Morphology, Molecular Characterization, and Phylogeny of Travassosius rufus Khalil, 1922 (Strongylidea: Trichostrongylidae), a Parasite from Endangered Sino-Mongolian Beaver (Castor fiber birulai) in Xinjiang, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Observation
2.3. Molecular Analysis
2.3.1. DNA Extraction, PCR Amplification, and Sequencing
2.3.2. DNA Annotation and Data Analysis
Sequence Analysis
2.4. Phylogenetic Tree Construction
3. Results
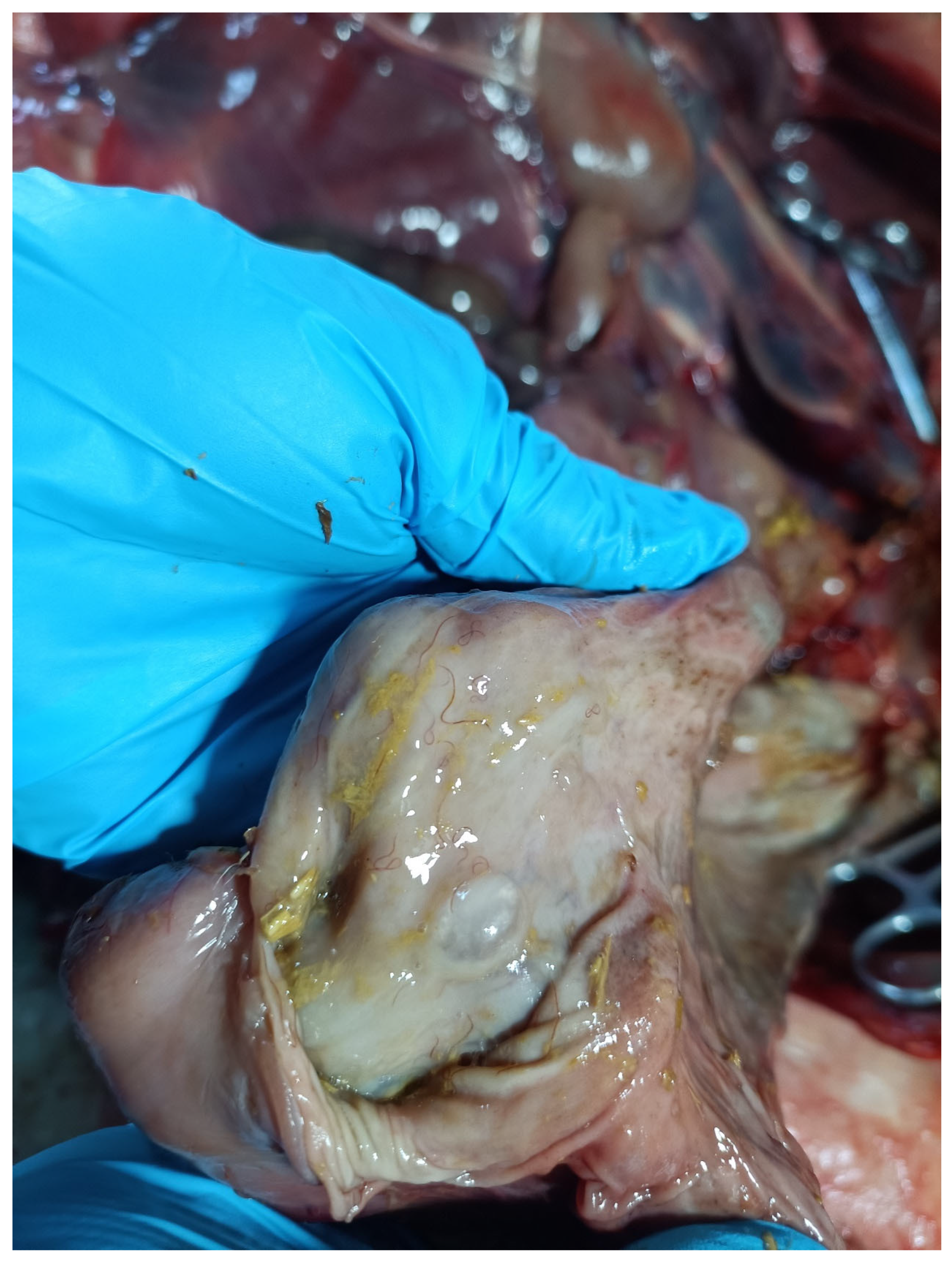
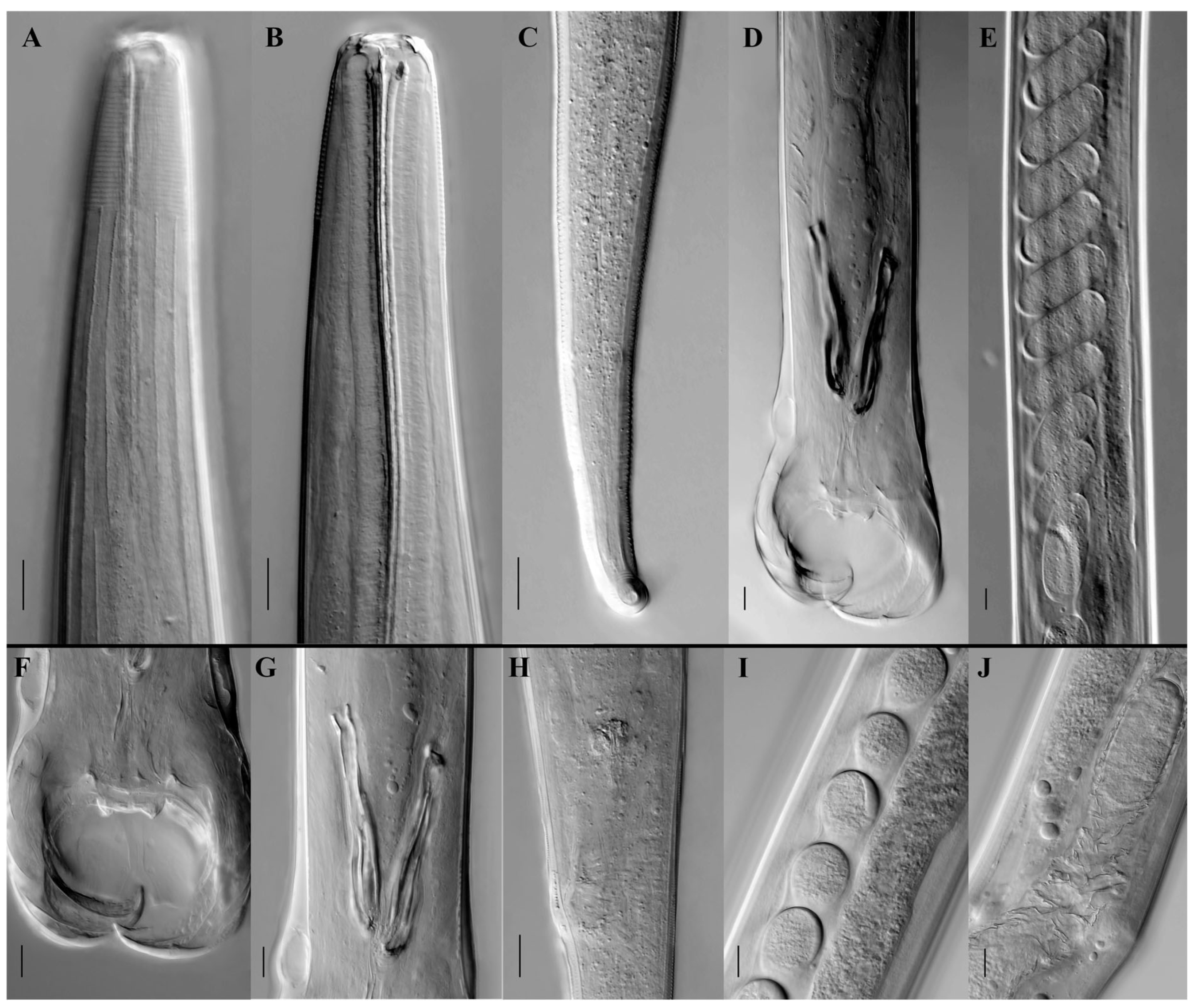
3.1. Morphological and Taxonomic Study of Travassosius rufus in Sino-Mongolian Beaver Based on Classification Methods
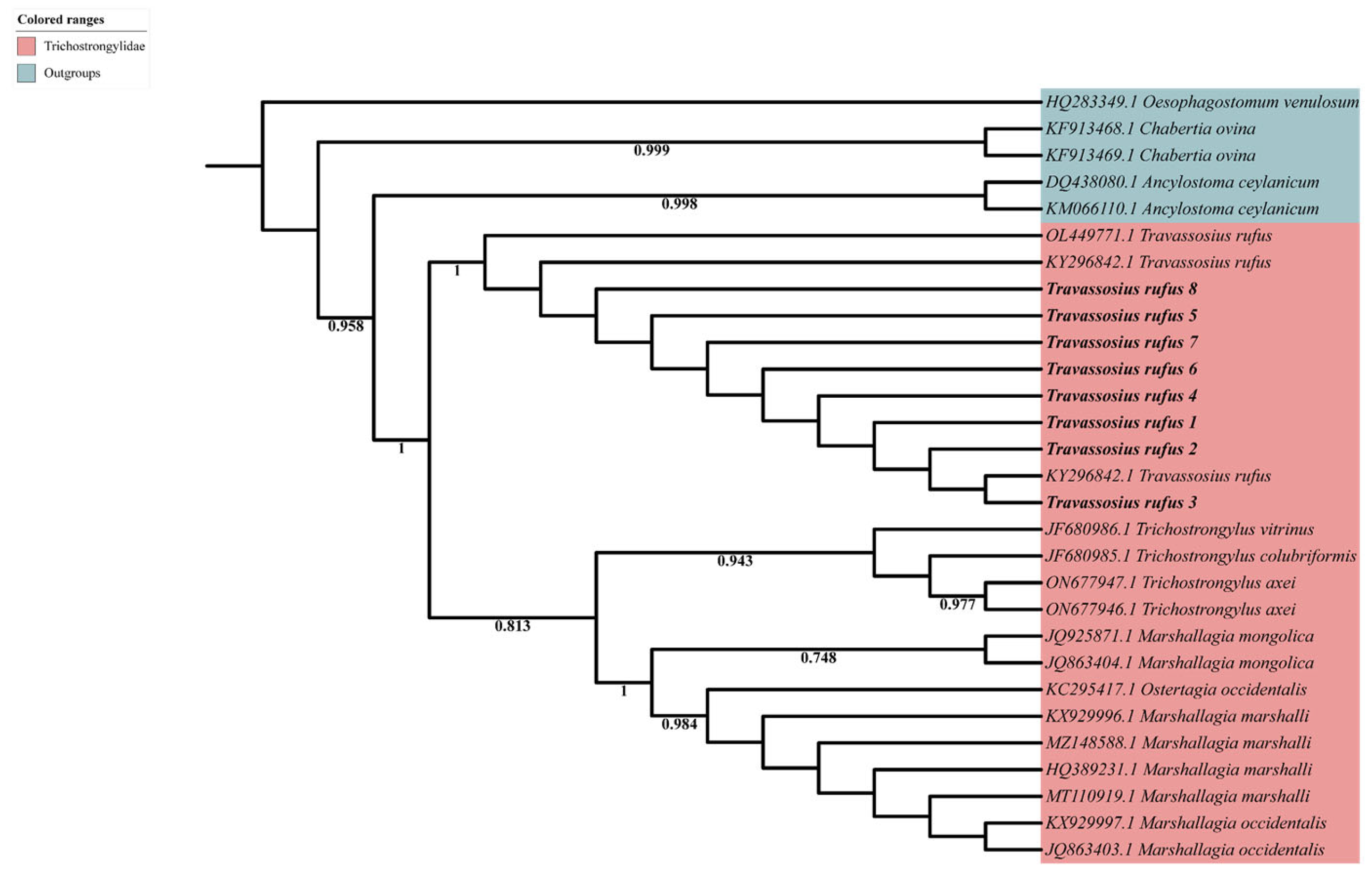
3.2. Molecular Taxonomy and Phylogenetic Position of Travassosius rufus in Sino-Mongolian Beaver Based on Sanger Sequencing
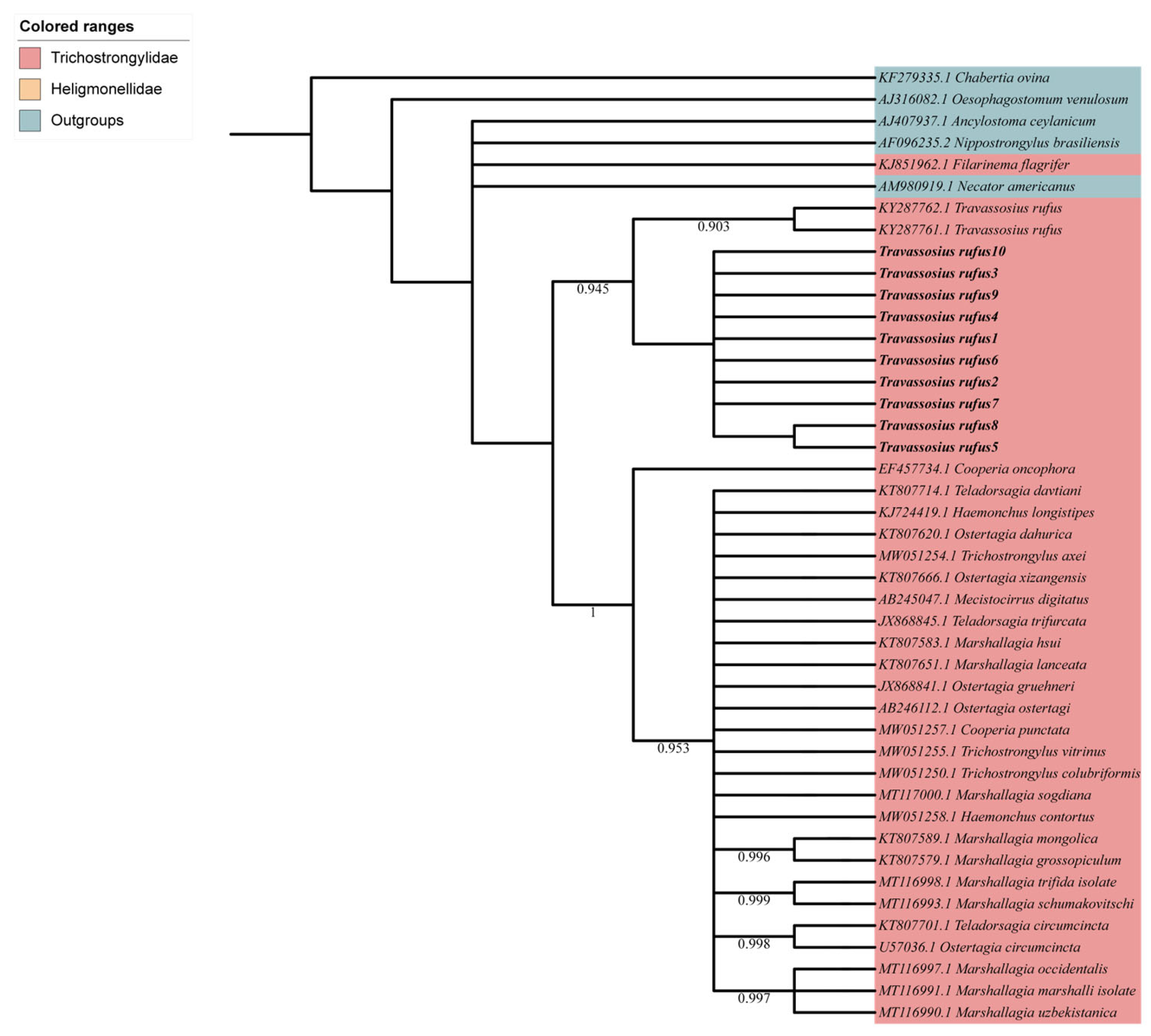
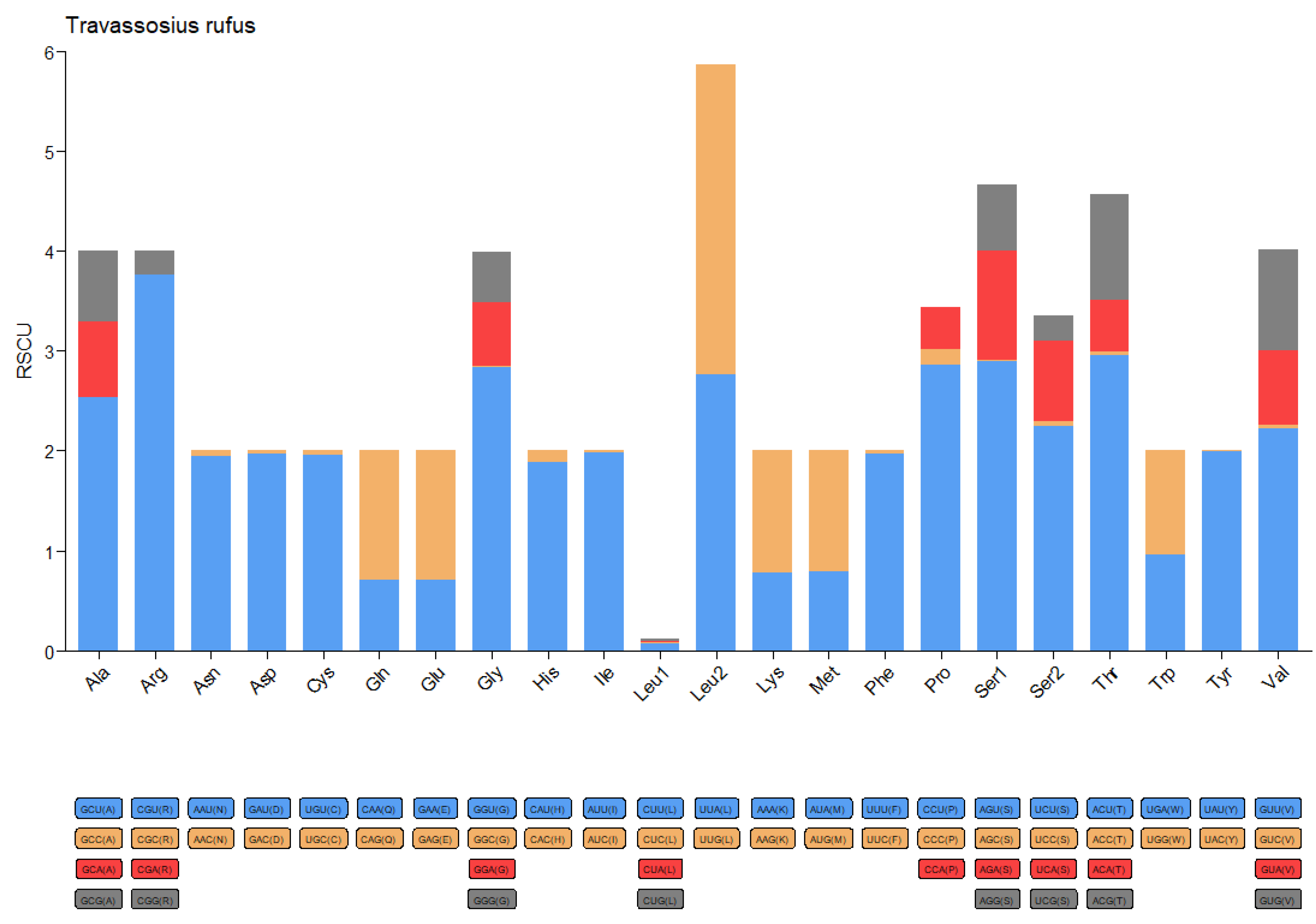
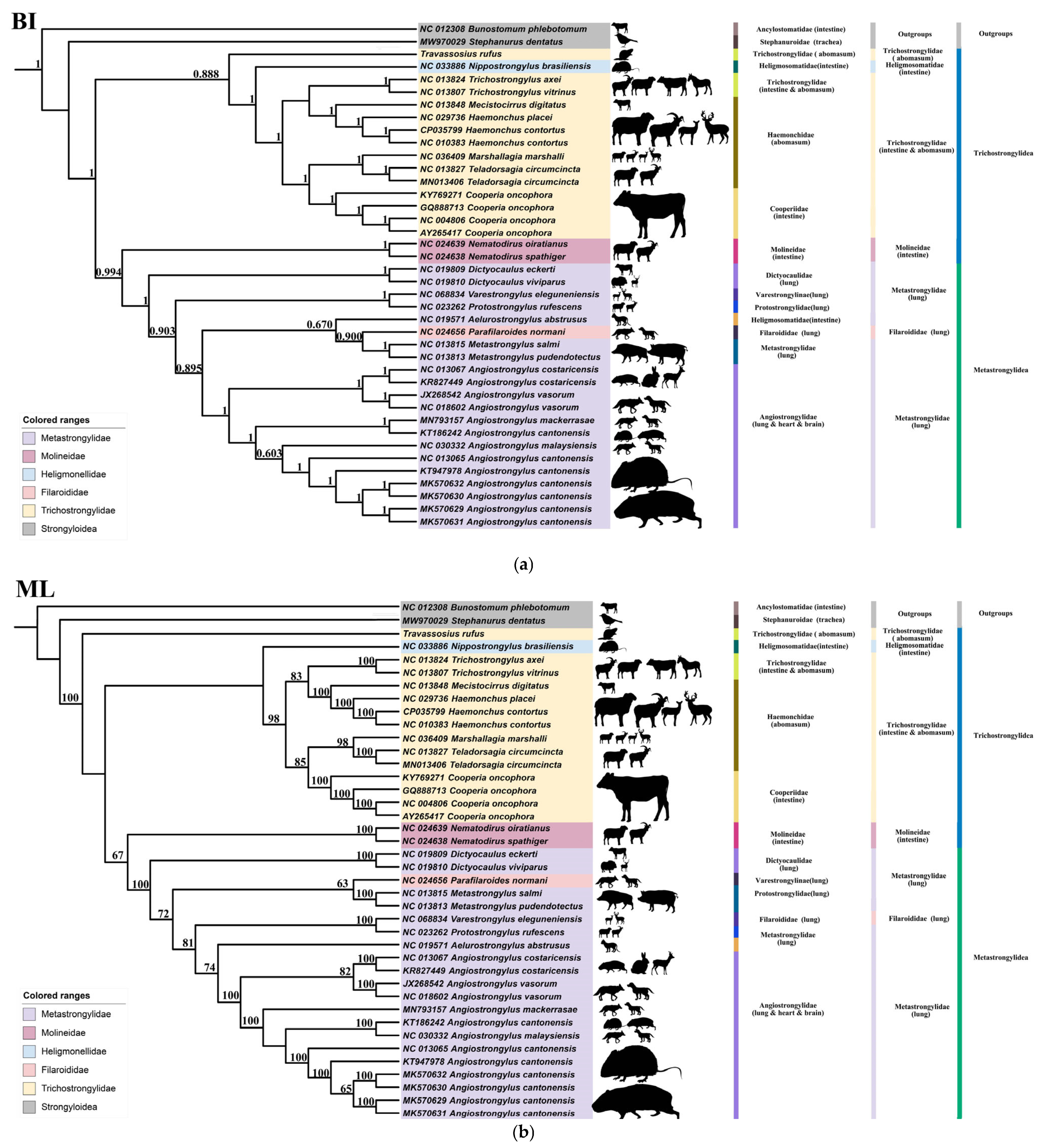
3.3. Phylogenetic and Micro-Taxonomic Classification of Travassosius rufus Based on Whole-Genome Sequencing
4. Discussion
4.1. Taxonomy of Travassosius rufus in Beavers Based on Morphology
4.2. Molecular Taxonomy and Phylogenetic Status of Travassosius rufus Based on DNA Barcoding
4.3. Molecular Taxonomy and Phylogenetic Status of Travassosius rufus Based on Genome Sequencing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H. The distribution and ecological characteristics of the beaver (Castor fiber L.) in China. J. Shandong Univ. (Nat. Sci.) 1981, 4, 103–109. [Google Scholar]
- Chu, H.; Jiang, Z. Distribution and Conservation of the Sino-Mongolian Beaver (Castor fiber birulai) in China. Oryx 2009, 43, 197–202. [Google Scholar] [CrossRef]
- Djoshkin, W.; Safonow, W. Die Biber der Alten und Neuen Welt; Ziemsen Verlag: Wittenberg, Germany, 1972. [Google Scholar]
- Raškauskaitė, M.; Šimkevičius, K. Eurasian Beaver (Castor fiber L.) Population in Asu Science and Teaching Hunting Area and Beaver Dams Rebuild Intensity; Vytautas Magnus University: Kaunas, Lithuania, 2018. [Google Scholar]
- Gridan, A.; Niță, D.; Ionescu, G.; Popa, M.; Pașca, C. Distribution, structural and functional characteristics of beaver dams (Castor fiber): Case study. Black River Basin 2017, 22, 88–92. [Google Scholar]
- Nica, A.; Petrea, M.-S.; Simionov, I.-A.; Antache, A.; Cristea, V. Ecological impact of european beaver. Castor fiber. Anim. Sci. 2022, 65, 640–647. [Google Scholar]
- Brazier, R.E.; Puttock, A.; Graham, H.A.; Auster, R.E.; Davies, K.H.; Brown, C.M.L. Beaver: Nature’s Ecosystem Engineers. Wires Water 2021, 8, e1494. [Google Scholar] [CrossRef]
- Bashinskiy, I.V. Beavers in Lakes: A Review of Their Ecosystem Impact. Aquat. Ecol. 2020, 54, 1097–1120. [Google Scholar] [CrossRef]
- Liu, D.; Huang, X.; Chu, H.; Liu, Y.; Zhang, F.; Chen, G.; Qi, Y. Activity Rhythms of Sino-Mongolia Beaver (Caster fiber birulai) Measured with Infrared Camera Traps in Xinjiang, China. Arid. Zone Res. 2015, 32, 205–211. [Google Scholar]
- Nolet, B.A.; Rosell, F. Comeback of the beaver Castor fiber: An overview of old and new conservation problems. Biol. Conserv. 1998, 83, 165–173. [Google Scholar] [CrossRef]
- Ahlen, P.A.; Sjöberg, G.; Stéen, M. Parasitic Fauna of Eurasian Beavers (Castor fiber) in Sweden (1997–1998). Acta Vet. Scand. 2021, 63, 23. [Google Scholar] [CrossRef]
- Sainsbury, A.; Common, S. NECR540 Revised Disease Risk Analysis for the Conservation Translocation of the Eurasian Beaver (Castor fiber) to England; Natural England: York, UK, 2024. [Google Scholar]
- Jasmer, D.P.; Goverse, A.; Smant, G. Parasitic Nematode Interactions with Mammals and Plants. Annu. Rev. Phytopathol. 2003, 41, 245–270. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Fenton, A.; Petchey, O.L.; Jones, T.R.; Barber, R.; Pedersen, A.B. Stability of Within-Host-Parasite Communities in a Wild Mammal System. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. On the Discovery of the Genus Travassosius from China (Strongylidea: Trichostrongylidae). Acta Zootaxonomica Sin. 1992, 4, 492–494. [Google Scholar]
- Chilton, N.B.; Huby-Chilton, F.; Gasser, R.B.; Beveridge, I. The Evolutionary Origins of Nematodes within the Order Strongylida Are Related to Predilection Sites within Hosts. Mol. Phylog. Evol. 2006, 40, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Traversa, D.; Giangaspero, A.; Galli, P.; Paoletti, B.; Otranto, D.; Gasser, R.B. Specific Identification of Habronema Microstoma and Habronema Muscae (Spirurida, Habronematidae) by PCR Using Markers in Ribosomal DNA. Mol. Cell Probes 2004, 18, 215–221. [Google Scholar] [CrossRef]
- McDonnell, A.; Love, S.; Tait, A.; Lichtenfels, J.R.; Matthews, J.B. Phylogenetic Analysis of Partial Mitochondrial Cytochrome Oxidase c Subunit I and Large Ribosomal RNA Sequences and Nuclear Internal Transcribed Spacer I Sequences from Species of Cyathostominae and Strongylinae (Nematoda, Order Strongylida). Parasites Parasitol. 2000, 121, 649–659. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of Nucleotide Composition at Fourfold Degenerate Sites of Animal Mitochondrial Genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Jex, A.R.; Waeschenbach, A.; Hu, M.; van Wyk, J.A.; Beveridge, I.; Littlewood, D.T.J.; Gasser, R.B. The Mitochondrial Genomes of Ancylostoma caninum and Bunostomum phlebotomum—Two Hookworms of Animal Health and Zoonotic Importance. BMC Genom. 2009, 10, 79. [Google Scholar] [CrossRef]
- Audebert, F.; Durette-Desset, M.C. Do lagomorphs play a relay role in the evolution of the Trichostrongylina nematodes? Parasite 2007, 14, 183–197. [Google Scholar] [CrossRef][Green Version]
- Underwood, W.J.; Blauwiekel, R.; Delano, M.L. Chapter 15-Biology and diseases of ruminants (sheep, goats, and cattle). In Laboratory Animal Medicine; Academic Press: Cambridge, MA, USA, 2015; Volume 519, pp. 623–694. [Google Scholar]
- Van Der Veer, M.; De Vries, E. A Single Nucleotide Polymorphism Map of the Mitochondrial Genome of the Parasitic Nematode Cooperia Oncophora. Parasitology 2004, 128, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Jex, A.; Hu, M.; Littlewood, D.; Waeschenbach, A.; Gasser, R.B. Using 454 technology for long-PCR based sequencing of the complete mitochondrial genome from single Haemonchus contortus (Nematoda). BMC Genom. 2008, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Loiola dos Santos, L.; Prosdocimi, F.; Costa Barroso Lima, N.; Rodrigues da Costa, I.; Cunha Cardoso, D.; Gonçalves Drummond, M.; dos Santos Alves Figueiredo Brasil, B.; Bastianetto, E.; Aparecida Andrade de Oliveira, D. Comparative Genomics and Phylogenomics of Trichostrongyloidea Mitochondria Reveal Insights for Molecular Diagnosis and Evolutionary Biology of Nematode Worms. Gene Rep. 2017, 9, 65–73. [Google Scholar] [CrossRef]
- Sun, M.M.; Han, L.; Zhang, F.K.; Zhou, D.H.; Wang, S.Q.; Ma, J.; Zhu, X.Q.; Liu, G.H. Characterization of the Complete Mitochondrial Genome of Marshallagia Marshalli and Phylogenetic Implications for the Superfamily Trichostrongyloidea. Parasitol. Res. 2018, 117, 307–313. [Google Scholar] [CrossRef]
- Palevich, N.; Maclean, P.H.; Choi, Y.J.; Mitreva, M. Characterization of the Complete Mitochondrial Genomes of Two Sibling Species of Parasitic Roundworms, Haemonchus contortus and Teladorsagia circumcincta. Front. Genet. 2020, 11, 573395. [Google Scholar] [CrossRef]
- Zhao, G.H.; Jia, Y.Q.; Cheng, W.Y.; Zhao, W.; Bian, Q.Q.; Liu, G.H. Characterization of the Complete Mitochondrial Genomes of Nematodirus oiratianus and Nematodirus spathiger of Small Ruminants. Parasites Vectors 2014, 7, 319. [Google Scholar] [CrossRef]
- Li, K.; Shahzad, M.; Zhang, H.; Mehmood, K.; Jiang, X.; Luo, H.; Zhang, L.; Dong, X.; Li, J. Characterization of the Complete Mitochondrial Genome of Metastrongylus salmi (M. salmi) Derived from Tibetan Pigs in Tibet, China. Acta Parasitol. 2018, 63, 280–286. [Google Scholar] [CrossRef]
- Gasser, R.B.; Jabbar, A.; Mohandas, N.; Höglund, J.; Hall, R.S.; Littlewood, T.J.; Jex, A.R. Assessment of the Genetic Relationship between Dictyocaulus Species from Bos taurus and Cervus elaphus Using Complete Mitochondrial Genomic Datasets. Parasites Vectors 2012, 5, 241. [Google Scholar] [CrossRef]
- Jabbar, A.; Jex, A.R.; Mohandas, N.; Hall, R.S.; Littlewood, D.T.J.; Gasser, R.B. The Mitochondrial Genome of Aelurostrongylus abstrusus-Diagnostic, Epidemiological and Systematic Implications. Gene 2013, 516, 294–300. [Google Scholar] [CrossRef]
- Jefferies, R.; Vrhovec, M.G.; Wallner, N.; Catalan, D.R. Aelurostrongylus abstrusus and Troglostrongylus sp. (Nematoda: Metastrongyloidea) Infections in Cats Inhabiting Ibiza, Spain. Vet. Parasitol. 2010, 173, 344–348. [Google Scholar] [CrossRef]
- Jabbar, A.; Mohandas, N.; Jex, A.R.; Gasser, R.B. The Mitochondrial Genome of Protostrongylus rufescens-Implications for Population and Systematic Studies. Parasites Vectors 2013, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Y.; Wang, L.; Wang, Z.; Zhu, P.; Hu, Z.; Gu, X.; He, R.; Xu, J.; Jing, B.; et al. Comprehensive Molecular Characterization of the Mitochondrial Genome of the Takin Lungworm Varestrongylus eleguneniensis (Strongylida: Protostrongylidae). Int. J. Mol. Sci. 2022, 23, 13597. [Google Scholar] [CrossRef] [PubMed]
- Verocai, G.G.; Kutz, S.J.; Simard, M.; Hoberg, E.P. Varestrongylus eleguneniensis sp. n. (Nematoda: Protostrongylidae): A Widespread, Multi-Host Lungworm of Wild North American Ungulates, with an Emended Diagnosis for the Genus and Explorations of Biogeography. Parasites Vectors 2014, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Zhang, Y.; Zhang, L.; Liu, Q.; Liu, H.X.; Hu, L.; Wei, F.R.; Steinmann, P.; Graeff-Teixeira, C.; Zhou, X.N.; et al. The Complete Mitochondrial Genome of the Rodent Intra-Arterial Nematodes Angiostrongylus cantonensis and Angiostrongylus costaricensis. Parasitol. Res. 2012, 111, 115–123. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Eamsobhana, P.; Lim, P.E. Complete Mitochondrial Genome of Angiostrongylus malaysiensis Lungworm and Molecular Phylogeny of Metastrongyloid Nematodes. Acta Trop. 2016, 161, 33–40. [Google Scholar] [CrossRef]
- Gasser, R.B.; Jabbar, A.; Mohandas, N.; Schnyder, M.; Deplazes, P.; Littlewood, D.T.J.; Jex, A.R. Mitochondrial Genome of Angiostrongylus vasorum: Comparison with Congeners and Implications for Studying the Population Genetics and Epidemiology of This Parasite. Infect. Genet. Evol. 2012, 12, 1884–1891. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Traub, R.J.; Mohandas, N.; Aland, K.V.; Reid, S.A.; McCarthy, J.S.; Jones, M.K. The Mitochondrial Genome of Angiostrongylus mackerrasae as a Basis for Molecular, Epidemiological and Population Genetic Studies. Parasites Vectors 2015, 8, 473. [Google Scholar] [CrossRef]
- Sultana, T.; Han, H.; Park, J.K. Comparison of complete mitochondrial genomes of pine wilt nematode Bursaphelenchus xylophilus and Bursaphelenchus mucronatus (Nematoda: Aphelenchoidea) and development of a molecular tool for species identification. Gene. 2013, 520, 39–46. [Google Scholar] [CrossRef]
- Jabbar, A.; Mohandas, N.; Gasser, R.B. Characterisation of the mitochondrial genome of Parafilaroides normani (lungworm) of Arctocephalus pusillus doriferus (Australian fur seal). Parasitol. Res. 2014, 113, 3049–3055. [Google Scholar] [CrossRef]
- Deng, Y.P.; Zhang, X.L.; Li, L.Y.; Yang, T.; Liu, G.H.; Fu, Y.T. Characterization of the Complete Mitochondrial Genome of the Swine Kidney Worm Stephanurus dentatus (Nematoda: Syngamidae) and Phylogenetic Implications. Vet Parasitol 2021, 295, 109475. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.V.D.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Chapin, E.A. New nematodes from Northern American mammals. J. Agric. Res. 1925, 30, 677–681. [Google Scholar]
- Cameron, T.W.M. On the morphology and parasitic development of Travassosius rufus Khalil, 1922, a trichostrongyle parasite of the Canadian beaver (Castor canadensis canadensis). Libro Jubil. L. Travassos Rio De Jan. 1938, 103–106. [Google Scholar]
- Smith, H.; Archibald, R. On the incidence of gastrointestinal parasites in Nova Scotia beaver. Can. J. Zool. 1967, 45, 659–661. [Google Scholar] [CrossRef]
- Bush, A.; Samuel, W.M. The Genus Travassosius Khalil, 1922 (Nematoda, Trichostrongyloidea) in Beaver, Castor spp.: A Review and Suggestion for Speciation. Can. J. Zool. 1978, 56, 7. [Google Scholar] [CrossRef]
- Saccone, C.; De Giorgi, C.; Gissi, C.; Pesole, G.; Reyes, A. Evolutionary Genomics in Metazoa: The Mitochondrial DNA as a Model System. Gene 1999, 238, 195–209. [Google Scholar] [CrossRef]
- Brown, T.A.; Cecconi, C.; Tkachuk, A.N.; Bustamante, C.; Clayton, D.A. Replication of Mitochondrial DNA Occurs by Strand Displacement with Alternative Light-Strand Origins, Not via a Strand-Coupled Mechanism. Genes. Dev. 2005, 19, 2466–2476. [Google Scholar] [CrossRef]
- Formaggioni, A.; Luchetti, A.; Plazzi, F. Mitochondrial Genomic Landscape: A Portrait of the Mitochondrial Genome 40 Years after the First Complete Sequence. Life 2021, 11, 663. [Google Scholar] [CrossRef]
- Hu, M.; Gasser, R.B. Mitochondrial Genomes of Parasitic Nematodes–Progress and Perspectives. Trends Parasitol. 2006, 22, 78–84. [Google Scholar] [CrossRef]
- Hu, M.; Chilton, N.B.; Gasser, R.B. The Mitochondrial Genomics of Parasitic Nematodes of Socio-Economic Importance: Recent Progress, and Implications for Population Genetics and Systematics. Adv. Parasitol. 2003, 56, 133–212. [Google Scholar]
- Lagisz, M.; Poulin, R.; Nakagawa, S. You Are Where You Live: Parasitic Nematode Mitochondrial Genome Size Is Associated with the Thermal Environment Generated by Hosts. J. Evol. Biol. 2013, 26, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Palevich, N.; Britton, C.; Kamenetzky, L.; Mitreva, M.; de Moraes Mourão, M.; Bennuru, S.; Quack, T.; Scholte, L.L.S.; Tyagi, R.; Slatko, B.E. Tackling Hypotheticals in Helminth Genomes. Trends Parasitol. 2018, 34, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Rand, D.M. The units of selection on mitochondrial DNA. Annu. Rev. Ecol. Syst. 2001, 32, 415–448. [Google Scholar] [CrossRef]
- Gendron, E.M.S.; Qing, X.; Sevigny, J.L.; Li, H.; Liu, Z.; Blaxter, M.; Powers, T.O.; Thomas, W.K.; Porazinska, D.L. Comparative Mitochondrial Genomics in Nematoda Reveal Astonishing Variation in Compositional Biases and Substitution Rates Indicative of Multi-Level Selection. BMC Genom. 2024, 25, 615. [Google Scholar] [CrossRef]
| Gene | Primer Name | Primer Sequence (5′→3′) | References |
|---|---|---|---|
| 18S | 18S-F | AAAGATTAAGCCATGCA | [16] |
| 18S-R | GCAGGTTCACCTACAGAT | ||
| ITS | ITS-F | GAGTCGATGAAGAACGCAG | [17] |
| ITS-R | GAATCCTGGTTAGTTTCTTTTCCT | ||
| COI | COI-F | TTTTTTGGGCATCCTGAGGTTTAT | [18] |
| COI-R | TAAAGAAAGAACATAATGAAAATG |
| Measurement Item | Travassosius rufus | |
|---|---|---|
| Female | Male | |
| Number (individuals) | 3 | 3 |
| Body length (mm) | 9.89–11.87 | 8.69–10.477 |
| Body width (mm) | 0.142–0.161 | 0.112–0184 |
| Esophagus length (mm) | 0.512–0.559 | 0.452–0.552 |
| Nerve ring to head (mm) | 0.260–0.282 | 0.272–0.340 |
| Excretory pore to head (mm) | 0.332–0.396 | 0.310–0.385 |
| Cervical papillae to head (mm) | 0.436–0.468 | 0.396–0.479 |
| Vulva to tail tip (mm) | 2.247–2.831 | —— |
| Size of eggs (μm) | 78.10–89.56 × 40.18–52.78 | —— |
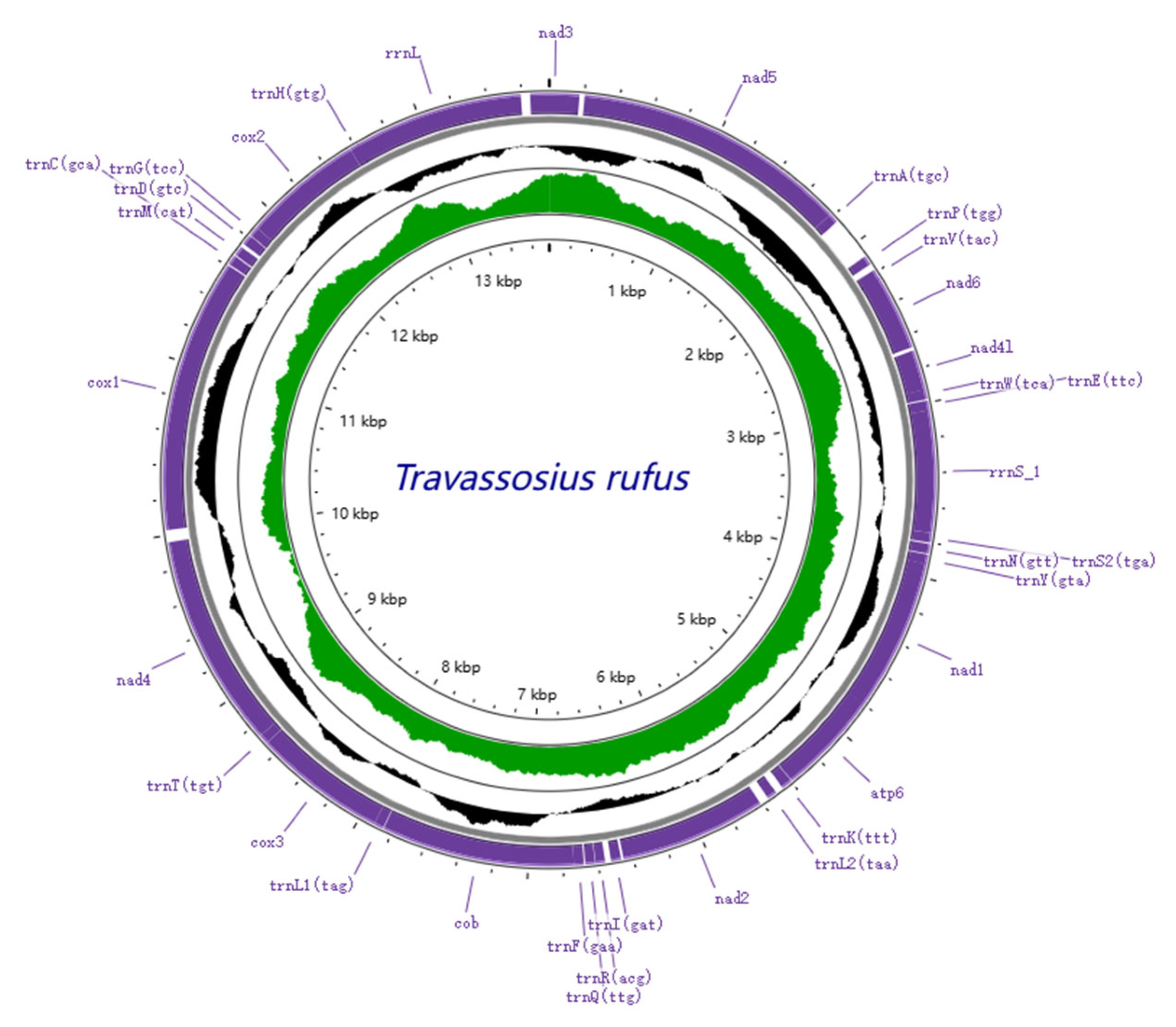
| Gene | Start | End | Length | Strand | Gap or Overlap | Start Codon/Stop Codon |
|---|---|---|---|---|---|---|
| cox1 | 1 | 1578 | 1578 | + | 8 | ATT/TAG |
| tRNA-Cys | 1585 | 1640 | 56 | + | 10 | |
| tRNA-Met | 1649 | 1706 | 58 | + | 23 | |
| tRNA-Asp | 1728 | 1785 | 58 | + | 6 | |
| tRNA-Gly | 1790 | 1845 | 56 | + | 2 | |
| cox2 | 1846 | 2541 | 696 | + | 1 | GTT/TAA |
| tRNA-His | 2541 | 2596 | 56 | + | 3 | |
| rrnL | 2598 | 3561 | 964 | + | 4 | |
| nad3 | 3564 | 3899 | 336 | + | 30 | ATG/TAG |
| nad5 | 3928 | 5509 | 1582 | + | 2 | ATT/T |
| tRNA-Ala | 5510 | 5565 | 56 | + | 255 | ATT/TTT |
| tRNA-Pro | 5819 | 5872 | 54 | + | 40 | |
| tRNA-Val | 5911 | 5966 | 56 | + | 1 | |
| nad6 | 5966 | 6400 | 435 | + | 20 | TTG/TAA |
| nad4L | 6419 | 6649 | 231 | + | 3 | ATT/TTT |
| tRNA-Trp | 6651 | 6707 | 57 | + | 9 | |
| tRNA-Glu | 6715 | 6770 | 56 | + | −2 | |
| rrnS | 6767 | 7471 | 705 | + | 2 | |
| tRNA-Ser2 | 7472 | 7524 | 53 | + | 12 | |
| tRNA-Asn | 7535 | 7590 | 56 | + | 8 | |
| tRNA-Tyr | 7597 | 7651 | 55 | + | 1 | |
| nad1 | 7651 | 8526 | 876 | + | −3 | TTG/TAA |
| atp6 | 8522 | 9121 | 600 | + | 5 | ATT/TAA |
| tRNA-Lys | 9125 | 9186 | 62 | + | 48 | |
| tRNA-Leu2 | 9233 | 9288 | 56 | + | 2 | |
| tRNA-Ser | 9289 | 9340 | 52 | + | 2 | |
| nad2 | 9341 | 10,183 | 843 | + | 15 | TTG/TAA |
| tRNA-Ile | 10,197 | 10,255 | 59 | + | 35 | |
| tRNA-Arg | 10,289 | 10,343 | 55 | + | 5 | |
| tRNA-Gln | 10,347 | 10,401 | 55 | + | 10 | |
| tRNA-Phe | 10,410 | 10,465 | 56 | + | 2 | |
| cytb | 10,466 | 11,578 | 1113 | + | 5 | ATA/TAA |
| tRNA-Leu1 | 11,582 | 11,636 | 55 | + | 2 | |
| cox3 | 11,637 | 12,404 | 768 | + | 0 | ATT/TGT |
| tRNA-Thr | 12,403 | 12,457 | 55 | + | 2 | |
| nad4 | 12,458 | 13,687 | 1230 | + | GTG/TAA |
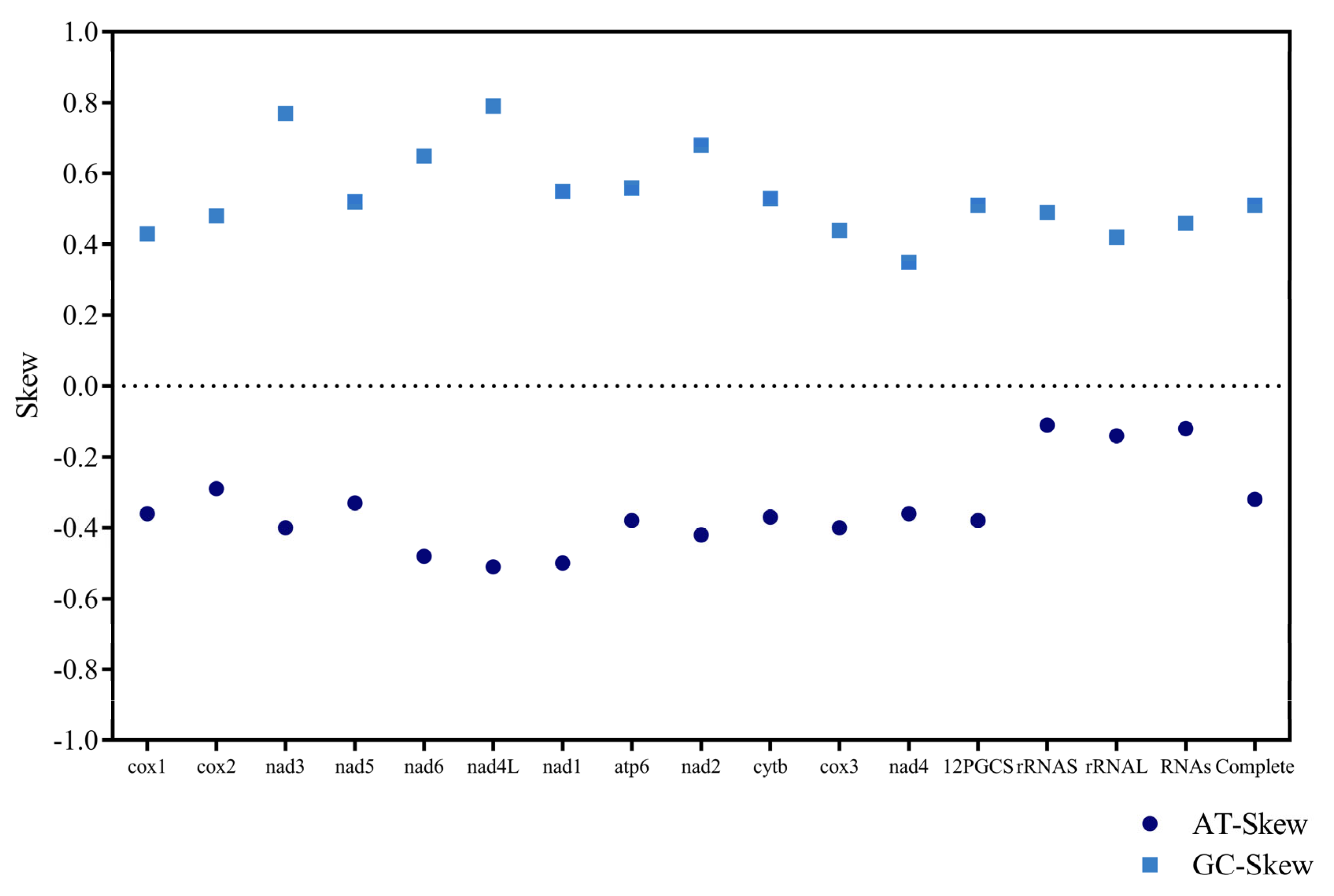
| Regions | Size(bp) | T(U) | C | A | G | AT(%) | GC(%) | GT(%) | AT Skew | GC Skew |
|---|---|---|---|---|---|---|---|---|---|---|
| cox1 | 1576 | 45.9 | 9.3 | 21.6 | 23.1 | 67.5 | 32.3 | 69 | −0.36 | 0.43 |
| cox2 | 696 | 45.0 | 7.8 | 25.0 | 22.3 | 70.0 | 30.1 | 67.3 | −0.29 | 0.48 |
| nad3 | 336 | 51.8 | 3.0 | 22.0 | 23.2 | 73.8 | 26.2 | 75 | −0.40 | 0.77 |
| nad5 | 1561 | 51.2 | 5.4 | 26.0 | 17.3 | 77.2 | 22.7 | 68.5 | −0.33 | 0.52 |
| nad6 | 435 | 56.6 | 4.1 | 19.8 | 19.5 | 76.4 | 23.6 | 76.1 | −0.48 | 0.65 |
| nad4L | 231 | 57.1 | 2.6 | 18.6 | 21.6 | 75.7 | 24.2 | 78.7 | −0.51 | 0.79 |
| nad1 | 875 | 51.9 | 6.9 | 17.4 | 23.9 | 69.3 | 30.8 | 75.8 | −0.50 | 0.55 |
| atp6 | 599 | 50.3 | 6.0 | 22.5 | 21.2 | 72.8 | 27.2 | 71.5 | −0.38 | 0.56 |
| nad2 | 841 | 53.5 | 4.0 | 21.6 | 20.8 | 75.1 | 24.8 | 74.3 | −0.42 | 0.68 |
| cytb | 1110 | 47.8 | 7.2 | 21.8 | 23.2 | 69.6 | 30.4 | 71 | −0.37 | 0.53 |
| cox3 | 768 | 50.9 | 7.7 | 21.6 | 19.8 | 72.5 | 27.5 | 70.7 | −0.40 | 0.44 |
| nad4 | 1228 | 52.0 | 7.6 | 24.7 | 15.7 | 76.7 | 23.3 | 67.7 | −0.36 | 0.35 |
| coding 1 | 3419 | 48.3 | 5.1 | 24.1 | 22.6 | 72.3 | 27.7 | 70.9 | −0.33 | 0.63 |
| coding 2 | 3419 | 47.7 | 8.7 | 24.1 | 19.4 | 71.9 | 28.1 | 67.2 | −0.33 | 0.38 |
| coding 3 | 3418 | 55.0 | 6.1 | 19.0 | 19.9 | 74.0 | 26.0 | 74.9 | −0.49 | 0.53 |
| PCGs | 10,256 | 50.3 | 6.6 | 22.5 | 20.6 | 72.8 | 27.2 | 70.9 | −0.38 | 0.51 |
| rrnL | 967 | 43.1 | 5.7 | 34.5 | 16.6 | 77.6 | 22.3 | 59.7 | −0.11 | 0.49 |
| rrnS | 703 | 42.5 | 7.3 | 32.3 | 17.9 | 74.8 | 25.2 | 60.4 | −0.14 | 0.42 |
| rRNAs | 1670 | 42.9 | 6.3 | 33.6 | 17.2 | 76.5 | 23.5 | 60.1 | −0.12 | 0.46 |
| tRNAs | 1232 | 42.6 | 5.9 | 34.8 | 16.6 | 77.4 | 22.5 | 59.2 | −0.10 | 0.48 |
| Complete mitochondrial genome | 13,720 | 48.6 | 6.4 | 25.3 | 19.7 | 73.9 | 26.1 | 68.3 | −0.32 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Chu, W.; Zhang, D.; Li, K.; Huang, W.; Li, X. Morphology, Molecular Characterization, and Phylogeny of Travassosius rufus Khalil, 1922 (Strongylidea: Trichostrongylidae), a Parasite from Endangered Sino-Mongolian Beaver (Castor fiber birulai) in Xinjiang, China. Animals 2025, 15, 1339. https://doi.org/10.3390/ani15091339
Jia H, Chu W, Zhang D, Li K, Huang W, Li X. Morphology, Molecular Characterization, and Phylogeny of Travassosius rufus Khalil, 1922 (Strongylidea: Trichostrongylidae), a Parasite from Endangered Sino-Mongolian Beaver (Castor fiber birulai) in Xinjiang, China. Animals. 2025; 15(9):1339. https://doi.org/10.3390/ani15091339
Chicago/Turabian StyleJia, Huiping, Wenwen Chu, Dong Zhang, Kai Li, Wenpu Huang, and Xiaoyun Li. 2025. "Morphology, Molecular Characterization, and Phylogeny of Travassosius rufus Khalil, 1922 (Strongylidea: Trichostrongylidae), a Parasite from Endangered Sino-Mongolian Beaver (Castor fiber birulai) in Xinjiang, China" Animals 15, no. 9: 1339. https://doi.org/10.3390/ani15091339
APA StyleJia, H., Chu, W., Zhang, D., Li, K., Huang, W., & Li, X. (2025). Morphology, Molecular Characterization, and Phylogeny of Travassosius rufus Khalil, 1922 (Strongylidea: Trichostrongylidae), a Parasite from Endangered Sino-Mongolian Beaver (Castor fiber birulai) in Xinjiang, China. Animals, 15(9), 1339. https://doi.org/10.3390/ani15091339





