The Effect of Poppy Oil on Egg Production and Calcium Metabolism in Japanese Quail
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Animals
2.3. Dietary Treatments
2.4. Measurements and Samplings
2.5. Feed and Excreta Analyses
2.6. Calculation of Ca Retention
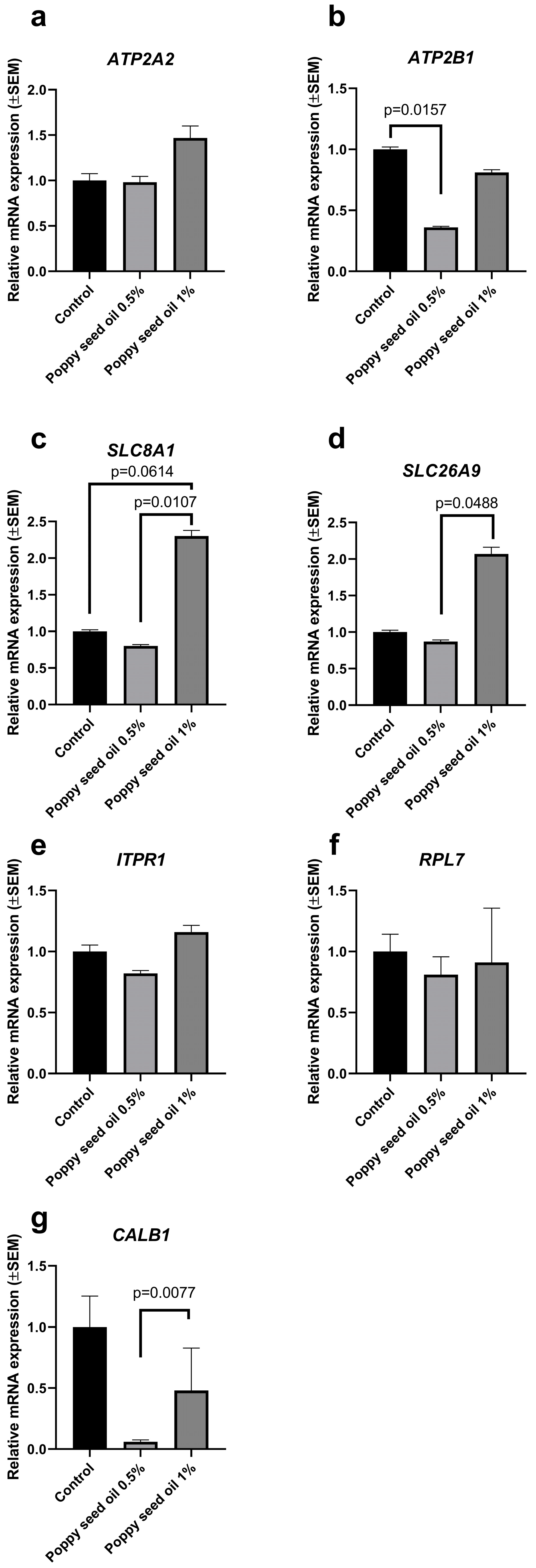
2.7. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR (qPCR) Assays
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahim, M.A.; Ayub, H.; Sehrish, A.; Ambreen, S.; Khan, F.A.; Itrat, N.; Nazir, A.; Shoukat, A.; Shoukat, A.; Ejaz, A.; et al. Essential Components from Plant Source Oils: A Review on Extraction, Detection, Identification, and Quantification. Molecules 2023, 28, 6881. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Espírito Santo, L.; Machado, S.; Pardo, J.E.; Oliveira, M.B.P.P. Nutritional and Chemical Characterization of Poppy Seeds, Cold-Pressed Oil, and Cake: Poppy Cake as a High-Fibre and High-Protein Ingredient for Novel Food Production. Foods 2022, 11, 3027. [Google Scholar] [CrossRef]
- Tavlı, Ö.F. Papaver somniferum L. In Novel Drug Targets with Traditional Herbal Medicines; Gürağaç Dereli, F.T., Ilhan, M., Belwal, T., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Shah, H.A.; Khan, S.; Ahmed, M.M. Unravelling the Medicinal Secrets of Khashkhaash (Papaver somniferum L.) Seeds: A Powerful Blend of Unani Wisdom and Modern Science. J. Drug Deliv. Ther. 2025, 15, 155–158. [Google Scholar] [CrossRef]
- Muhammad, A.; Akhtar, A.; Aslam, S.; Khan, R.S.; Ahmed, Z.; Khalid, N. Review on physicochemical, medicinal and nutraceutical properties of poppy seeds: A potential functional food ingredient. Funct. Foods Health Dis. 2021, 11, 522–547. [Google Scholar] [CrossRef]
- Midilli, M.; Bayram, I.; Erol, H.; Cetingul, I.S.; Cakir, S.; Calikoglu, E.; Kiralan, M. The Effects of Dietary Poppy Seed Oil and Sunflower Oil on Performance, Reproduction and Egg Quality Parameters and Fatty Acid Profile of Egg Yolk in the Japanese quail. J. Anim. Vet. Adv. 2009, 8, 379–384. [Google Scholar]
- Bayram, İ.; Çetingül, İ.S.; Yardımcı, M.; Şahin, E.H.; Akkaya, A.B.; Uyarlar, C. Effects of Poppy Seed Oil Supplementation in Diets on Egg Production, Egg Quality and Some Blood Parameters in Laying Hens. Kocatepe Vet. J. 2008, 1, 37–42. [Google Scholar]
- Akinci, Z.; Bayram, I. Effects of Poppy Seed Meal on Egg Production and Hatching Results in Quail (Coturnix coturnix japonica). Res. Vet. Sci. 2003, 75, 141–147. [Google Scholar] [CrossRef]
- Fidan, F.; Küçükkurt, İ.; Sözbilir, N.B.; Eryavuz, A.; Bayram, İ.; Çetingül, S.; Yardımcı, M. Effects of Supplementation of Poppy Seed and Poppy Seed Oil at Various Quantities on Oxidant-Antioxidant Balance in Laying Hens. Kocatepe Vet. J. 2010, 3, 1–5. [Google Scholar]
- Jan, Y.; Peer, L.A. Papaver somniferum: Phytochemistry, Biological Activity and Toxicology; a review. Int. J. Bot. Stud. 2021, 6, 753–757. [Google Scholar]
- Hungarian Committee on Nutrient Recommendations, Codex Pabularis Hungaricus II. Magyar Takarmánykódex II; A/3 Nyomdaipari és Kiadói Szolgáltató Kft: Budapest, Hungary, 2004; ISBN 963860970503. [Google Scholar]
- ISO 6496; Animal Feeding Stuff—Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Vernier, Switzerland, 1999.
- ISO 5984; Animal Feeding Stuff—Determination of Crude Ash. International Organization for Standardization: Vernier, Switzerland, 2022.
- Al-Masri, M.R. Absorption and Endogenous Excretion of Phosphorus in Growing Broiler Chicks, as Influenced by Calcium and Phosphorus Ratios in Feed. Br. J. 1995, 74, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.V.; Couroussé, N.; Crochet, S.; Coustham, V. Identification of Reference Genes for Quantitative Gene Expression Studies in Three Tissues of Japanese Quail. Genes 2019, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Lančaričová, A.; Havrlentová, M.; Muchová, D.; Bednárová, A. Oil Content and Fatty Acids Composition of Poppy Seeds Cultivated in Two Localities of Slovakia. Agriculture (Pol’nohospodárstvo) 2016, 62, 19–27. [Google Scholar] [CrossRef]
- Luhmer, K.; Schulze-Kaysers, N.; Feuereisen, M.; Wirth, L.; Maretzky, F.; Wüst, M.; Blum, H.; Dörr, E.; Pude, R. Fatty Acid Composition, Tocopherols, Volatile Compounds, and Sensory Evaluation of Low Morphine Yielding Varieties of Poppy (Papaver somniferum L.) Seeds and Oils. J. Agric. Food Chem. 2021, 69, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Atalay, C. Determination of Seed and Oil Properties of Some Poppy (Papaver somniferum L.) Varieties. Grasas Y Aceites 2006, 57, 169–174. [Google Scholar] [CrossRef]
- Coelho, L.M.; Alves Leão, A.P.; Bernardes, L.F.; Veiga Alves, V.; Gomes Martins, B.; Vogas Peixoto, J.; José Pereira, L.; José Fassani, É.; Ribeiro Alvarenga, R.; Gilberto Zangeronimo, M. Reproductive Aspects of Japanese quails (Coturnix coturnix japonica) Hatched from Eggs Incubated Under Different Light Colors. Theriogenology 2021, 170, 67–76. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Mohammed, H.; Hassan, R.A.; Swelum, A.A.; Saadeldin, I.M. Does Light Intensity Affect the Behavior, Welfare, Performance, Meat Quality, Amino Acid Profile, and Egg Quality of Japanese quails? Poult. Sci. 2019, 98, 3093–3102. [Google Scholar] [CrossRef]
- Pizzolante, C.C.; Faltarone, A.B.G.; Garcia, E.A.; Saldanha, E.S.P.B.; Deodato, A.P.; Sherer, M.R.; Mendes, A.A.; Mori, C.; Pelicia, K. Production Performance and Egg Quality of Quails (Coturnix japonica) During Several Periods of the Day. Braz. J. Poult. Sci. 2006, 8, 149–152. [Google Scholar] [CrossRef]
- Sharifi, M.R.; Shams Shargh, M.; Hassani, S.; Senobar, H.; Jenabi, S. The Effects of Dietary Nonphytate Phosphorus Levels and Phytase on Laying Performance and Egg Quality Parameters of Japanese quails (Coturnix coturnix japonica). Arch. Geflügelk. 2012, 76, 13–19. [Google Scholar] [CrossRef]
- Buğdaycı, K.E.; Gümüş, H.; Oğuz, M.N.; Karakaş Oğuz, F.; Gülle, İ. Effects of Mediterranean Mussel Shell (Mytilus galloprovincialis) on Performance and Egg Quality in Laying Quails. Acta Vet. Eurasia 2019, 45, 22–29. [Google Scholar] [CrossRef]
- Navarro, M.P.; Murillo, A. Calcium Balance in the Quail (Coturnix coturnix japonica): 1. Influence of Sex and Diethylstilbestrol. Poult. Sci. 1976, 55, 2201–2209. [Google Scholar] [CrossRef]
- Kaur, S.; Mandal, A.B.; Singh, K.B.; Kadam, M.M.; Elangovan, A.V. Response of Laying Japanese quails to Graded Levels of Essential Amino Acids Profile with Reduced Dietary Protein. J. Sci. Food Agric. 2007, 87, 751–759. [Google Scholar] [CrossRef]
- Sünder, A.; Wilkens, M.; Böhm, V.; Liebert, F. Egg Yolk Colour in Organic Production as Affected by Feeding—Consequences for Farmers and Consumers. Food Chem. 2022, 382, 131854. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Deng, X.; Ji, X.; Liu, N.; Cai, H. Sources, Dynamics in vivo, and Application of Astaxanthin and Lutein in Laying Hens: A Review. Anim. Nutr. 2023, 13, 324–333. [Google Scholar] [CrossRef]
- Hammershøj, M.; Johansen, N.F. Review: The Effect of Grass and Herbs in Organic Egg Production on Egg Fatty Acid Composition, Egg Yolk Colour and Sensory Properties. Livest. Sci. 2016, 194, 37–43. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Cui, B.; Wang, M.; Fu, H.; Wang, Y. Carotenoid-Enriched Oil Preparation and Stability Analysis During Storage: Influence of Oils’ Chain Length and Fatty Acid Saturation. LWT 2021, 151, 112163. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian Eggshell Mineralization: Biochemical and Functional Characterization of Matrix Proteins. Comptes. Rendus. Palevol. 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Brionne, A.; Nys, Y.; Hennequet-Antier, C.; Gautron, J. Hen Uterine Gene Expression Profiling During Eggshell Formation Reveals Putative Proteins Involved in the Supply of Minerals or in the Shell Mineralization Process. BMC Genom. 2014, 15, 220. [Google Scholar] [CrossRef]
- Bahadoran, S.; Samani, A.D.; Hassanpour, H. Effect of Heat Stress on the Gene Expression of Ion Transporters/channels in the Uterus of Laying Hens During Eggshell Formation. Stress 2018, 21, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jonchère, V.; Brionne, A.; Gautron, J.; Nys, Y. Identification of Uterine Ion Transporters for Mineralisation Precursors of the Avian Eggshell. BMC Physiol. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Nys, Y.; Roy, N. Chapter 22—Calcium Homeostasis and Eggshell Biomineralization in Female Chicken, Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: London, UK, 2019; pp. 361–382. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Jin, Y.; Zhuang, Z.; Huang, X.; Li, K.; Wang, S.; Xia, W.; Ruan, D.; Wang, S.; et al. Dietary Zinc Supplementation Affects Eggshell Quality and Ultrastructure in Commercial Laying Ducks by Influencing Calcium Metabolism. Poult. Sci. 2022, 101, 101539. [Google Scholar] [CrossRef]
- Bar, A. Differential Regulation of Calbindin in the Calcium Transporting Organs of Birds with high Calcium Requirements. J. Poult. Sci. 2009, 46, 267–285. [Google Scholar] [CrossRef]
- Narayanan, D.; Adebiyi, A.; Jaggar, J.H. Inositol Trisphosphate Receptors in Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2190–H2210. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Xia, W.G.; Chen, W.; Abouelezz, K.F.M.; Ruan, D.; Wang, S.; Zhang, Y.N.; Huang, X.B.; Li, K.C.; Zheng, C.T.; et al. Effects of Capsaicin on Laying Performance, Follicle Development, and Ovarian Antioxidant Capacity in Aged Laying Ducks. Poult. Sci. 2021, 100, 100901. [Google Scholar] [CrossRef]
- Chen, W.; Xia, W.G.; Ruan, D.; Wang, S.; Abouelezz, K.F.M.; Wang, S.L.; Zhang, Y.N.; Zheng, C.T. Dietary Calcium Deficiency Suppresses Follicle Selection in Laying Ducks Through Mechanism Involving Cyclic Adenosine Monophosphate-mediated Signaling Pathway. Animal 2020, 14, 2100–2108. [Google Scholar] [CrossRef]
- Wang, J.W.; Chen, W.; Kang, X.T.; Huang, Y.Q.; Tian, Y.D.; Wang, Y.B. Identification of Differentially Expressed Genes Induced by Energy Restriction Using Annealing Control Primer System from the Liver and Adipose Tissues of Broilers. Poult. Sci. 2012, 91, 972–978. [Google Scholar] [CrossRef]

| Treatment/Composition (%) | |||
|---|---|---|---|
| Ingredients | Control | 0.5% Poppy Oil | 1.0% Poppy Oil |
| corn | 25.79 | 25.79 | 25.79 |
| wheat | 30.00 | 30.00 | 30.00 |
| soybean meal, 46% | 30.04 | 30.04 | 30.04 |
| sunflower oil | 4.70 | 4.20 | 3.70 |
| poppy oil | 0.00 | 0.50 | 1.00 |
| Mono calcium phosphate | 1.62 | 1.62 | 1.62 |
| limestone | 5.65 | 5.65 | 5.65 |
| salt | 1.05 | 1.05 | 1.05 |
| DL-Met | 0.15 | 0.15 | 0.15 |
| SiO2 | 0.50 | 0.50 | 0.50 |
| premix a | 0.50 | 0.50 | 0.50 |
| Total | 100.0 | 100.0 | 100.0 |
| Nutrients | Nutrient content | ||
| crude protein, % b | 18.3 | 18.3 | 18.2 |
| ME c, MJ/kg | 12.13 | 12.13 | 12.13 |
| Lys, % | 1.04 | 1.04 | 1.04 |
| Met, % | 0.45 | 0.45 | 0.45 |
| Thr, % | 0.74 | 0.74 | 0.74 |
| Trp, % | 0.24 | 0.24 | 0.24 |
| Ca, % b | 2.03 | 2.14 | 2.23 |
| total P, % b | 0.70 | 0.78 | 0.74 |
| non phytate P, % | 0.35 | 0.35 | 0.35 |
| Na, % | 0.4 | 0.4 | 0.4 |
| Nutrient | Sunflower Oil | Poppy Oil |
|---|---|---|
| Myristic acid C14:0, % | 0.06 | 0.06 |
| Pentadecanoic acid C15:0, % | 0.01 | 0.01 |
| Palmitic acid C16:0, % | 6.12 | 8.77 |
| Palmitoleic acid C16:1, % | 0.07 | 0.14 |
| Margaric acid C17:0, % | 0.04 | 0.06 |
| Heptadecanoic acid C17:1, % | 0.03 | - |
| Stearic acid C18:0, % | 3.14 | 2.07 |
| Oleic acid C18:1c, % | 26.41 | 14.13 |
| Octadecanoic acid isomer C 18:1, % | 0.61 | 1.23 |
| Linoleic acid C18:2c, % | 60.82 | 72.81 |
| Arachidic acid C20:0, % | 0.21 | 0.09 |
| Eicosenoic acid C20:1, % | 0.14 | 0.07 |
| α-Linolenic acid C18:3n3, % | 1.44 | 0.56 |
| Behenic acid C22:0, % | 0.69 | - |
| Tricosanoic acid C23:0, % | 0.02 | - |
| Lignoceric acid C24:0, % | 0.20 | - |
| Ca, mg/kg | 44.5 | 37.6 |
| P, mg/kg | 85.6 | 0.50 |
| GenBank No. | Gene Symbol | Gene Name | Forward and Reverse Primer | Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|---|
| XM_015877724.2 | ATP2A2 | ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 | F: AGCGTTCAGAGAATCAAAGCAAG | 81 | 54.81 |
| R: ATCAGCAGGAACCTTGTCTCCA | 56.36 | ||||
| XM_015277056.2 | ATP2B1 | ATPase plasma membrane Ca2+ transporting 1 | F: CTCGGCGCTGCCCGGTG | 103 | 61.38 |
| R: CCATGACGAGCTGTGTTCCCCAA | 60.11 | ||||
| XM_032447330.1 | ITPR1 | inositol 1,4,5-trisphosphate receptor type 1 | F: ACAGCCAGAAGCAGGTGACCTTA | 118 | 58.74 |
| R: CGGCTTTGCTGCTTTCCAGAACT | 59.25 | ||||
| XM_015855985.2 | CALB1 | calbindin 1 | F: ACGACTCCGACGGCAATGGGTA | 120 | 60.93 |
| R: CCACAAAGGCTTTCATTTCGGGT | 57.12 | ||||
| XM_015885285.2 | SLC26A9 | solute carrier family 26 member 9 | F: GCTCTTCTCTCCGTGCCACCT | 106 | 59.15 |
| R: CGTTCGATGGCATAGCGGGGTC | 60.78 | ||||
| XM_032443672.1 | SLC8A1 | solute carrier family 8 member A1 | F: CACCTGTGGGGAGCTGGAGT | 113 | 58.67 |
| R: CCCCGATCTCTAGGTAGAAGGTCTT | 57.46 | ||||
| XM_015855784.2 | RPL7 | ribosomal protein L7 | F: ACTTTGTGGAGGGTGGAGATGCT | 96 | 58.77 |
| R: AAACTGCAGCTGGGCATCTGA | 57.62 | ||||
| XM_015873412.2 | GAPDH | glyceraldehyde-3-phosphate dehydrogenase | F: TCTCTGTTGTTGACCTGACCTG | 155 | 54.90 |
| R: ATGGCTGTCACCATTGAAGTC | 53.56 |
| Poppy Oil Inclusion | RMSE | p | |||
|---|---|---|---|---|---|
| Control | 0.5% | 1.0% | |||
| Number of eggs produced per week | 57.2 b | 62.3 ab | 66.3 a | 6.76 | 0.0004 |
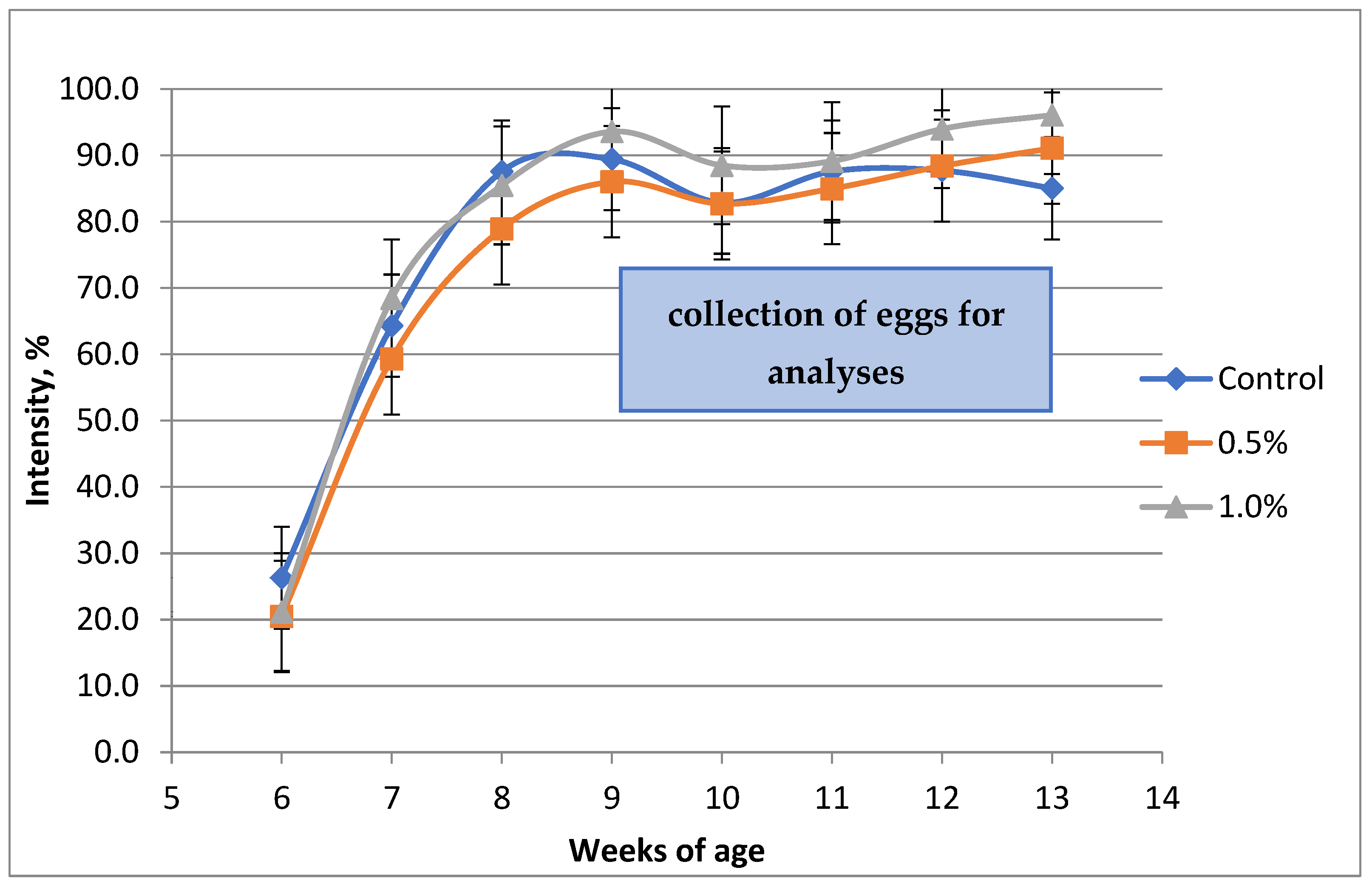
| Production intensity, % | 86.5 b | 86.6 b | 92.2 a | 6.78 | 0.0133 |
| Egg weight, g | 12.29 b | 12.59 a | 12.35 ab | 0.34 | 0.0160 |
| Egg mass per week, g | 703.4 b | 783.6 a | 818.1 a | 89.1 | 0.0005 |
| Feed conversion, g/egg | 39.8 | 40.1 | 38.1 | 3.50 | 0.1674 |
| Feed conversion kg/kg egg mass | 3.24 | 3.18 | 3.09 | 0.25 | 0.1645 |
| Ca retention, % | 9.45 b | 18.90 a | 21.62 a | 2.49 | 0.0114 |
| Traits of eggs used for strength analyses | |||||
| Egg weight, g | 12.7 | 12.7 | 12.7 | 0.53 | 0.9842 |
| Eggshell weight, g | 1.17 | 1.20 | 1.17 | 0.07 | 0.3318 |
| Eggshell thickness, µm | 268.3 ab | 268.5 a | 255.7 b | 16.7 | 0.0277 |
| Eggshell strength, N | 14.1 | 14.7 | 13.4 | 1.74 | 0.0834 |
| Yolk color c | 5.36 a | 4.67 b | 4.51 b | 0.52 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, C.; Ozsváth, X.; Csernus, B.; Gulyás, G.; Horváth, M.; Czeglédi, L.; Oláh, J.; Rizqoh, N.; Achille, G.; Posta, J. The Effect of Poppy Oil on Egg Production and Calcium Metabolism in Japanese Quail. Animals 2025, 15, 1348. https://doi.org/10.3390/ani15091348
Szabó C, Ozsváth X, Csernus B, Gulyás G, Horváth M, Czeglédi L, Oláh J, Rizqoh N, Achille G, Posta J. The Effect of Poppy Oil on Egg Production and Calcium Metabolism in Japanese Quail. Animals. 2025; 15(9):1348. https://doi.org/10.3390/ani15091348
Chicago/Turabian StyleSzabó, Csaba, Xénia Ozsváth, Brigitta Csernus, Gabriella Gulyás, Márta Horváth, Levente Czeglédi, János Oláh, Nafiatur Rizqoh, Gabriele Achille, and János Posta. 2025. "The Effect of Poppy Oil on Egg Production and Calcium Metabolism in Japanese Quail" Animals 15, no. 9: 1348. https://doi.org/10.3390/ani15091348
APA StyleSzabó, C., Ozsváth, X., Csernus, B., Gulyás, G., Horváth, M., Czeglédi, L., Oláh, J., Rizqoh, N., Achille, G., & Posta, J. (2025). The Effect of Poppy Oil on Egg Production and Calcium Metabolism in Japanese Quail. Animals, 15(9), 1348. https://doi.org/10.3390/ani15091348






