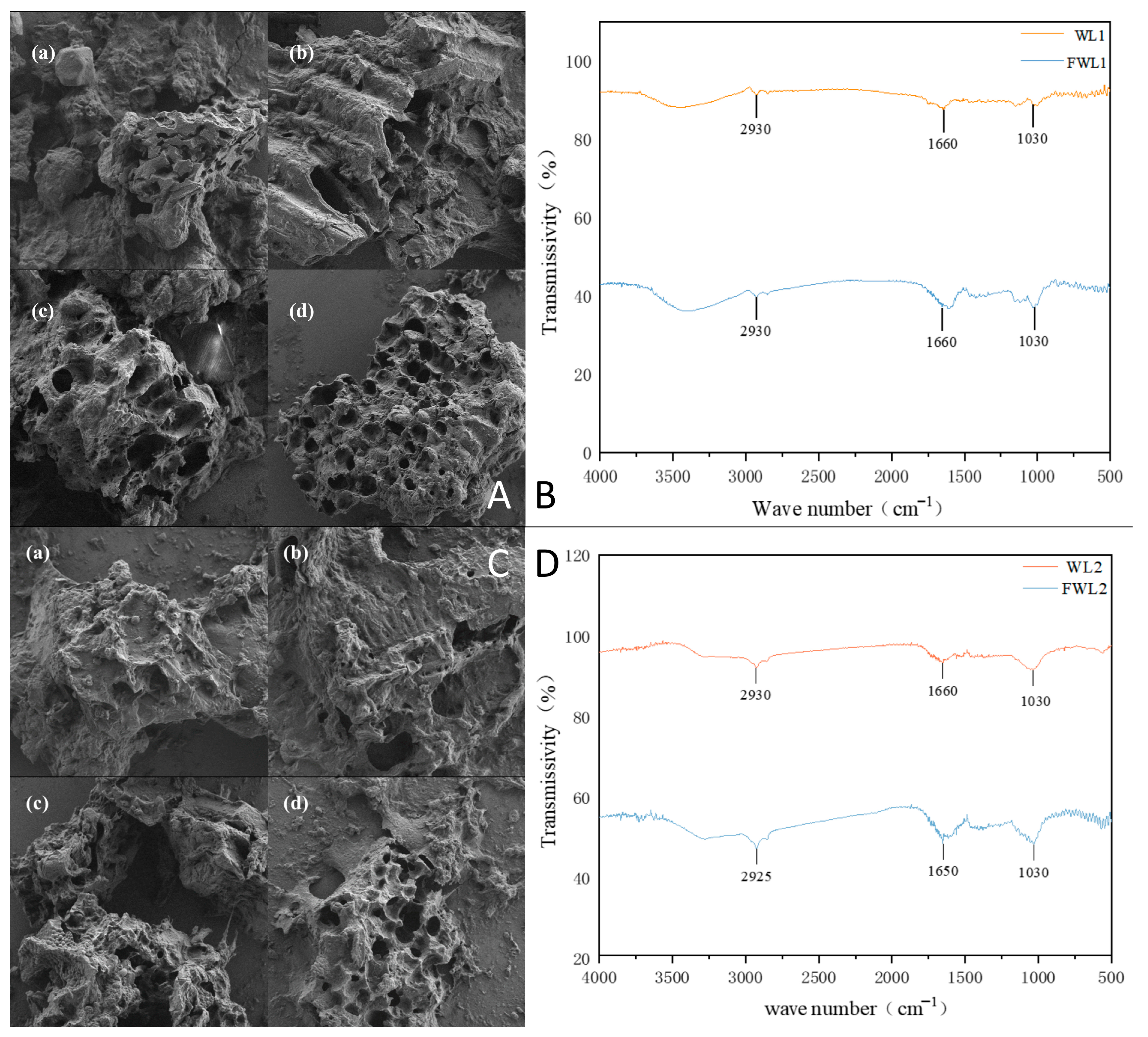
Observation via scanning electron microscopy revealed varying degrees of change in the surface structure of both types of distiller’s grains after fermentation. Following liquid fermentation treatment, the porosity increased, and the structure became looser compared to the original porous structure. Untreated distiller’s grains exhibited a compact and smooth surface, whereas the fermented grains showed a rougher surface and smaller particle size. This change might be related to the metabolites of the fermentation strains or the added protease [
29], or it could be due to the degradation and disruption of fibrous materials by cellulase and xylanase during fermentation, altering the surface structure [
30].
FTIR is a method combining Fourier transformation and infrared spectroscopy, commonly used to determine chemical functional groups, purity, and protein secondary structure, and is known for its high sensitivity, simplicity of operation, and detection sensitivity [
31]. The infrared spectra of the distiller’s grains showed an enhanced absorption peak in the amide I band (1600–1700 cm
−1) after fermentation. The amide I band is a marker band for protein secondary structure. For strong-aroma fresh distiller’s grains after treatment, the absorption peak shifted from around 1638 cm
−1 to near 1600 cm
−1. According to previously reported results, this suggests a change in the protein composition of strong-aroma grains, primarily characterized by a conversion involving arginine/glutamine. For soy-sauce-aroma grains, the absorption peak intensified at 1660 cm
−1, which corresponds to the C=O stretching vibration of glutamine [
32,
33]. Both distiller’s grains showed a significant enhancement of the absorption peak at 2930 cm
−1, attributed to C-H stretching vibrations. The peak intensification is likely due to the degradation of polysaccharides during fermentation, where chain breakage exposes more CH
2 and CH
3 groups [
34]. The fermented soy-sauce-aroma grains also exhibited a more pronounced absorption peak near 1060 cm
−1, influenced by the C-O-C ether bond stretching vibration of sugar units [
35]. Combining the single-factor fermentation results, it is plausible that microbial metabolites or xylanase decomposed hemicellulose, breaking down polysaccharides into smaller oligosaccharides or altering their structure.
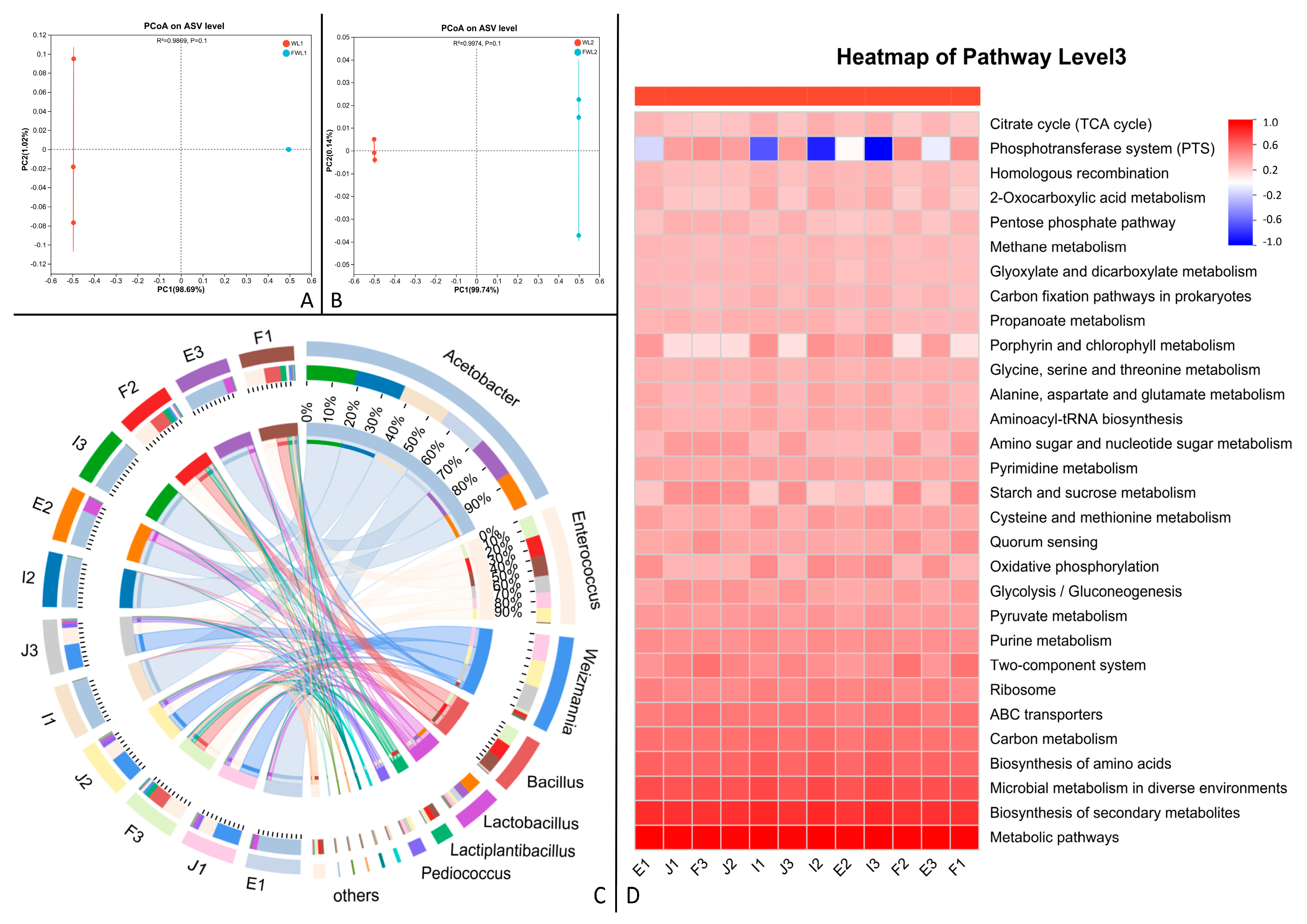
Microbial 16S rRNA high-throughput sequencing is used to study complex microbial communities, providing qualitative and quantitative (i.e., relative abundance) data through amplification and sequencing [
36]. This study compared changes in the microbial composition of the feed substrate before and after fermentation through species abundance, alpha diversity, beta diversity, and KEGG functional prediction analysis. Species abundance plots showed that the predominant bacterium in the unfermented distiller’s grains was
Acetobacter, while
Lactobacillus and
Bacillus were dominant in the fermented grains. Regarding species richness changes, the microbial abundance increased after fermentation for both distiller’s grains. This might be because the low pH of the untreated grains was unfavorable for microbial growth, whereas the post-fermentation environment was more suitable, allowing the inoculated
Lactobacillus and
Bacillus to proliferate. Combined with alpha diversity analysis and beta diversity PCoA plots, there was a clear trend for differences in microbial composition before and after fermentation for both distiller’s grains. The unfermented grains had a high ethanol content, and
Acetobacter readily proliferates in ethanol-rich substrates, consuming ethanol and converting it to acetic acid. High concentrations of acetic acid can damage bacterial cell membranes, inhibit the synthesis of cellular metabolites, and affect bacterial reproduction [
37,
38,
39]. This explains why the microbial diversity was relatively low in the untreated distiller’s grains before fermentation. After fermentation, the microbial community in the feed ingredients became more concentrated, with the strain composition stabilizing, primarily consisting of
Lactobacillus and
Bacillus.
Weissella which was also present utilizes glucose as a carbon source to produce lactic acid and thrives at a pH of 5.5 or lower [
40].
Lactobacillus plantarum can regulate sugar metabolism to produce more ATP and also alter amino acid metabolism in acidic environments [
41,
42,
43].
Weizmannia belongs to the
Bacillus genus, exhibits strong adaptability, and can survive in temperatures ranging from 35 to 50 °C [
44]. The metabolites of
Weizmannia can utilize polysaccharides and convert them to lactic acid [
45].
According to the KEGG functional prediction abundance heatmap analysis of the distiller’s grains, the phosphotransferase system (PTS) showed a positive correlation in the microbial analysis of the fermented grains. Combined with the species abundance plot, the genes for the PTS likely originated from the Bacillus genus [
46]. The PTS has very broad regulatory functions and is involved in nitrogen and carbon metabolism [
47].
Based on the findings, a detailed hypothesis for the synergistic mechanism is proposed. Primarily, xylanase and cellulase initially degrade hemicellulose and cellulose, breaking the fibrous structure and releasing fermentable sugars. Subsequently, B. subtilis further decomposes these polysaccharides, while S. cerevisiae and Lactobacillus utilize the sugars for growth. Lactobacillus rapidly produces lactic acid, lowering the pH to inhibit pathogens and stabilize the system. This cooperative action collectively enhances fiber degradation, increases crude protein, and improves the overall nutritional quality of the fermented product. This synergistic interaction effectively disrupted the lignocellulosic matrix, increased crude protein availability, and modified the physicochemical structure of the substrate.
4.2. Ileal Amino Acid Digestibility, Nutrient Digestibility, Digestible Energy, and Metabolizable Energy of the Two Fresh Distiller’s Grains in Growing Pigs
The improvement in ileal amino acid digestibility was poorer for strong-aroma distiller’s grains compared to soy-sauce-aroma grains. This is likely due to the higher crude fiber and lower CP content of the strong-aroma grains. Arabinoxylan, β-glucan, and mannan in hemicellulose are major components of NSPs. Monogastric animals lack enzymes to digest NSPs, and NSPs increase chyme viscosity in the digestive tract, hindering contact between digestive enzymes and nutrients in the feed, leading to faster passage through the digestive tract and reduced digestibility and utilization of nutrients by the animal [
48,
49]. Upadhaya et al. compared the ileal amino acid digestibility of diets with and without added β-mannanase. Their results showed that the digestibility of lysine improved most significantly in the treated diet, and the digestibility of arginine, histidine, valine, and glycine also increased significantly [
50]. Although only a few amino acids showed significant changes in AID for fermented strong-aroma grains in the current study, the improvement in SID for most amino acids was statistically significant. This might be due to the reduction in neutral detergent fiber content, which decreases endogenous amino acid losses in the ileum [
51]. Similar to the AID and SID results, the horizontal comparison between the two grain types still showed less ideal apparent digestibility for the strong-aroma grains, most probably originating from their nutritional characteristics, high crude fiber, and low protein content. Related experimental studies have reported that
Lactobacillus fermentation can alter the fiber structure of grains, thereby improving nutrient digestibility [
52,
53,
54]. The raw material of soy-sauce-aroma distiller’s grains has a relatively high CP content. During fermentation, proteases and microbial metabolites influenced the protein structure, enhancing CP digestibility. Similarly, some studies have also found that
Bacillus subtilis can alter the protein structure of soybean meal, increasing the acid-soluble protein content [
55]. Shi et al. used
Bacillus subtilis to ferment soybean meal, and their results showed increased CP digestibility of the fermented soybean meal [
56], consistent with the findings of this study. The changes in DE and ME after fermentation were significant for both distiller’s grains. Studies have shown that DE and ME are negatively correlated with the content of neutral detergent fiber and acid detergent fiber, and positively correlated with CP content [
57].