Integrative Transcriptomic and Metabolomic Analysis of Muscle and Liver Reveals Key Molecular Pathways Influencing Growth Traits in Zhedong White Geese
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Transcriptome Sequencing
2.3. Metabolomic Analysis
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. Growth Performance and Carcass Yield
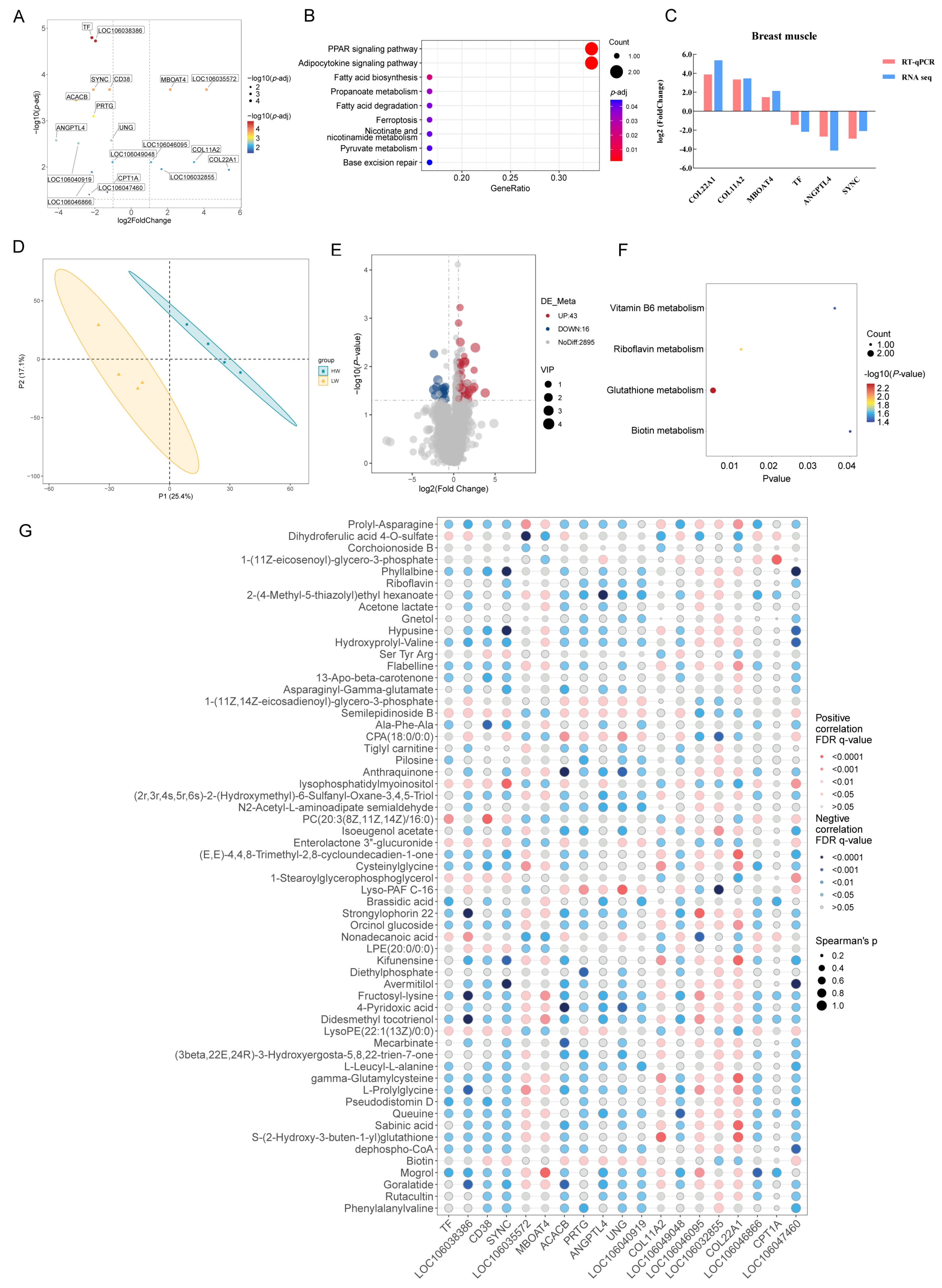
3.2. Transcriptomic and Metabolomic Profiles of Breast Muscle
3.3. Transcriptomic and Metabolomic Profiles of the Liver
3.4. The Interplay Between the Breast Muscle and Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, X.-W.; Wang, J.-W.; Zeng, F.-T.; Qiu, X.-P. Mitochondrial DNA Cleavage Patterns Distinguish Independent Origin of Chinese Domestic Geese and Western Domestic Geese. Biochem. Genet. 2006, 44, 237–245. [Google Scholar] [CrossRef]
- Heikkinen, M.E.; Ruokonen, M.; Alexander, M.; Aspi, J.; Pyhäjärvi, T.; Searle, J.B. Relationship between Wild Greylag and European Domestic Geese Based on Mitochondrial DNA. Anim. Genet. 2015, 46, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Haraf, G.; Wołoszyn, J.; Okruszek, A.; Goluch, Z.; Wereńska, M.; Teleszko, M. The Protein and Fat Quality of Thigh Muscles from Polish Goose Varieties. Poult. Sci. 2021, 100, 100992. [Google Scholar] [CrossRef]
- Krittanawong, C.; Narasimhan, B.; Wang, Z.; Virk, H.U.H.; Farrell, A.M.; Zhang, H.; Tang, W.H.W. Association Between Egg Consumption and Risk of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Am. J. Med. 2021, 134, 76–83.e2. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, S.; Fan, S.; Jin, Z.; Bao, Q.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of Growth Performance, Meat Quality, and Blood Biochemical Indexes of Yangzhou Goose under Different Feeding Patterns. Poult. Sci. 2024, 103, 103349. [Google Scholar] [CrossRef] [PubMed]
- Güller, I.; Russell, A.P. MicroRNAs in Skeletal Muscle: Their Role and Regulation in Development, Disease and Function. J. Physiol. 2010, 588, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Boz, M.A.; Oz, F.; Yamak, U.S.; Sarica, M.; Cilavdaroglu, E. The Carcass Traits, Carcass Nutrient Composition, Amino Acid, Fatty Acid, and Cholesterol Contents of Local Turkish Goose Varieties Reared in an Extensive Production System. Poult. Sci. 2019, 98, 3067–3080. [Google Scholar] [CrossRef]
- Shi, K.; Lu, Y.; Chen, X.; Li, D.; Du, W.; Yu, M. Effects of Ten-Eleven Translocation-2 (Tet2) on Myogenic Differentiation of Chicken Myoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 252, 110540. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, J.; He, X.; Zhang, X.; Luo, W. Whole-Transcriptome Sequencing Revealed the ceRNA Regulatory Network during the Proliferation and Differentiation of Goose Myoblast. Poult. Sci. 2024, 103, 104173. [Google Scholar] [CrossRef]
- Brockmöller, J.; Roots, I. Assessment of Liver Metabolic Function. Clinical Implications. Clin. Pharmacokinet. 1994, 27, 216–248. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, X.; Sun, D.; Han, G.; Wang, F.; Ye, M.; Wang, L.; Zou, H. Glycoproteomics Analysis of Human Liver Tissue by Combination of Multiple Enzyme Digestion and Hydrazide Chemistry. J. Proteome Res. 2009, 8, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, Y.; Javad, H.U.; Wang, R.; Zhou, Z.; Huang, Y.; Shu, X.; Li, C. Growth Performance of and Liver Function in Heat-Stressed Magang Geese Fed the Antioxidant Zinc Ascorbate and Its Potential Mechanism of Action. Biol. Trace Elem. Res. 2024, 203, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, Z.; Yang, Z.F.; He, X.X.; Zhang, C.Y.; Yang, H.M.; Rose, S.P.; Wang, Z.Y. Effects of Different Dietary Starch Sources on Growth and Glucose Metabolism of Geese. Poult. Sci. 2023, 102, 102362. [Google Scholar] [CrossRef] [PubMed]
- De Bandt, J.-P.; Jegatheesan, P.; Tennoune-El-Hafaia, N. Muscle Loss in Chronic Liver Diseases: The Example of Nonalcoholic Liver Disease. Nutrients 2018, 10, 1195. [Google Scholar] [CrossRef]
- Zhu, X.; Shao, B.; Guo, Y.; Gao, L.; Zhang, H.; Chen, W.; Wang, Y.; Gao, G.; Huang, Y. Incidence Rate of Angel Wing and Its Effect on Wing Bone Development and Serum Biochemical Parameters in Geese. Poult. Sci. 2021, 100, 101450. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a Unified Platform for Metabolomics Data Processing, Analysis and Interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Kong, J.; Yao, Z.; Chen, J.; Zhao, Q.; Li, T.; Dong, M.; Bai, Y.; Liu, Y.; Lin, Z.; Xie, Q.; et al. Comparative Transcriptome Analysis Unveils Regulatory Factors Influencing Fatty Liver Development in Lion-Head Geese under High-Intake Feeding Compared to Normal Feeding. Vet. Sci. 2024, 11, 366. [Google Scholar] [CrossRef]
- Charvet, B.; Guiraud, A.; Malbouyres, M.; Zwolanek, D.; Guillon, E.; Bretaud, S.; Monnot, C.; Schulze, J.; Bader, H.L.; Allard, B.; et al. Knockdown of Col22a1 Gene in Zebrafish Induces a Muscular Dystrophy by Disruption of the Myotendinous Junction. Development 2013, 140, 4602–4613. [Google Scholar] [CrossRef]
- Petrany, M.J.; Swoboda, C.O.; Sun, C.; Chetal, K.; Chen, X.; Weirauch, M.T.; Salomonis, N.; Millay, D.P. Single-Nucleus RNA-Seq Identifies Transcriptional Heterogeneity in Multinucleated Skeletal Myofibers. Nat. Commun. 2020, 11, 6374. [Google Scholar] [CrossRef]
- Kim, M.; Franke, V.; Brandt, B.; Lowenstein, E.D.; Schöwel, V.; Spuler, S.; Akalin, A.; Birchmeier, C. Single-Nucleus Transcriptomics Reveals Functional Compartmentalization in Syncytial Skeletal Muscle Cells. Nat. Commun. 2020, 11, 6375. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, M.; Sun, H.; Chen, Q.; Yan, D.; Dong, X.; Pan, Y.; Lu, S. Genome-Wide Association Study of Growth Traits in a Four-Way Crossbred Pig Population. Genes 2022, 13, 1990. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Han, K.; Wu, Y.; Bai, H.; Ke, Q.; Pu, F.; Wang, Y.; Xu, P. Genome-Wide Association Study of Growth and Body-Shape-Related Traits in Large Yellow Croaker (Larimichthys crocea) Using ddRAD Sequencing. Mar. Biotechnol. 2019, 21, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J.T.; Bult, C.J.; Kadin, J.A.; Richardson, J.E.; Blake, J.A. The Mouse Genome Database (MGD): From Genes to Mice—A Community Resource for Mouse Biology. Nucleic Acids Res. 2005, 33, D471–D475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, J.; Lyu, Y.; Zhang, D.; Reddi, K.K.; Sun, F.; Yi, J.; Liu, C.; Li, H.; Yao, H.; Dai, J.; et al. Genomic Characteristics and Selection Signatures in Indigenous Chongming White Goat (Capra hircus). Front. Genet. 2020, 11, 901. [Google Scholar] [CrossRef]
- Mattijssen, F.; Alex, S.; Swarts, H.J.; Groen, A.K.; van Schothorst, E.M.; Kersten, S. Angptl4 Serves as an Endogenous Inhibitor of Intestinal Lipid Digestion. Mol. Metab. 2014, 3, 135–144. [Google Scholar] [CrossRef]
- Son, Y.; Lorenz, W.W.; Paton, C.M. Linoleic Acid-Induced ANGPTL4 Inhibits C2C12 Skeletal Muscle Differentiation by Suppressing Wnt/β-Catenin. J. Nutr. Biochem. 2023, 116, 109324. [Google Scholar] [CrossRef]
- McDaniel, A.H.; Li, X.; Tordoff, M.G.; Bachmanov, A.A.; Reed, D.R. A Locus on Mouse Chromosome 9 (Adip5) Affects the Relative Weight of the Gonadal but Not Retroperitoneal Adipose Depot. Mamm. Genome 2006, 17, 1078–1092. [Google Scholar] [CrossRef]
- Elbitar, S.; Renard, M.; Arnaud, P.; Hanna, N.; Jacob, M.-P.; Guo, D.-C.; Tsutsui, K.; Gross, M.-S.; Kessler, K.; Tosolini, L.; et al. Pathogenic Variants in THSD4, Encoding the ADAMTS-like 6 Protein, Predispose to Inherited Thoracic Aortic Aneurysm. Genet. Med. 2021, 23, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Seifi Moroudi, R.; Ansari Mahyari, S.; Vaez Torshizi, R.; Lanjanian, H.; Masoudi-Nejad, A. Identification of New Genes and Quantitative Trait Locis Associated with Growth Curve Parameters in F2 Chicken Population Using Genome-Wide Association Study. Anim. Genet. 2021, 52, 171–184. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Satoh, A.; Yabe, S.; Furusawa, M.; Tokushige, N.; Tezuka, H.; Mikami, M.; Iwata, W.; Shingyouchi, A.; Matsuzaka, T.; et al. Hepatic CREB3L3 Controls Whole-Body Energy Homeostasis and Improves Obesity and Diabetes. Endocrinology 2014, 155, 4706–4719. [Google Scholar] [CrossRef]
- Ruppert, P.M.M.; Park, J.-G.; Xu, X.; Hur, K.Y.; Lee, A.-H.; Kersten, S. Transcriptional Profiling of PPARα−/− and CREB3L3−/− Livers Reveals Disparate Regulation of Hepatoproliferative and Metabolic Functions of PPARα. BMC Genom. 2019, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Heni, M.; Wagner, R.; Ketterer, C.; Böhm, A.; Linder, K.; Machicao, F.; Machann, J.; Schick, F.; Hennige, A.M.; Stefan, N.; et al. Genetic Variation in NR1H4 Encoding the Bile Acid Receptor FXR Determines Fasting Glucose and Free Fatty Acid Levels in Humans. J. Clin. Endocrinol. Metab. 2013, 98, E1224–E1229. [Google Scholar] [CrossRef]
- Cogburn, L.A.; Trakooljul, N.; Chen, C.; Huang, H.; Wu, C.H.; Carré, W.; Wang, X.; White, H.B. Transcriptional Profiling of Liver during the Critical Embryo-to-Hatchling Transition Period in the Chicken (Gallus gallus). BMC Genom. 2018, 19, 695. [Google Scholar] [CrossRef]
- Li, H.; Yu, Q.; Li, T.; Shao, L.; Su, M.; Zhou, H.; Qu, J. Rumen Microbiome and Metabolome of Tibetan Sheep (Ovis Aries) Reflect Animal Age and Nutritional Requirement. Front. Vet. Sci. 2020, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Yuan, Z.H.; Li, F.D.; Yue, X.P. Integrating Transcriptome and Metabolome to Identify Key Genes Regulating Important Muscular Flavour Precursors in Sheep. Animal 2022, 16, 100679. [Google Scholar] [CrossRef]
- Rashan, E.H.; Bartlett, A.K.; Khana, D.B.; Zhang, J.; Jain, R.; Smith, A.J.; Baker, Z.N.; Cook, T.; Caldwell, A.; Chevalier, A.R.; et al. ACAD10 and ACAD11 Enable Mammalian 4-Hydroxy Acid Lipid Catabolism. bioRxiv 2024, 2024.01.09.574893. [Google Scholar] [CrossRef]
- Yew, M.J.; Heywood, S.E.; Ng, J.; West, O.M.; Pal, M.; Kueh, A.; Lancaster, G.I.; Myers, S.; Yang, C.; Liu, Y.; et al. ACAD10 Is Not Required for Metformin’s Metabolic Actions or for Maintenance of Whole-body Metabolism in C57BL/6J Mice. Diabetes Obes. Metab. 2024, 26, 1731–1745. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Hao, J.; Xie, H.; Shimizu, K.; Li, R.; Zhang, C. Natural Product Mogrol Attenuates Bleomycin-Induced Pulmonary Fibrosis Development through Promoting AMPK Activation. J. Funct. Foods 2021, 77, 104280. [Google Scholar] [CrossRef]
- Tanaka, C.; Harada, N.; Teraoka, Y.; Urushizaki, H.; Shinmori, Y.; Onishi, T.; Yotsumoto, Y.; Ito, Y.; Kitakaze, T.; Inui, T.; et al. Mogrol Stimulates G-Protein-Coupled Bile Acid Receptor 1 (GPBAR1/TGR5) and Insulin Secretion from Pancreatic β-Cells and Alleviates Hyperglycemia in Mice. Sci. Rep. 2024, 14, 3244. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Jin, S.; Ishii, G. Isolation and Structural Elucidation of 4-(Beta-D-Glucopyranosyldisulfanyl)Butyl Glucosinolate from Leaves of Rocket Salad (Eruca sativa L.) and Its Antioxidative Activity. Biosci. Biotechnol. Biochem. 2004, 68, 2444–2450. [Google Scholar] [CrossRef] [PubMed]


| Nutrition Composition | Geese Diets | |
|---|---|---|
| 0–28 Days | 29–70 Days | |
| Ingredients, % | ||
| Corn grain | 58.96 | 66.03 |
| Soybean meal | 26.67 | 15.81 |
| Bran | 2.30 | 2.10 |
| Alfalfa meal | 7.10 | 11.05 |
| Soybean oil | 1.85 | 2.00 |
| Limestone | 0.65 | 0.50 |
| CaHPO4 | 1.06 | 1.10 |
| L-lysine HCl | 0.11 | 0.21 |
| DL-methionine | 0.10 | 0.00 |
| NaCl | 0.20 | 0.20 |
| Premix 1 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Nutritional level, % | ||
| Metabolic energy, Mcal/kg | 2.90 | 3.00 |
| Crude protein | 19.00 | 15.00 |
| Calcium | 0.65 | 0.60 |
| Digestibility phosphorus | 0.30 | 0.30 |
| Lysine | 1.00 | 0.85 |
| Methionine + cysteine | 0.60 | 0.50 |
| Gene | Accession No. | Primer Sequence (5′–3′) |
|---|---|---|
| COL11A2 | XM_066986897.1 | catccagctgcccaagaaga |
| ctgcttgagggagttgaggg | ||
| COL22A1 | XM_066990776.1 | aggactgaggcacaaagagc |
| cactgtatcgcaccacacct | ||
| TF | XM_013186329.3 | ggaccccaaaaccaaatgcc |
| catccagacacagcagctca | ||
| SYNC | XM_048049583.2 | ggcgactacttccaggagtg |
| gcactccttcgtcaccttga | ||
| MBOAT4 | XM_013196938.3 | gttgcaaagctcctctaccg |
| tcaaggtagcacaggacagg | ||
| ANGPTL4 | XM_048077583.2 | cttcaggcagctacccttct |
| atggtggtggacttcagagg | ||
| THSD4 | XM_013177461.3 | gctgaattgccgtgccatag |
| ccagacacaaccttgcaagc | ||
| SOX6 | XM_066997629.1 | gctttccctgacatgcacaa |
| aggtacgttttggtcgaggt | ||
| FGFRL1 | XM_048048078.2 | aggttccgaatccttcagca |
| acctggctgttctttcctga | ||
| SLC25A30 | XM_066988573.1 | tggaatgatgcatgcactgg |
| ctcccgaaagaatgccacac | ||
| CNST | XM_066995523.1 | aaaagagacagctggggagc |
| tcgtcatcatcatcgggctg | ||
| CREB3L3 | XM_048077817.2 | ccagaaccaagagctgcaga |
| ggacctggagaaaactcgca | ||
| NR1H4 | XM_048061114.2 | ccatgttcctccgttcagct |
| agcgcgtattcttcctgtgt | ||
| UAP1 | XM_067001427.1 | atcgggttctgcttggagaa |
| cggtggtgaagaagtggttg | ||
| LOC106049048 | XM_048054800.2 | aggcttgccggtcatagttc |
| cgggttccagttttgcagtg | ||
| PCK1 | XM_013190722.3 | gcagccatgagatctgaagc |
| ttttctccatagccaggcca | ||
| GAPDH | XM_067004670.1 | gagggtagtgaaggctgctg |
| accatcaagtccaccacacg |
| Item | LW | HW |
|---|---|---|
| BW at D1 (g) | 132.25 ± 9.29 | 127.25 ± 1.71 |
| BW at D70 (g) | 3579.25 ± 269.53 b | 4328.00 ± 47.83 a |
| Average daily gain (g) | 49.24 ± 3.98 b | 60.01 ± 0.68 a |
| Percentage of half-eviscerated weight (%) | 75.18 ± 8.02 | 69.96 ± 2.35 |
| Percentage of eviscerated weight (%) | 88.63 ± 8.51 | 84.15 ± 2.26 |
| Head yield (%) | 4.39 ± 0.41 | 4.17 ± 0.16 |
| Brain yield (‰) | 2.45 ± 0.30 | 2.07 ± 0.14 |
| Breast muscle yield (%) | 7.48 ± 1.52 | 6.99 ± 0.95 |
| Heart yield (‰) | 6.90 ± 0.83 | 6.48 ± 0.21 |
| Liver yield (%) | 2.37 ± 0.66 | 3.15 ± 0.54 |
| Gallbladder yield (‰) | 1.0 ± 0.43 | 0.98 ± 0.34 |
| Gizzard yield (‰) | 5.25 ± 0.43 | 5.39 ± 0.41 |
| Glandular stomach yield (‰) | 5.04 ± 0.71 | 6.25 ± 2.40 |
| Pancreas yield (%) | 3.36 ± 0.14 | 4.01 ± 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Zhou, X.; Dai, J.; Gao, Y.; Gao, L.; Shen, Y.; Chen, S. Integrative Transcriptomic and Metabolomic Analysis of Muscle and Liver Reveals Key Molecular Pathways Influencing Growth Traits in Zhedong White Geese. Animals 2025, 15, 1341. https://doi.org/10.3390/ani15091341
Shi K, Zhou X, Dai J, Gao Y, Gao L, Shen Y, Chen S. Integrative Transcriptomic and Metabolomic Analysis of Muscle and Liver Reveals Key Molecular Pathways Influencing Growth Traits in Zhedong White Geese. Animals. 2025; 15(9):1341. https://doi.org/10.3390/ani15091341
Chicago/Turabian StyleShi, Kai, Xiao Zhou, Jiuli Dai, Yuefeng Gao, Linna Gao, Yangyang Shen, and Shufang Chen. 2025. "Integrative Transcriptomic and Metabolomic Analysis of Muscle and Liver Reveals Key Molecular Pathways Influencing Growth Traits in Zhedong White Geese" Animals 15, no. 9: 1341. https://doi.org/10.3390/ani15091341
APA StyleShi, K., Zhou, X., Dai, J., Gao, Y., Gao, L., Shen, Y., & Chen, S. (2025). Integrative Transcriptomic and Metabolomic Analysis of Muscle and Liver Reveals Key Molecular Pathways Influencing Growth Traits in Zhedong White Geese. Animals, 15(9), 1341. https://doi.org/10.3390/ani15091341





