Morphological, Molecular and Phylogenetic Characterization of Ceratomyxa nemiptera sp. nov. (Myxozoa: Ceratomyxidae) Infecting Nemipterus virgatus Houttuyn, 1782 in the East China Sea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
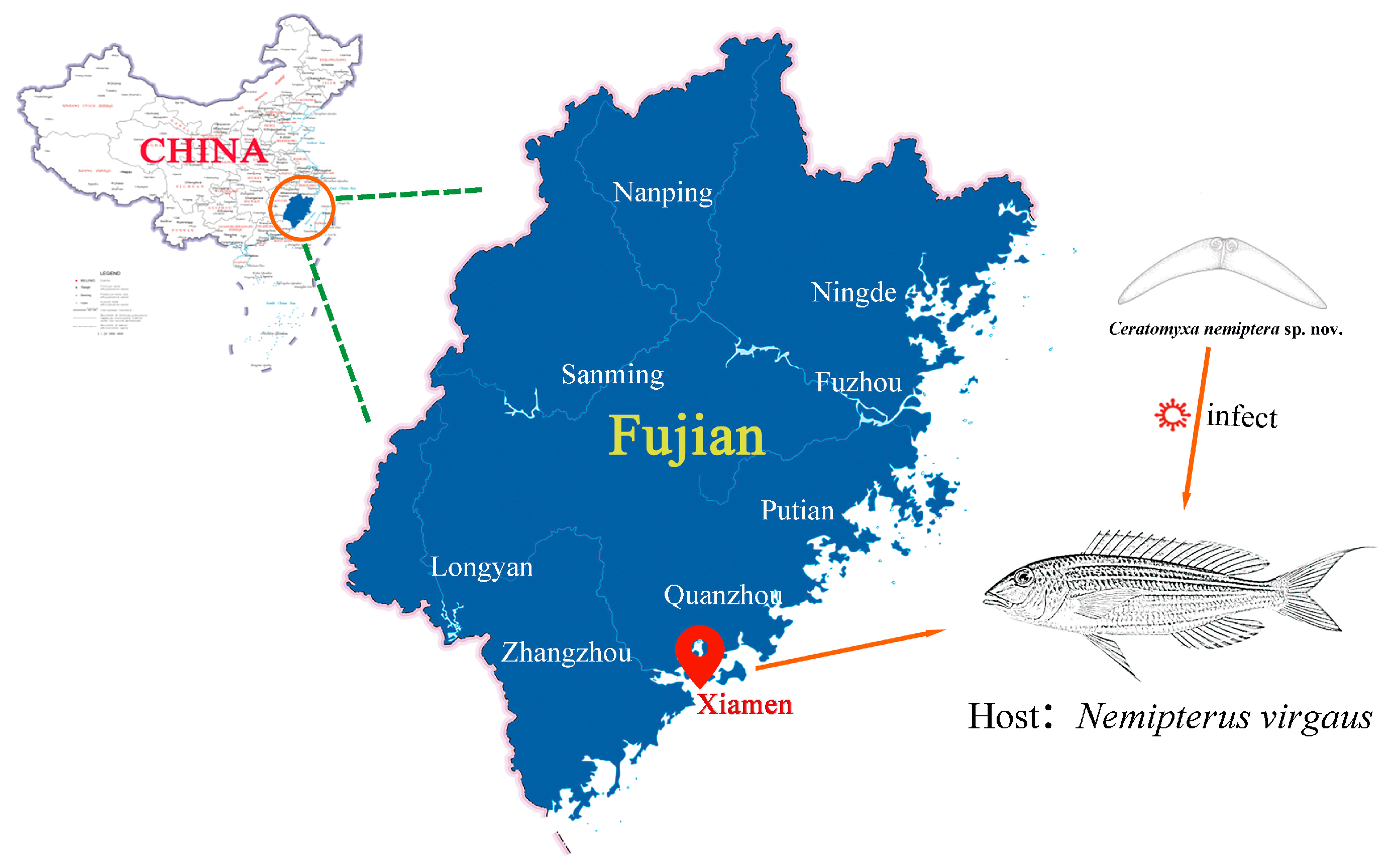
2.1. Sample Collection and Morphological Analysis
2.2. DNA Extraction and Amplification
2.3. Molecular and Phylogenetic Analysis
3. Results
3.1. Taxonomic Summary
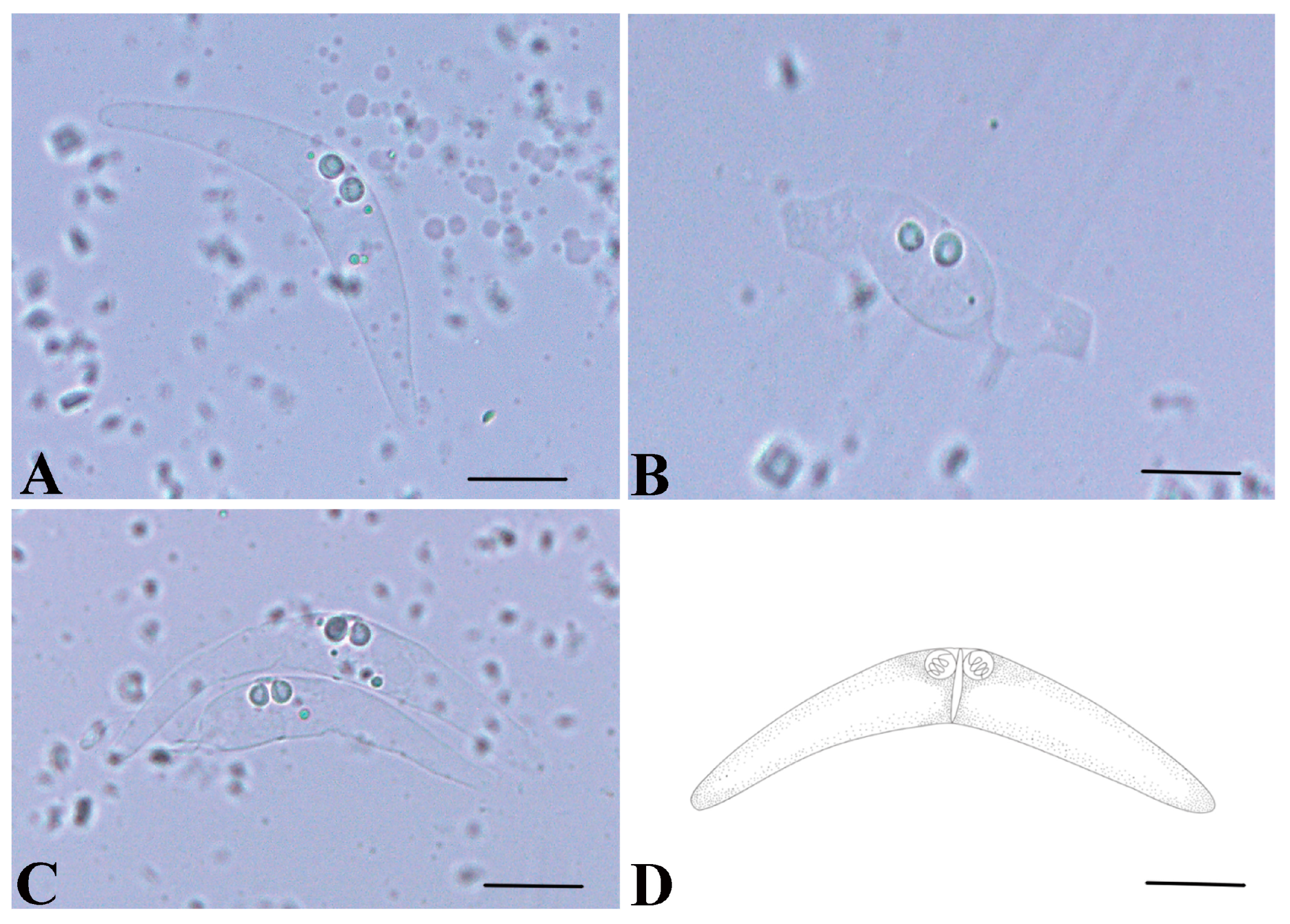
3.2. Morphological Description
3.3. Remarks
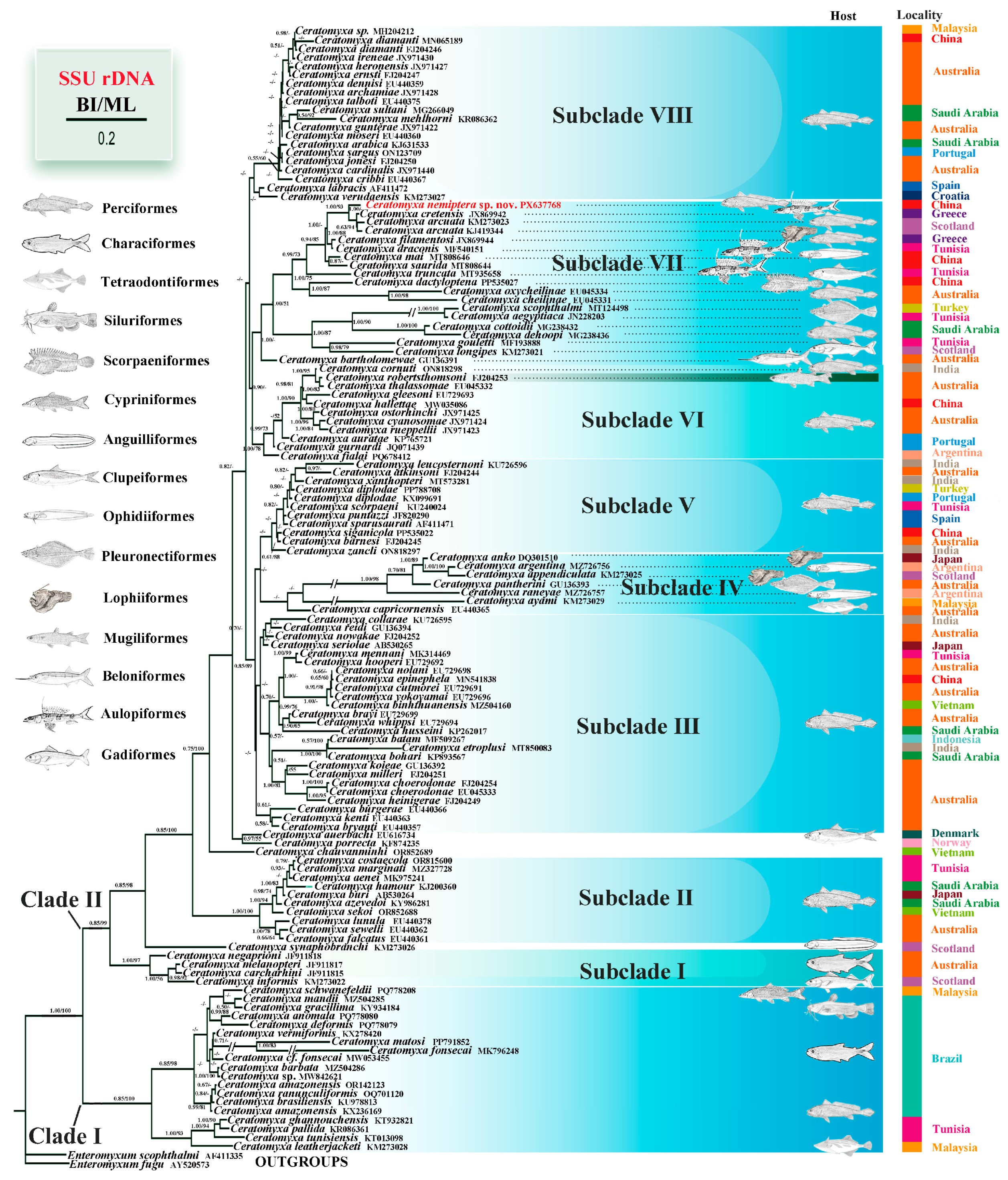
3.4. Molecular and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okamura, B.; Gruhl, A.; Bartholomew, J.L. An introduction to myxozoan evolution, ecology and development. In Myxozoan Evolution, Ecology and Development; Okamura, B., Gruhl, A., Bartholomew, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–20. [Google Scholar] [CrossRef]
- Zatti, S.A.; Atkinson, S.D.; Maia, A.A.M.; Bartholomew, J.L.; Adriano, E.A. Ceratomyxa gracillima sp. nov. (Cnidaria: Myxosporea) provides evidence of panmixia and ceratomyxid radiation in the Amazon basin. Parasitology 2018, 145, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.L.; Andree, K.B.; Bartholomew, J.L.; El-Matbouli, M.; Desser, S.S.; Devlin, R.H.; Feist, S.W.; Hedrick, R.P.; Hoffmann, R.W.; Khattra, J.; et al. Recent advances in our knowledge of the Myxozoa. J. Eukaryot. Microbiol. 2001, 48, 395–413. [Google Scholar] [CrossRef]
- Okamura, B.; Hartigan, A.; Naldoni, J. Extensive uncharted biodiversity: The parasite dimension. Integr. Comp. Biol. 2018, 58, 1132–1145. [Google Scholar] [CrossRef]
- Whipps, C.M.; Atkinson, S.D.; Hoeksema, B.W. World List of Myxozoa. 2026. Available online: https://www.marinespecies.org/myxozoa (accessed on 2 January 2026).
- Heiniger, H.; Gunter, N.L.; Adlard, R.D. Relationships between four novel ceratomyxid parasites from the gall bladders of labrid fishes from Heron Island, Queensland, Australia. J. Parasitol. 2008, 94, 158–165. [Google Scholar] [CrossRef]
- Zhang, D.D.; Zhao, Y.J.; Yang, S.H.; Yang, C.Z. Morphological and molecular identification of a novel species, Ceratomyxa siganicola sp. nov. (Myxozoa: Ceratomyxidae) from Siganus fuscescens, in East China Sea. Acta Parasitol. 2019, 64, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, Y.J.; Yang, S.H.; Yang, C.Z. A new record of Ceratomyxa diamanti from China and comparison of its different geographic strains. Acta Parasitol. 2020, 65, 312–318. [Google Scholar] [CrossRef]
- Eiras, J.C.; Cruz, C.; Saraiva, A. Synopsis of the species of Ceratomyxa Thélohan, 1892 (Cnidaria, Myxosporea, Ceratomyxidae) described between 2007 and 2017. Syst. Parasitol. 2018, 95, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Z.; Ma, H.G.; Huang, Y.M.; Atkinson, S.D.; Zhao, Y.J.; Bartholomew, J.L. Morphological and genetic analysis of Ceratomyxa saurida Zhao et al. 2015 and Ceratomyxa mai sp. nov. (Myxozoa: Ceratomyxidae) from the East China Sea. Int. J. Syst. Evol. Microbiol. 2023, 73, 289–298. [Google Scholar] [CrossRef]
- Yang, C.Z.; Yang, Y.T.; Chen, X.; Yang, S.H.; Zhou, Y.; Ma, H.G.; Liu, Y.; Zhao, Y.J. Integrative taxonomy and phylogenetic analyses of two Ceratomyxa species (Myxozoa, Ceratomyxidae) from the China Sea, including a new species description. ZooKeys 2025, 1250, 359–378. [Google Scholar] [CrossRef]
- Lom, J.; Dyková, I. Myxozoan genera: Definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006, 53, 1–36. [Google Scholar] [CrossRef]
- Gunter, N.L.; Adlard, R.D. The demise of Leptotheca Thélohan, 1895 (Myxozoa: Myxosporea: Ceratomyxidae) and assignment of its species to Ceratomyxa Thélohan, 1892 (Myxosporea: Ceratomyxidae), Ellipsomyxa Køie, 2003 (Myxosporea: Ceratomyxidae), Myxobolus Bütschli, 1882 and Sphaerospora Thélohan, 1892 (Myxosporea: Sphaerosporidae). Syst. Parasitol. 2010, 75, 81–104. [Google Scholar] [CrossRef]
- Rocha, S.; Rangel, L.F.; Casal, G.; Severino, R.; Soares, F.; Rodrigues, P.; Santos, M.J. Occurrence of two myxosporean parasites in the gall bladder of white seabream Diplodus sargus (L.) (Teleostei, Sparidae), with the morphological and molecular description of Ceratomyxa sargus sp. nov. PeerJ 2023, 11, e14599. [Google Scholar] [CrossRef]
- Heiniger, H.; Adlard, R.D. Molecular identification of cryptic species of Ceratomyxa Thélohan, 1892 (Myxosporea: Bivalvulida) including the description of eight novel species from apogonid fishes (Perciformes: Apogonidae) from Australian waters. Acta Parasitol. 2013, 58, 342–360. [Google Scholar] [CrossRef]
- Surendran, S.; Chandran, A.; Vijayagopal, P.; Sanil, N.K. Morphological and molecular characterization of Ceratomyxa xanthopteri sp. nov. (Myxosporea: Ceratomyxidae) from the marine ornamental fish Acanthurus xanthopterus Valenciennes 1835 (Acanthuridae) off Vizhinjam coast, Kerala. Parasitol. Res. 2021, 120, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.B.; Li, Y.Z.; Zhao, X.Y.; Chen, Y.Z.; Jin, X.S. Acoustic assessment of five groups commercial fish in South China Sea. Acta Oceanol. Sin. 2006, 28, 128–134. [Google Scholar]
- Tomochi, H.; Li, Y.C.; Tran, B.T.; Yanagida, T.; Sato, H. Three Unicapsula species (Myxosporea: Trilosporidae) of Asian marine fishes, including the description of Unicapsula setoensis sp. nov. in the yellowfin goby (Acanthogobius flavimanus) from the Inland Sea of Japan. Parasitol. Res. 2014, 113, 3807–3816. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Inoue, K.; Zhang, J.Y.; Sato, H. New records of three commercial fish hosts for two Unicapsula spp. And Kudoa megacapsula (Myxozoa: Myxosporea: Multivalvulida). Parasitol. Res. 2022, 121, 3133–3145. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Zhang, C.L.; Cheng, Q.T. Fauna of East China Sea Fishes; Science Press: Beijing, China, 1963. [Google Scholar]
- Wang, Y.F.; Ma, C.Y.; Song, X.J.; Li, M.Y.; Zhang, H.Y. Assessment of fish diversity in the East China Sea hairtail national aquatic germplasm resources conservation zone using DNA barcoding. Glob. Ecol. Conserv. 2024, 53, e03013. [Google Scholar] [CrossRef]
- Chen, W.; Yang, C.Z.; Whipps, C.M.; Peng, Z.G.; Zhao, Y.J. Taxonomy on three novel species of Sphaeromyxa Thélohan 1892 (Myxozoa, Bivalvulida, Sphaeromyxidae) with insight into the evolution of the genus. Parasitol. Res. 2020, 119, 1493–1503. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Zhao, Y.J.; Chen, X.; Wang, M.; Peng, J.J.; Cui, X.J.; Yang, C.Z. Taxonomy and phylogeny of Myxobolus branchiostegi sp. nov. (Myxozoa, Myxosporea) infecting the gallbladder of Branchiostegus argentatus Cuvier, 1830 from the East China Sea. ZooKeys 2025, 101, 2357–2367. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, F. Morphological and molecular characterization of Henneguya cystigena n. sp. (Cnidaria, Myxosporea) parasitizing the alimentary tract of yellowfin seabream, Acanthopagrus latus, in the East China Sea. Parasite 2025, 32, 51. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, X.; Zheng, C.; Wang, X.Y.; Buchmann, K.; Yin, F. Coinfection of large yellow croaker Larimichthys crocea by Trypanosoma sp. (Euglenozoa: Kinetoplastea) and Ceratomyxa xiangshanensis n. sp. (Cnidaria: Myxosporea) in offshore net cage systems in the East China Sea. Parasitol. Int. 2026, 111, 103167. [Google Scholar] [CrossRef]
- Huang, Y.M.; Zhao, Y.J.; Zhou, Y.; Yang, C.Z. First molecular evidence of Ceratomyxa epinephela (Myxozoa: Ceratomyxidae) and its genetic variation from different host species. Acta Hydrobiol. Sin. 2020, 44, 1263–1269. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhou, Y.; Kent, M.L.; Whipps, C.M. Replacement of the preoccupied name Davisia Laird 1953 and description of a new myxozoan species (Myxosporea: Sinuolineidae) from Sebastiscus marmoratus (Cuvier, 1829) in the East China Sea. J. Parasitol. 2008, 94, 269–279. [Google Scholar] [CrossRef]
- Yang, C.Z.; Zhou, Y.; Zhao, Y.J.; Huang, W.; Huang, C. Erection of Unicapsulocaudum mugilum gen. et sp. nov. (Myxozoa: Ceratomyxidae) based on its morphological and molecular data. J. Nat. Hist. 2017, 51, 457–467. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Ma, C.L.; Song, W. Illustrated guide to the identification of pathogenetic protozoa in mariculture. II diagnostic methods for the myxosporean. J. Ocean Univ. Qingdao 2001, 31, 681–688. [Google Scholar]
- Barta, J.R.; Martin, D.S.; Liberator, P.A.; Dashkevicz, M.; Anderson, J.W.; Feighner, S.D.; Elbrecht, A.; Perkins-Barrow, A.; Jenkins, M.C.; Danforth, H.D.; et al. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997, 83, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher sequence analysis tools framework in 2024. Nucleic Acids Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.C. Synopsis of the species of Ceratomyxa Thélohan, 1892 (Myxozoa: Myxosporea: Ceratomyxidae). Syst. Parasitol. 2006, 65, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.A.; Yokoyama, H.; Ogawa, K. Description and phylogeny of Ceratomyxa anko sp. n. and Zschokkella lophii sp. n. from the Japanese anglerfish, Lophius litulon (Jordan). J. Fish Dis. 2008, 31, 921–930. [Google Scholar] [CrossRef]
- Azizi, R.; Yemmen, C.; Rangel, L.F.; Santos, M.J.; Bahri, S. Morphology, seasonality and molecular characterization of Ceratomyxa draconis sp. nov. parasite of Trachinus draco (L.) from the Bay of Bizerte, Tunisia. Parasitol. Res. 2020, 119, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.B.; Kwon, S.R.; Kim, S.K.; Nam, Y.K.; Kim, K.H. Ultrastructure and development of Ceratomyxa protopsettae Fujita, 1923 (Myxosporea) in the gallbladder of cultured olive flounder, Paralichthys olivaceus. Acta Protozool. 2004, 43, 241–250. [Google Scholar]
- Bittencourt, L.S.; da Silva, D.T.; Hamoy, I.; de Carvalho, A.A.; da Silva, M.F.; Videira, M.; Carvalho, J.C.T.; Matos, E. Morphological and Phylogenetic Features of Ceratomyxa macapaensis sp. nov. (Myxozoa: Ceratomyxidae) in Mesonauta festivus Heckel, 1840 (Cichliformes: Cichlidae) from the eastern Amazon region. Acta Parasitol. 2022, 67, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.L.; Adriano, E.A.; Franzolin, G.N.; Zatti, S.A.; Naldoni, J. A novel Ceratomyxa species (Myxozoa: Cnidaria) infecting an Amazonian catfish. Parasitol. Int. 2022, 89, 102582. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, J.; Yang, C.Z.; Zhao, Y.J. Myxobolus jialingensis sp. nov. (Myxozoa: Myxobolidae) infecting urinary bladder and hepatopancreas of yellowhead catfish Tachysurus fulvidraco from China. Zootaxa 2020, 4819, 179–186. [Google Scholar] [CrossRef]
- Zhang, B.; Tu, X.; Gu, Z.M. Myxobolus shuifuensis sp. n. (Myxozoa: Myxobolidae) infecting the exotic mrigal Cirrhinus mrigala feral in China. Parasitol. Int. 2023, 94, 102732. [Google Scholar] [CrossRef]
- Samshuri, M.A.; Borkhanuddin, M.H. Myxobolus acanthogobii Hoshina, 1952 and Myxobolus selari sp. nov. (Myxosporea: Myxobolidae) infecting brain of commercial fishes in Terengganu, Malaysia. Syst. Parasitol. 2024, 101, 39–52. [Google Scholar] [CrossRef]
- Chen, W.; Whipps, C.M.; Qing, X.Y.; Yang, C.Z.; Zhao, Y.J. A novel amphibian myxosporean, Cystodiscus chongqingensis sp. nov., infecting the Asiatic toad (Bufo gargarizans) from China. J. Parasitol. 2025, 111, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, W.; Pu, H.C.; Zhou, Y.; Zhao, Y.J.; Yang, C.Z. Morphological, Molecular and Phylogenetic Characterization of Auerbachia megacapsula sp. nov. (Myxozoa: Coccomyxidae) from Larimichthys crocea Richardson, 1846. Acta Parasitol. 2025, 70, 177–185. [Google Scholar] [CrossRef]
- Forró, B.; Eszterbauer, E. Correlation between host specificity and genetic diversity for the muscle-dwelling fish parasite Myxobolus pseudodispar: Examples of myxozoan host-shift? Folia Parasitol. 2016, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holzer, A.S.; Bartošová-Sojková, P.; Born-Torrijos, A.; Lövy, A.; Hartigan, A.; Fiala, I. The joint evolution of the Myxozoa and their alternate hosts: A cnidarian recipe for success and vast biodiversity. Mol. Ecol. 2018, 27, 1651–1666. [Google Scholar] [CrossRef] [PubMed]
- Elloumi, A.; Rangel, L.F.; Santos, M.J.; Bahri, S. Myxidium tunisiensis sp. nov. (Myxosporea: Myxidiidae) infecting the rough skate Raja radula Delaroche, 1908 (Rajiformes: Rajidae) from North East Tunisia. Parasitol. Res. 2023, 122, 19–33. [Google Scholar] [CrossRef]
- Carlson, C.J.; Hopkins, S.; Bell, K.C.; Doña, J.; Godfrey, S.S.; Kwak, M.L.; Lafferty, K.D.; Moir, M.L.; Speer, K.A.; Strona, G.; et al. A global parasite conservation plan. Biol. Conserv. 2020, 250, 108596. [Google Scholar] [CrossRef]
- Petit, R.J.; Excoffier, L. Gene flow and species delimitation. Trends Ecol. Evol. 2009, 24, 386–393. [Google Scholar] [CrossRef]
- Edwards, S.V.; Robin, V.V.; Ferrand, N.; Moritz, C. The evolution of comparative phylogeography: Putting the Geography (and More) into Comparative Population Genomics. Genome Biol. Evol. 2022, 14, e176. [Google Scholar] [CrossRef]
- Mu, Q.Y.; Yu, C.C.; Wang, Y.; Han, T.S.; Wang, H.; Ding, W.N.; Zhang, Q.Y.; Low, S.L.; Zheng, Q.J.; Peng, C.; et al. Comparative phylogeography of Acanthocalyx (Caprifoliaceae) reveals distinct genetic structures in the Himalaya–Hengduan Mountains. Alpine Bot. 2022, 132, 153–168. [Google Scholar] [CrossRef]
- Nieberding, C.M.; Olivieri, I. Parasites: Proxies for host genealogy and ecology? Trends Ecol. Evol. 2007, 22, 156–165. [Google Scholar] [CrossRef]
- Sweet, A.D.; Johnson, K.P. The role of parasite dispersal in shaping a host–parasite system at multiple evolutionary scales. Mol. Ecol. 2018, 27, 5104–5119. [Google Scholar] [CrossRef]
- Lei, H.P.; Jakovlić, I.; Zhou, S.; Liu, X.; Yan, C.; Jin, X.; Wang, B.; Li, W.X.; Wang, G.T.; Zhang, D. Geography, phylogeny and host switch drive the coevolution of parasitic Gyrodactylus flatworms and their hosts. Parasites Vectors 2024, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Zatti, S.A.; Araújo, B.L.; Adriano, E.A.; Maia, A.A. A new freshwater Ceratomyxa species (Myxozoa: Ceratomyxidae) parasitizing a sciaenid fish from the Amazon Basin, Brazil. Parasitol. Int. 2023, 97, 102796. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.D.; Whitaker, D.J.; Kent, M.L. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 1999, 172, 163–196. [Google Scholar] [CrossRef]
| Species | SL | ST | PCL | PCW | PFT | PA (°) | Host | Locality | References |
|---|---|---|---|---|---|---|---|---|---|
| Ceratomyxa nemiptera sp. nov. | 6.2 ± 0.6 (5.4–6.9) | 44.8 ± 4.6 (38.5–53.1) | 2.8 ± 0.2 (2.4–3.1) | 2.3 ± 0.2 (1.9–2.6) | 2–3 | 131.6 ± 14.6 (103.3–153.0) | Nemipterus virgatus Houttuyn, 1782 | East China Sea, PR China | Present work |
| Ceratomyxa fistulariae Kpatcha, Diebakate, Faye & Toguebaye, 1996 | 10.2 (10–12) | 39.6 (38.8–40) | 5.2 (4.5–5.5) | - | - | - | Fistularia petimba Lacepède, 1803 | Atlantic Ocean, Senegal | [36] |
| Ceratomyxa anko Freeman, Yokoyama & Ogawa, 2008 | 10.8 (9.7–11.9) | 41.9 (36.9–47.2) | 4.6 (4.1–5.3) | - | - | - | Lophius litulon Jordan, 1902 | Fukushima Prefecture, Japan | [37] |
| Ceratomyxa draconis Azizi, Yemmen, Rangel, Santos & Bahri, 2020 | 7.4 ± 0.77 (6.4–8.0) | 30.8 ± 1.65 (28.8–32.8) | 3.3 ± 0.2 (3.6–4.0) | - | - | 120–156 | Trachinus draco Linnaeus, 1758 | Bay of Bizerte, Tunisia | [38] |
| Ceratomyxa protopsettae Fujita, 1923 | 11.64 ± 0.95 (10–12) | 46.63 ± 5.8 (50–65) | 4.15 ± 0.34 | - | 5–6 | - | Paralichthys olivaceus Temminck & Schlegel, 1846 | East Sea, South Korea | [39] |
| Ceratomyxa macapaensis Bittencourt, 2022 | 4.2 ± 0.5 | 22.75 ± 0.3 | 1.86 ± 0.3 | 1.63 ± 0.1 | 3–4 | - | Mesonauta festivus Heckel, 1840 | Piririm River, Amapá, Brazil | [40] |
| Ceratomyxa mandii Bruno, 2022 | 4.6 ± 0.5 (3.4–5.5) | 31.2 ± 2.3 (26.2–36.3) | 1.8 ± 0.3 (1.0–2.5) | 1.9 ± 0.3 (1.2–2.4) | 3–4 | 162 ± 10.4 (143–178) | Pimelodina flavipinnis Steindachner, 1876 | Amazon River, Amazonas, Brazil | [41] |
| Ceratomyxa mai Yang, Huang, Atkinson, Bartholomew, Ma & Zhao, 2023 | 9.2 ± 0.5 (8.1–9.9) | 20.9 ± 1.9 (17.3–24.7) | 2.6 ± 0.2 (2.4–2.9) | 2.7 ± 0.2 (2.4–3.3) | - | 142.2 ± 8.2 (125.8–158.2) | Saurida elongata Temminck & Schlegel, 1846 | East China Sea, PR China | [10] |
| Ceratomyxa saurida Zhao, Al-Farraj, AL-Rasheid & Song, 2015 | 9.0 ± 0.7 (8.0–10.6) | 42.5 ± 4.0 (38.1–54.6) | 3.3 ± 0.3 (3.0–3.7) | - | - | 156.8 ± 11.4 (138.1–176.3) | Saurida elongata Temminck & Schlegel, 1846 | East China Sea, PR China | [10] |
| Ceratomyxa siganicola Zhang, Zhao, Yang & Yang, 2019 | 5.6 ± 0.5 (4.8–6.5) | 19.1 ± 1.8 (16.0–22.1) | 2.7 ± 0.2 (2.1–3.0) | - | - | 177.1 ± 0.5 (175.2–178.4) | Siganas fuscescens Houttuyn, 1782 | East China Sea, PR China | [7] |
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Ceratomyxa nemiptera sp. nov. | 93.56% | 93.38% | 92.49% | 78.88% | 75.74% | 76.86% | |
| Ceratomyxa arcuata | 0.0637 | 97.55% | 93.28% | 79.90% | 78.32% | 77.72% | |
| Ceratomyxa cretensis | 0.0681 | 0.0151 | 92.46% | 78.70% | 76.52% | 77.85% | |
| Ceratomyxa draconis | 0.0751 | 0.0643 | 0.0738 | 78.52% | 74.93% | 76.61% | |
| Ceratomyxa anko | 0.2320 | 0.2000 | 0.2190 | 0.2400 | 70.52% | 72.83% | |
| Ceratomyxa mandii | 0.2790 | 0.2410 | 0.2670 | 0.2850 | 0.3420 | 93.45% | |
| Ceratomyxa macapaensis | 0.2300 | 0.2200 | 0.2250 | 0.2370 | 0.2980 | 0.6400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, P.; Zhou, Y.; Tan, X.; Zhao, Y.; Yang, C. Morphological, Molecular and Phylogenetic Characterization of Ceratomyxa nemiptera sp. nov. (Myxozoa: Ceratomyxidae) Infecting Nemipterus virgatus Houttuyn, 1782 in the East China Sea. Animals 2026, 16, 166. https://doi.org/10.3390/ani16020166
Li P, Zhou Y, Tan X, Zhao Y, Yang C. Morphological, Molecular and Phylogenetic Characterization of Ceratomyxa nemiptera sp. nov. (Myxozoa: Ceratomyxidae) Infecting Nemipterus virgatus Houttuyn, 1782 in the East China Sea. Animals. 2026; 16(2):166. https://doi.org/10.3390/ani16020166
Chicago/Turabian StyleLi, Pingping, Yang Zhou, Xiaoping Tan, Yuanjun Zhao, and Chengzhong Yang. 2026. "Morphological, Molecular and Phylogenetic Characterization of Ceratomyxa nemiptera sp. nov. (Myxozoa: Ceratomyxidae) Infecting Nemipterus virgatus Houttuyn, 1782 in the East China Sea" Animals 16, no. 2: 166. https://doi.org/10.3390/ani16020166
APA StyleLi, P., Zhou, Y., Tan, X., Zhao, Y., & Yang, C. (2026). Morphological, Molecular and Phylogenetic Characterization of Ceratomyxa nemiptera sp. nov. (Myxozoa: Ceratomyxidae) Infecting Nemipterus virgatus Houttuyn, 1782 in the East China Sea. Animals, 16(2), 166. https://doi.org/10.3390/ani16020166





