Health Status of Skopelos Goats and Its Impact on Milk Yield Under Intensive and Extensive Farming Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms and Animals Involved
2.2. Data Recording
2.3. Statistical Analysis
3. Results
3.1. Morbidity Frequency Measures
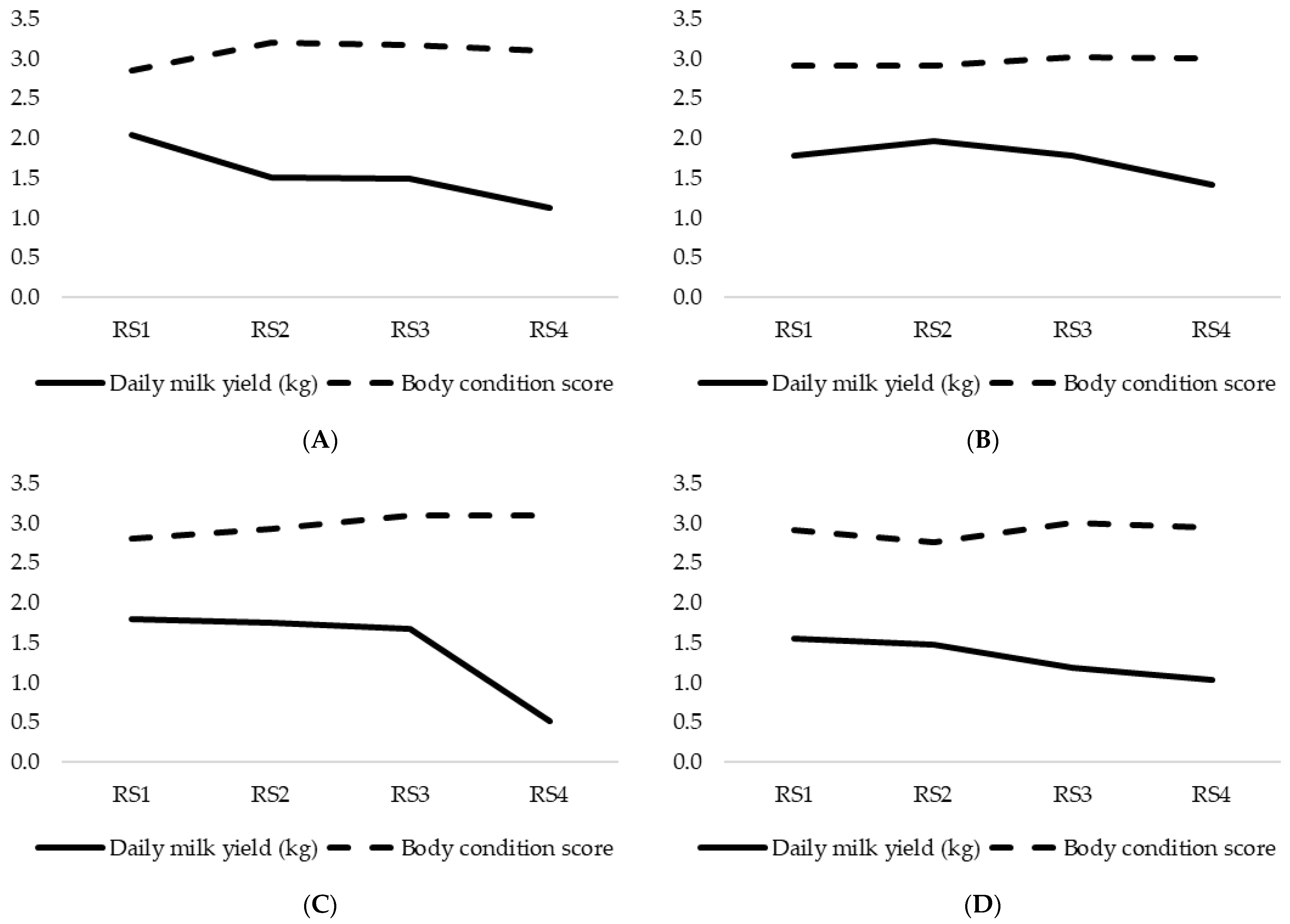
3.2. Milk Yield and Body Condition Score Variation Throughout Lactation Period
3.3. Mixed Linear Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AWIN | Animal welfare indicators |
| BCS | Body condition score |
| CI | Cumulative incidence |
| DMY | Daily milk yield |
| EFSA | European Food Safety Authority |
| EMM | Estimated marginal means |
| ICAR | Internation committee for animal recordings |
| IMI | Intramammary infection |
| NA | Not applicable |
| PDO | Protected designation of origin |
| RS | Recording session |
| RS1 | Recording session 1 |
| RS2 | Recording session 2 |
| RS3 | Recording session 3 |
| RS4 | Recording session 4 |
| SE | Standard error |
References
- ELSTAT Agricultural—Livestock Census. Available online: https://www.statistics.gr/el/agricultural-2021 (accessed on 25 February 2025).
- Eurostat Goats Population—Annual Data. Available online: https://ec.europa.eu/eurostat/databrowser/view/apro_mt_lsgoat$defaultview/default/table?lang=en (accessed on 19 February 2025).
- Gelasakis, A.I.; Rose, G.; Giannakou, R.; Valergakis, G.E.; Theodoridis, A.; Fortomaris, P.; Arsenos, G. Typology and Characteristics of Dairy Goat Production Systems in Greece. Livest. Sci. 2017, 197, 22–29. [Google Scholar] [CrossRef]
- Escareño, L.; Salinas-Gonzalez, H.; Wurzinger, M.; Iñiguez, L.; Sölkner, J.; Meza-Herrera, C. Dairy Goat Production Systems. Trop. Anim. Health Prod. 2012, 45, 17–34. [Google Scholar] [CrossRef]
- Gaspar, P.; Escribano, A.J.; Mesías, F.J.; Escribano, M.; Pulido, A.F. Goat Systems of Villuercas-Ibores Area in SW Spain: Problems and Perspectives of Traditional Farming Systems. Small Rumin. Res. 2011, 97, 1–11. [Google Scholar] [CrossRef]
- Lu, C.D.; Miller, B.A. Current Status, Challenges and Prospects for Dairy Goat Production in the Americas. Asian-Australas. J. Anim. Sci. 2019, 32, 1244–1255. [Google Scholar] [CrossRef]
- Anzuino, K.; Bell, N.J.; Bazeley, K.J.; Nicol, C.J. Assessment of Welfare on 24 Commercial UK Dairy Goat Farms Based on Direct Observations. Vet. Rec. 2010, 167, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Gelasakis, A.I.; Valergakis, G.E.; Arsenos, G. Health and Welfare of Indigenous Goat Breeds from Dairy Farms in Greece. In Sustainable Goat Production in Adverse Environments: Volume I; Simões, J., Gutiérrez, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 223–246. [Google Scholar]
- Anzuino, K.; Knowles, T.G.; Lee, M.R.F.; Grogono-Thomas, R. Survey of Husbandry and Health on UK Commercial Dairy Goat Farms. Vet. Rec. 2019, 185, 267. [Google Scholar] [CrossRef] [PubMed]
- Arsoy, D. Herd Management and Welfare Assessment of Dairy Goat Farms in Northern Cyprus by Using Breeding, Health, Reproduction, and Biosecurity Indicators. Trop. Anim. Health Prod. 2020, 52, 71–78. [Google Scholar] [CrossRef]
- Sevi, A.; Casamassima, D.; Pulina, G.; Pazzona, A. Factors of Welfare Reduction in Dairy Sheep and Goats. Ital. J. Anim. Sci. 2009, 8, 81–101. [Google Scholar] [CrossRef]
- Can, E.; Vieira, A.; Battini, M.; Mattiello, S.; Stilwell, G. On-Farm Welfare Assessment of Dairy Goat Farms Using Animal-Based Indicators: The Example of 30 Commercial Farms in Portugal. Acta Agric. Scand. A Anim. Sci. 2016, 66, 43–55. [Google Scholar] [CrossRef]
- Battini, M.; Stilwell, G.; Vieira, A.; Barbieri, S.; Canali, E.; Mattiello, S. On-FarmWelfare Assessment Protocol for Adult Dairy Goats in Intensive Production Systems. Animals 2015, 5, 934–950. [Google Scholar] [CrossRef]
- Muri, K.; Stubsjøen, S.M.; Valle, P.S. Development and Testing of an On-Farm Welfare Assessment Protocol for Dairy Goats. Anim. Welf. 2013, 22, 385–400. [Google Scholar] [CrossRef]
- Hempstead, M.N.; Lindquist, T.M.; Shearer, J.K.; Shearer, L.C.; Cave, V.M.; Plummer, P.J. Welfare Assessment of 30 Dairy Goat Farms in the Midwestern United States. Front. Vet. Sci. 2021, 8, 646715. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Statement on the Use of Animal-Based Measures to Assess the Welfare of Animals. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2767 (accessed on 26 February 2025).
- Marcone, G.; Carnovale, F.; Arney, D.; De Rosa, G.; Napolitano, F. A Simple Method for On-Farm Evaluation of Sheep Welfare Using Animal-Based Indicators. Small Rumin. Res. 2022, 208, 106636. [Google Scholar] [CrossRef]
- Battini, M.; Vieira, A.; Barbieri, S.; Ajuda, I.; Stilwell, G.; Mattiello, S. Invited Review: Animal-Based Indicators for on-Farm Welfare Assessment for Dairy Goats. J. Dairy Sci. 2014, 97, 6625–6648. [Google Scholar] [CrossRef]
- Cabiddu, A.; Branca, A.; Decandia, M.; Pes, A.; Santucci, P.M.; Masoero, F.; Calamari, L. Relationship between Body Condition Score, Metabolic Profile, Milk Yield and Milk Composition in Goats Browsing a Mediterranean Shrubland. Livest. Prod. Sci. 1999, 61, 267–273. [Google Scholar] [CrossRef]
- Gráff, M.; Mikó, E.; Zádori, B.; Csanádi, J. The Relationship between Body Condition and Milk Composition in Dairy Goats. Adv. Res. Life Sci. 2018, 2, 26–29. [Google Scholar] [CrossRef]
- Vacca, G.; Carcangiu, V.; Dettori, M.; Bini, P. Relationships between Body Condition Score, Milk Yield and Milk Composition of Sarda Goat. J. Anim. Feed. Sci. 2004, 13, 705–708. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Angelidis, A.S.; Giannakou, R.; Filioussis, G.; Kalamaki, M.S.; Arsenos, G. Bacterial Subclinical Mastitis and Its Effect on Milk Yield in Low-Input Dairy Goat Herds. J. Dairy Sci. 2016, 99, 3698–3708. [Google Scholar] [CrossRef]
- Koop, G.; van Werven, T.; Schuiling, H.J.; Nielen, M. The Effect of Subclinical Mastitis on Milk Yield in Dairy Goats. J. Dairy Sci. 2010, 93, 5809–5817. [Google Scholar] [CrossRef]
- Contreras, A.; Corrales, J.C.; Sierra, D.; Marco, J. Prevalence and Aetiology of Non-Clinical Intramammary Infection in Murciano-Granadina Goats. Small Rumin. Res. 1995, 17, 71–78. [Google Scholar] [CrossRef]
- Da Silva, J.B.; Fagundes, G.M.; Soares, J.P.G.; Fonseca, A.H. Dairy Goat Health Management and Milk Production on Organic and Conventional System in Brazil. Semin. Cienc. Agrar. 2013, 34, 1273–1280. [Google Scholar] [CrossRef]
- Delgado-Pertíñez, M.; Guzmán-Guerrero, J.L.; Mena, Y.; Castel, J.M.; González-Redondo, P.; Caravaca, F.P. Influence of Kid Rearing Systems on Milk Yield, Kid Growth and Cost of Florida Dairy Goats. Small Rumin. Res. 2009, 81, 105–111. [Google Scholar] [CrossRef]
- Sandrucci, A.; Bava, L.; Tamburini, A.; Gislon, G.; Zucali, M. Management Practices and Milk Quality in Dairy Goat Farms in Northern Italy. Ital. J. Anim. Sci. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Tiezzi, F.; Tomassone, L.; Mancin, G.; Cornale, P.; Tarantola, M. The Assessment of Housing Conditions, Management, Animal-Based Measure of Dairy Goats’ Welfare and Its Association with Productive and Reproductive Traits. Animals 2019, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.; Merin, U.; Silanikove, N. Changes in Milk Composition as Affected by Subclinical Mastitis in Goats. J. Dairy Sci. 2004, 87, 1719–1726. [Google Scholar] [CrossRef]
- Díaz, J.R.; Romero, G.; Muelas, R.; Alejandro, M.; Peris, C. Effect of Intramammary Infection on Milk Electrical Conductivity in Murciano-Granadina Goats. J. Dairy Sci. 2012, 95, 718–726. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Angelidis, A.; Giannakou, R.; Arsenos, G. Bacterial Subclinical Mastitis and Its Effect on Milk Quality Traits in Low-input Dairy Goat Herds. Vet. Rec. 2018, 183, 449. [Google Scholar] [CrossRef]
- AWIN. AWIN Welfare Assessment Protocol for Goats; 2015; Available online: https://air.unimi.it/handle/2434/269102 (accessed on 17 April 2025).
- International Committee for Animal Recording. Procedure 1 of Section 2 of ICAR—Guideliness—Computing 24-Hour Yield. Available online: https://www.icar.org/guidelines/ (accessed on 17 April 2025).
- Tillack, A.; Merle, R.; Müller, K.E.; Hoedemaker, M.; Jensen, K.C.; Bartel, A.; Oehm, A.W.; Klawitter, M.; Stock, A. The Relationship between Lameness Prevalence and Pasture Access in 659 Dairy Herds in Germany. PLoS ONE 2024, 19, e0305536. [Google Scholar] [CrossRef]
- Battini, M.; Renna, M.; Giammarino, M.; Battaglini, L.; Mattiello, S. Feasibility and Reliability of the AWIN Welfare Assessment Protocol for Dairy Goats in Semi-Extensive Farming Conditions. Front. Vet. Sci. 2021, 8, 731927. [Google Scholar] [CrossRef]
- Larrondo, C.; Leiva, J.; de la Cruz-Cruz, L. Dairy Goat Welfare in Semi-Intensive Production Systems and Drought Conditions. Anim. Welf. 2021, 30, 371–379. [Google Scholar] [CrossRef]
- Huxley, J.N. Impact of Lameness and Claw Lesions in Cows on Health and Production. Livest. Sci. 2013, 156, 64–70. [Google Scholar] [CrossRef]
- van Soest, F.J.S.; Mourits, M.C.M.; Blanco-Penedo, I.; Duval, J.; Fall, N.; Krieger, M.; Sjöstrom, K.; Hogeveen, H. Farm-Specific Failure Costs of Production Disorders in European Organic Dairy Herds. Prev. Vet. Med. 2019, 168, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Džermeikaitė, K.; Krištolaitytė, J.; Anskienė, L.; Šertvytytė, G.; Lembovičiūtė, G.; Arlauskaitė, S.; Girdauskaitė, A.; Rutkauskas, A.; Baumgartner, W.; Antanaitis, R. Effects of Lameness on Milk Yield, Milk Quality Indicators, and Rumination Behaviour in Dairy Cows. Agriculture 2025, 15, 286. [Google Scholar] [CrossRef]
- Alawneh, J.I.; Laven, R.A.; Stevenson, M.A. The Effect of Lameness on the Fertility of Dairy Cattle in a Seasonally Breeding Pasture-Based System. J. Dairy Sci. 2011, 94, 5487–5493. [Google Scholar] [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The Hidden Cost of Disease: II. Impact of the First Incidence of Lameness on Production and Economic Indicators of Primiparous Dairy Cows. J. Dairy Sci. 2021, 104, 7944–7955. [Google Scholar] [CrossRef]
- ELGO Μηνιαία Παραδοθείσα Ποσότητα Και Μέση Τιμή Νωπού ΠΡOΒΕΙOΥ & ΓΙΔΙΝOΥ Γάλακτος Hμερολογιακού Έτους 2024. Available online: https://www.elgo.gr/ (accessed on 27 February 2025).
- Jaques, N.; Turner, S.A.; Vallée, E.; Heuer, C.; Lopez-Villalobos, N. The Effect of Lameness on Milk Production of Dairy Goats. Animals 2023, 13, 1728. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Arsenos, G.; Valergakis, G.E.; Banos, G. Association of Lameness with Milk Yield and Lactation Curves in Chios Dairy Ewes. J. Dairy Res. 2015, 82, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N. Review on Mastitis and Its Economic Effect. Can. J. Sci. Res. 2017, 6, 13–22. [Google Scholar]
- Ruiz-Romero, R.A.; Ghavipanje, N.; Vargas-Bello-Pérez, E. The Role of Non-Aureus Staphylococcus in Small Ruminant Mastitis: A Systemic Review on Etiological Agents, Risk Factors, Virulence Determinants, and Novel Treatments. Small Rumin. Res. 2025, 245, 107475. [Google Scholar] [CrossRef]
- Arteche-Villasol, N.; Fernández, M.; Gutiérrez-Expósito, D.; Pérez, V. Pathology of the Mammary Gland in Sheep and Goats. J. Comp. Pathol. 2022, 193, 37–49. [Google Scholar] [CrossRef]
- Min, B.R.; Tomita, G.; Hart, S.P. Effect of Subclinical Intramammary Infection on Somatic Cell Counts and Chemical Composition of Goats’ Milk. J. Dairy Res. 2007, 74, 204–210. [Google Scholar] [CrossRef]
- Gosselin, V.B.; Lovstad, J.; Dufour, S.; Adkins, P.R.F.; Middleton, J.R. Use of MALDI-TOF to Characterize Staphylococcal Intramammary Infections in Dairy Goats. J. Dairy Sci. 2018, 101, 6262–6270. [Google Scholar] [CrossRef]
- Koop, G.; De Vliegher, S.; De Visscher, A.; Supré, K.; Haesebrouck, F.; Nielen, M.; van Werven, T. Differences between Coagulase-Negative Staphylococcus Species in Persistence and in Effect on Somatic Cell Count and Milk Yield in Dairy Goats. J. Dairy Sci. 2012, 95, 5075–5084. [Google Scholar] [CrossRef]
- Vouraki, S.; Gelasakis, A.I.; Fotiadou, V.; Banos, G.; Arsenos, G. Repeatability of Health and Welfare Traits and Correlation with Performance Traits in Dairy Goats Reared under Low-Input Farming Systems. Vet. Sci. 2022, 9, 289. [Google Scholar] [CrossRef]
- de Cremoux, R.; Legris, M.; Clément, V.; Bailly-Salins, A.; Minier, M. Study of the Relationship between the Presence of Milky Cysts, Udder Imbalances, Udder Morphological Traits and Somatic Cell Counts. Small Rumin. Res. 2024, 231, 107203. [Google Scholar] [CrossRef]
- Vikøren, T.; Lillehaug, A.; Åkerstedt, J.; Bretten, T.; Haugum, M.; Tryland, M. A Severe Outbreak of Contagious Ecthyma (Orf) in a Free-Ranging Musk Ox (Ovibos Moschatus) Population in Norway. Vet. Microbiol. 2008, 127, 10–20. [Google Scholar] [CrossRef] [PubMed]
- External Swellings. In Diseases of the Goat; Matthews, J., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 131–142. [Google Scholar]
- Lawan, Z.; Bala, J.A.; Bukar, A.M.; Balakrishnan, K.N.; Mangga, H.K.; Abdullah, F.F.J.; Noordin, M.M.; Mohd-Azmi, M.L. Contagious Ecthyma: How Serious Is the Disease Worldwide? Anim. Health Res. Rev. 2021, 22, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.; IJpelaar, J. Updated Estimates of the Costs Associated with Thirty Four Endemic Livestock Diseases in Great Britain: A Note. J. Agric. Econ. 2005, 56, 135–144. [Google Scholar] [CrossRef]
- Silva Salas, M.Á.; Mondragón-Ancelmo, J.; Jiménez Badillo, M.D.R.; Rodríguez Licea, G.; Napolitano, F. Assessing Dairy Goat Welfare in Intensive or Semi-Intensive Farming Conditions in Mexico. J. Dairy Sci. 2021, 104, 6175–6184. [Google Scholar] [CrossRef]
- Kim, Y.; Ku, J.Y.; Park, K.M.; Baek, J.; Choi, K.S.; Park, J. Comparison of Blood Profiles between Housed and Grazing Korean Indigenous Cattle (Hanwoo). Can. J. Vet. Res. 2024, 88, 33–37. [Google Scholar]
- Anaemia. In Diseases of the Goat, 4th ed.; Matthews, J., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 275–283. [Google Scholar]
- Cebra, C.; Cebra, M. Diseases of the Hematologic, Immunologic, and Lymphatic Systems (Multisystem Diseases). In Sheep and Goat Medicine, 2nd ed.; Pugh, D.G., Baird, A.N., Eds.; Elsevier Saunders: Maryland Heights, MO, USA, 2012; pp. 466–502. [Google Scholar]
- Lima, A.R.C.; Silveira, R.M.F.; Castro, M.S.M.; De Vecchi, L.B.; Fernandes, M.H.M.D.R.; de Resende, K.T. Relationship between Thermal Environment, Thermoregulatory Responses and Energy Metabolism in Goats: A Comprehensive Review. J. Therm. Biol. 2022, 109, 103324. [Google Scholar] [CrossRef] [PubMed]
- Leite, L.O.; Stamm, F.O.; Souza, R.A.; Camarinha Filho, J.A.; Garcia, R.C.M. On-Farm Welfare Assessment in Dairy Goats in the Brazilian Northeast. Arq. Bras. Med. Vet. Zootec. 2020, 72, 2308–2320. [Google Scholar] [CrossRef]
- Battini, M.; Peric, T.; Ajuda, I.; Vieira, A.; Grosso, L.; Barbieri, S.; Stilwell, G.; Prandi, A.; Comin, A.; Tubaro, F.; et al. Hair Coat Condition: A Valid and Reliable Indicator for on-Farm Welfare Assessment in Adult Dairy Goats. Small Rumin. Res. 2015, 123, 197–203. [Google Scholar] [CrossRef]
- Nenadović, K.; Ilić, T.; Jovanović, N.; Bugarski, D.; Vučinić, M. Welfare of Native Goat Breeds in Serbia—Emphasis on Parasitological Infections. Front. Vet. Sci. 2021, 8, 678880. [Google Scholar] [CrossRef]
- Smith, M.C.; Sherman, D.M. Respiratory System. In Goat Medicine; Smith, M.C., Sherman, D.M., Eds.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 339–376. [Google Scholar]
- Esmaeili, H.; Joghataei, S.M.; Khanjari, A.; Khiyabani, F.H.A. Comprehensive Survey of Caseous Lymphadenitis in Sheep and Goats Flocks of Iran: Clinical, Bacteriological, and Molecular Insights. Small Rumin. Res. 2025, 246, 107490. [Google Scholar] [CrossRef]
- Battini, M.; Barbieri, S.; Vieira, A.; Stilwell, G.; Mattiello, S. Results of Testing the Prototype of the AWIN Welfare Assessment Protocol for Dairy Goats in 30 Intensive Farms in Northern Italy. Ital. J. Anim. Sci. 2016, 15, 283–293. [Google Scholar] [CrossRef]
- Leite, L.O.; Stamm, F.D.O.; Maceno, M.A.C.; Camarinha Filho, J.A.; Garcia, R.D.C.M. On-Farm Welfare Assessment in Meat Goat Does Raised in Semi-Intensive and Extensive Systems in Semiarid Regions of Ceará, Northeast, Brazil. Ciênc. Rural 2020, 50, e20190745. [Google Scholar] [CrossRef]
- Mattiello, S.; Battini, M.; Mantova, E.; Noè, L.; Grosso, L.; Barbieri, S. Evidence of Poor Welfare in Goats with External Abscesses. A Preliminary Study. Large Anim. Rev. 2018, 24, 113–118. [Google Scholar]
- Ruiz, H.; Ferrer, L.M.; Ramos, J.J.; Baselga, C.; Alzuguren, O.; Tejedor, M.T.; de Miguel, R.; Lacasta, D. The Relevance of Caseous Lymphadenitis as a Cause of Culling in Adult Sheep. Animals 2020, 10, 1962. [Google Scholar] [CrossRef]
- Vieira, A.; Battini, M.; Can, E.; Mattiello, S.; Stilwell, G. Inter-Observer Reliability of Animal-Based Welfare Indicators Included in the Animal Welfare Indicators Welfare Assessment Protocol for Dairy Goats. Animal 2018, 12, 1942–1949. [Google Scholar] [CrossRef]
- Caroprese, M.; Casamassima, D.; Rassu, S.P.G.; Napolitano, F.; Sevi, A. Monitoring the On-Farm Welfare of Sheep and Goats. Ital. J. Anim. Sci. 2009, 8, 343–354. [Google Scholar] [CrossRef]
- Berckmans, D. General Introduction to Precision Livestock Farming. Anim. Front. 2017, 7, 6–11. [Google Scholar] [CrossRef]
- Morgan-Davies, C.; Tesnière, G.; Gautier, J.M.; Jørgensen, G.H.M.; González-García, E.; Patsios, S.I.; Sossidou, E.N.; Keady, T.W.J.; McClearn, B.; Kenyon, F.; et al. Review: Exploring the Use of Precision Livestock Farming for Small Ruminant Welfare Management. Animal 2024, 18, 101233. [Google Scholar] [CrossRef]
- Schillings, J.; Bennett, R.; Rose, D.C. Exploring the Potential of Precision Livestock Farming Technologies to Help Address Farm Animal Welfare. Front. Anim. Sci. 2021, 2, 639678. [Google Scholar] [CrossRef]
- Aquilani, C.; Confessore, A.; Bozzi, R.; Sirtori, F.; Pugliese, C. Review: Precision Livestock Farming Technologies in Pasture-Based Livestock Systems. Animal 2022, 16, 100429. [Google Scholar] [CrossRef]
- Vayssade, J.A.; Arquet, R.; Bonneau, M. Automatic Activity Tracking of Goats Using Drone Camera. Comput. Electron. Agric. 2019, 162, 767–772. [Google Scholar] [CrossRef]
- Giovanetti, V.; Decandia, M.; Molle, G.; Acciaro, M.; Mameli, M.; Cabiddu, A.; Cossu, R.; Serra, M.G.; Manca, C.; Rassu, S.P.G.; et al. Automatic Classification System for Grazing, Ruminating and Resting Behaviour of Dairy Sheep Using a Tri-Axial Accelerometer. Livest. Sci. 2017, 196, 42–48. [Google Scholar] [CrossRef]
- Temenos, A.; Voulodimos, A.; Korelidou, V.; Gelasakis, A.I.; Kalogeras, D.; Doulamis, A.; Doulamis, N. Goat-CNN: A Lightweight Convolutional Neural Network for Pose-Independent Body Condition Score Estimation in Goats. J. Agric. Food Res. 2024, 16, 101174. [Google Scholar] [CrossRef]
- Castro-Costa, A.; Salama, A.A.K.; Moll, X.; Aguiló, J.; Caja, G. Using Wireless Rumen Sensors for Evaluating the Effects of Diet and Ambient Temperature in Nonlactating Dairy Goats. J. Dairy Sci. 2015, 98, 4646–4658. [Google Scholar] [CrossRef]
- Bonilla-Manrique, O.; Mánguez-Martín, R.; Moreno-Oyervides, A.; Waclawek, J.P.; Moser, H.; Giraud, E.; Martín-Mateos, P.; Lendl, B. Design of a Mid-Infrared Device Using HPSDS for Multi-Gas Detection inside Barns. In Proceedings of the Optica Sensing Congress 2023 (AIS, FTS, HISE, Sensors, ES), Munich, Germany, 30 July–3 August 2023; Optica Publishing Group: Washington, DC, USA, 2023; p. STu2D.6. [Google Scholar]
- Korelidou, V.; Grbovic, Z.; Pavlovic, D.; Simovic, I.; Panic, M.; Temenos, A.; Gelasakis, A.I. Clinical Assessment of Dairy Goats’ Udder Health Using Infrared Thermography. Animals 2025, 15, 658. [Google Scholar] [CrossRef]
- Chiavaccini, L.; Gupta, A.; Anclade, N.; Chiavaccini, G.; De Gennaro, C.; Johnson, A.N.; Portela, D.A.; Romano, M.; Vettorato, E.; Luethy, D. Automated Acute Pain Prediction in Domestic Goats Using Deep Learning-Based Models on Video-Recordings. Sci. Rep. 2024, 14, 27104. [Google Scholar] [CrossRef] [PubMed]
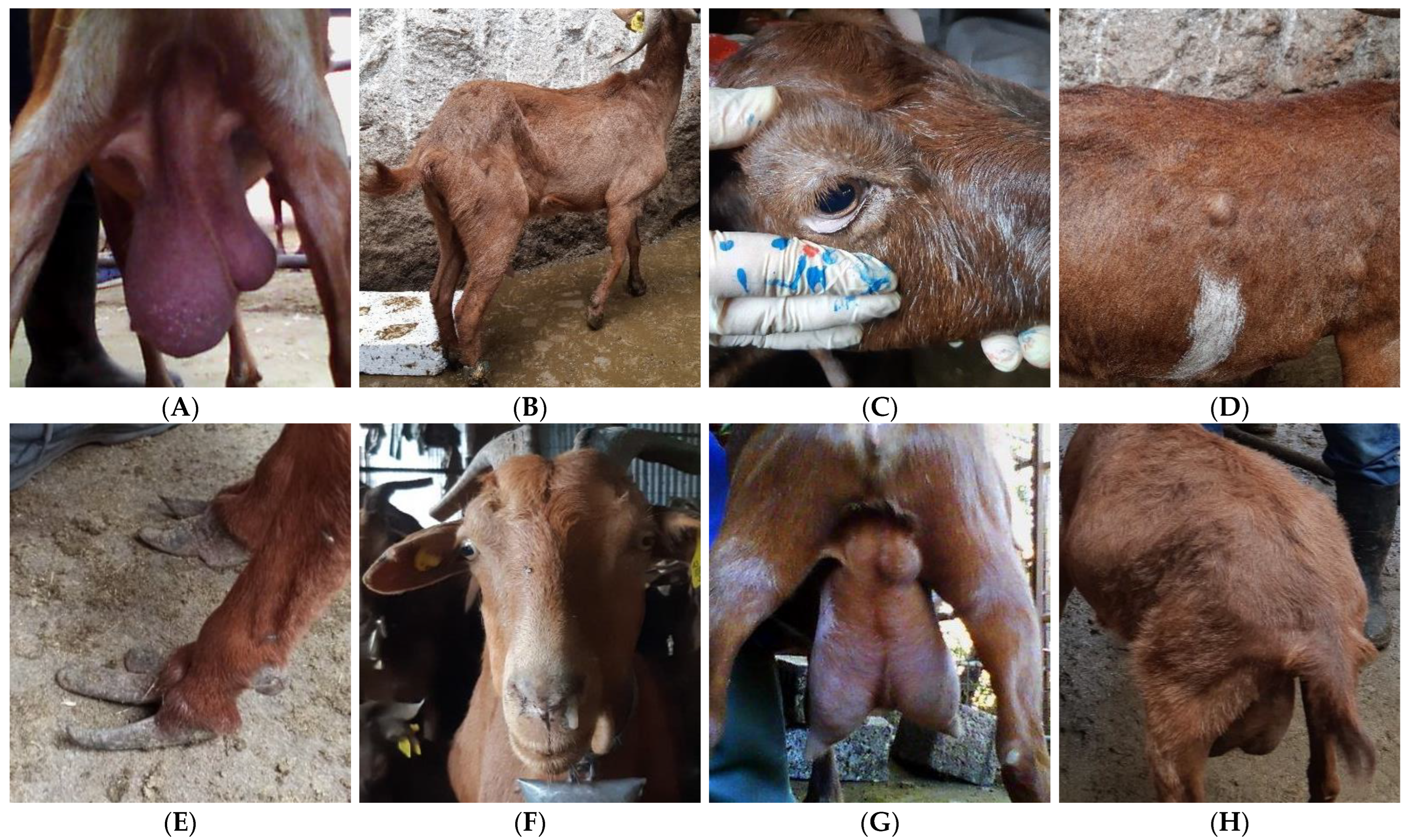
| Farm A (Intensive Farm) | Farm B (Extensive Farm) | |
|---|---|---|
| Production | ||
| Milk production/doe/lactation (210 days), kg | 450 | 350 |
| Total annual milk production, tn | 75 | 85 |
| Average milk fat content, % | 4.0 | 4.3 |
| Average milk protein content, % | 3.7 | 3.9 |
| Average somatic cell count (cells/mL) | 5 × 105–1 × 106 | 5 × 105–1 × 106 |
| Management | ||
| Duration of milking period, d | 200 | 150 |
| Duration of suckling period, d | 60 | 90 |
| Type of milking | Machine milking (Milkplan, Herringbone milking parlor 2 × 24 with silicone rubber cups) | Hand-milking |
| Milking machine parameters | Vacuum level: 40 kPa Pulsation rate: 90 pulses per minute Pulsation ratio: 50% Automatic removal of clusters | Not applicable |
| Cleaning routine of milking machine after every milking | Rinse with water → alkaline detergent (or acid detergent (twice a week) → water → drying | Not applicable |
| Number of milkings per day | 2 (07:00 and 19:00) | 2 (06:00 and 18:00) |
| Pre-milking preparation | No | No |
| Milk stripping | Yes | No |
| Post-dipping of teats | Yes (iodine-based) | No |
| Reproduction | ||
| Fecundity, n | 1.6 | 1.5 |
| Services per conception | ≤1.5 | ≤1.5 |
| Culling rate due to infertility, % | <5 | <5 |
| Average litter size at weaning | 1.4 | 1.3 |
| Artificial suckling | Yes | No |
| Infrastructures and housing | ||
| Type of building | Shed with openings | Shed with openings |
| Type of floor | Earthen | Earthen |
| Type of bedding | Straw | No bedding |
| Ventilation adequacy | Good | Medium |
| Lighting adequacy | Good | Medium |
| Type of waterers | Automatic | Barrels |
| Stocking density, m2/goat | 1.2 | - |
| Feeding and nutrition | ||
| Feedings per day | 2 | 2 |
| Grazing | No | Yes |
| Duration of grazing, h | Zero-grazing | 8–12 |
| Type of grazing land | Not applicable | Natural grasslands, shrublands/woodlands, cultivated pasturelands |
| Concentrates in milking goats (16% crude protein, kg/day) * | 1.0–1.2 | 0.5–1.0 |
| Concentrates in dry goats (14% crude protein, kg/day) | 0.5 | 0.5 |
| Roughages (hay) in milking goats (18% crude protein, kg/day) * | 0.8–1.9 | 0.0–0.5 |
| Roughages (hay) in dry goats (17% crude protein, kg/day) | 0.4 | 0.0–0.3 |
| Incidence of health and welfare issues | ||
| Clinical acidosis, % | ≤2 | ≤2 |
| Pregnancy toxemia, % | ≤2 | ≤2 |
| Metritis, % | ≤3 | ≤3 |
| Clinical mastitis, % | ≤5 | ≤5 |
| Subclinical mastitis, % | 10–20 | 10–20 |
| Retained placenta, % | ≤2 | ≤2 |
| Abortion, % | ≤3 | ≤3 |
| Lameness, % | ≤5 | ≤5 |
| Indicator | Description | Levels | |
|---|---|---|---|
| Head | Anemia | Pale mucus membrane | 3 |
| Mouth lesions | Papules and scabs around the mouth and lips | 2 | |
| Jaw swelling | Swelling and painful site during palpation at the premolar and molar teeth in the lower or upper jaw | 2 | |
| Ocular discharge | Discharges from the eye | 2 | |
| Nasal discharge | Discharges from the nasal cavity | 2 | |
| Limbs | Lameness | Impaired gait | 5 * |
| Arthritis | Swollen or inflamed limb joints | 2 | |
| Overgrown hooves | Excess horn tissue on claws | 3 | |
| Udder and teats | Cysts | Cysts on udder or teats | 2 |
| Skin lesions | Disrupted integrity of the skin | 2 | |
| Abscesses | Swollen, pus-filled cavities | 2 | |
| Signs of acute clinical intramammary infections | Painful, hot, hard, and swollen udder | 2 | |
| Fibrosis | Hard and fibrotic udder parenchyma at palpation | 2 | |
| Asymmetry | Uneven udder halves | 3 | |
| Swollen supra-mammary lymph nodes | Enlarged lymph nodes | 5 ** | |
| Other | Fecal soiling | Manure accumulation below the tail | 2 |
| Poor hair coat quality | Matted, rough, scurfy, uneven, shaggy hair coat | 2 | |
| Vaginitis | Inflammation of vagina | 2 | |
| Meteorism | Bloated rumen | 2 | |
| Body abscesses | Abscess at any part of the body, except for the udder | 2 | |
| Swollen body lymph nodes | Enlarged parotid, prescapular, prefemoral, or/and submandibular lymph nodes | 5 ** | |
| Body condition score | Nutritional status assessed by the palpation of the lumbar area and the estimation of the fat coverage | 5 *** | |
| Cough | Cough | 2 | |
| Abnormal respiration | Dyspnoea |
| Year 1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Point Prevalence (%) | Period Prevalence (%) | |||||||||
| RS1 | RS2 | RS3 | RS4 | |||||||
| Farm A n = 103 | Farm B n = 132 | Farm A n = 104 | Farm B n = 132 | Farm A n = 106 | Farm B n = 127 | Farm A n = 103 | Farm B n = 121 | Farm A n = 106 | Farm B n = 133 | |
| Lameness | 0.0 | 0.0 | 3.8 | 0.8 | 0.0 | 0.8 | 1.0 | 0.8 | 3.8 | 1.5 |
| Overgrown hooves | 3.0 | 0.0 | 21.2 | 2.3 | 14.2 | 1.6 | 1.0 | 0.0 | 29.2 | 3.8 |
| Anemia | NA | NA | 11.5 | 5.3 | 1.9 | 37.0 | 4.9 | 35.5 | 15.1 | 48.9 |
| Mouth scabs, papules | 0.0 | 0.0 | 22.1 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 22.6 | 0.0 |
| Jaw swelling | 1.0 | 0.0 | 2.9 | 0.0 | 5.7 | 0.8 | 4.9 | 1.7 | 9.4 | 2.3 |
| Nasal discharge | 0.0 | 0.0 | 0.0 | 0.0 | 4.7 | 0.8 | 0.0 | 13.2 | 4.7 | 12.8 |
| Teat cysts | 0.0 | 9.1 | 0.0 | 0.8 | 2.8 | 3.9 | 0.0 | 1.7 | 2.8 | 12.0 |
| Udder abscesses | 2.0 | 6.1 | 1.9 | 4.5 | 0.9 | 1.6 | 1.0 | 1.7 | 4.7 | 10.5 |
| Udder cysts | 0.0 | 0.8 | 1.9 | 3.0 | 0.0 | 4.7 | 0.0 | 0.0 | 1.9 | 8.3 |
| Udder fibrosis | 11.0 | 2.3 | 19.2 | 22.7 | 20.8 | 35.4 | 20.4 | 37.2 | 44.3 | 56.4 |
| Udder asymmetry | 16.0 | 10.6 | 45.2 | 43.2 | 51.9 | 36.2 | 19.4 | 47.9 | 74.5 | 75.2 |
| Swollen supra-mammary lymph nodes | 8.0 | 13.6 | 1.9 | 11.4 | 7.5 | 11.0 | 1.0 | 10.7 | 14.2 | 24.1 |
| Fecal soiling | 1.0 | 0.8 | 1.9 | 3.0 | 0.0 | 3.9 | 1.0 | 2.5 | 3.8 | 8.3 |
| Poor hair coat quality | 31.0 | 47.0 | 8.7 | 34.8 | 17.0 | 31.5 | 24.3 | 16.5 | 48.1 | 60.9 |
| Body abscesses | 8.0 | 23.5 | 35.6 | 22.7 | 35.8 | 22.0 | 33.0 | 14.9 | 50.0 | 45.1 |
| Swollen body lymph nodes (at least one) | NA | NA | 15.4 | 29.5 | 23.6 | 33.1 | 18.4 | 33.9 | 36.8 | 45.9 |
| Year 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Point Prevalence (%) | Period Prevalence (%) | |||||||||
| RS1 | RS2 | RS3 | RS4 | |||||||
| Farm A n = 118 | Farm B n = 97 | Farm A n = 126 | Farm B n = 108 | Farm A n = 128 | Farm B n = 99 | Farm A n = 125 | Farm B n = 98 | Farm A n = 145 | Farm B n = 114 | |
| Lameness | 2.5 | 0.0 | 2.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 |
| Overgrown hooves | 31.4 | 0.0 | 20.6 | 1.9 | 14.1 | 3.0 | 16.8 | 1.0 | 42.1 | 4.4 |
| Anemia | 7.6 | 44.3 | 6.3 | 50.0 | 8.6 | 46.5 | 1.6 | 33.7 | 16.6 | 71.9 |
| Jaw swelling | 2.5 | 0.0 | 1.6 | 0.9 | 3.1 | 0.0 | 3.2 | 0.0 | 5.5 | 0.9 |
| Nasal discharge | 0.0 | 26.8 | 0.8 | 9.3 | 5.5 | 5.1 | 24.8 | 29.6 | 25.5 | 43.9 |
| Teat cysts | 0.0 | 6.2 | 0.0 | 4.6 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 7.9 |
| Udder abscesses | 2.5 | 9.3 | 2.4 | 0.0 | 0.8 | 3.0 | 0.8 | 0.0 | 2.8 | 9.6 |
| Udder fibrosis | 31.4 | 13.4 | 22.2 | 43.5 | 16.4 | 39.4 | 24.0 | 35.7 | 48.3 | 68.4 |
| Udder asymmetry | 25.4 | 11.3 | 26.2 | 26.9 | 24.2 | 29.3 | 29.6 | 20.4 | 44.1 | 45.6 |
| Swollen supra-mammary lymph nodes | 33.9 | 27.8 | 27.0 | 38.0 | 22.7 | 24.2 | 9.6 | 17.3 | 45.5 | 51.8 |
| Fecal soiling | 1.7 | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 | 5.6 | 1.0 | 6.9 | 0.9 |
| Poor hair coat quality | 18.6 | 42.3 | 17.5 | 31.5 | 3.9 | 19.2 | 10.4 | 7.1 | 30.3 | 51.8 |
| Body abscesses | 28.0 | 16.5 | 23.0 | 19.4 | 32.0 | 27.3 | 28.8 | 25.5 | 44.1 | 38.6 |
| Swollen body lymph nodes (at least one) | NA | NA | 28.6 | 47.2 | 24.2 | 54.5 | 20.0 | 43.9 | 40.0 | 64.9 |
| Farm A (Intensive Farm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| New Cases (n) | Cumulative Incidence (%) | |||||||
| Year 1 | Year 2 | Year 1 | Year 2 | |||||
| RS2 | RS3 | RS4 | RS2 | RS3 | RS4 | |||
| Lameness | 3 | 0 | 1 | 0 | 0 | 0 | 4.0 (4/100) | 0.0 (0/101) |
| Overgrown hooves | 19 | 7 | 0 | 9 | 4 | 9 | 26.8 (26/97) | 31.0 (22/71) |
| Anemia | NA | 1 | 5 | 6 | 10 | 2 | 6.0 (6/100) | 18.9 (18/95) |
| Mouth scabs, papules | 21 | 1 | 0 | 0 | 0 | 0 | 22.0 (22/100) | 0.0 (0/103) |
| Jaw swelling | 3 | 4 | 3 | 1 | 2 | 1 | 10.0 (10/100) | 3.9 (4/102) |
| Nasal discharge | 0 | 5 | 0 | 1 | 4 | 27 | 5.0 (5/100) | 31.1 (32/103) |
| Udder fibrosis | 13 | 12 | 15 | 13 | 7 | 18 | 44.9 (40/89) | 55.1 (38/69) |
| Udder asymmetry | 34 | 25 | 8 | 12 | 11 | 15 | 79.8 (67/84) | 50.0 (38/76) |
| Swollen supra-mammary lymph nodes | 1 | 8 | 0 | 17 | 10 | 2 | 9.8 (9/92) | 42.0 (29/69) |
| Fecal soiling | 1 | 0 | 1 | 0 | 0 | 6 | 2.0 (2/99) | 5.9 (6/102) |
| Poor hair coat quality | 3 | 13 | 13 | 13 | 3 | 9 | 41.4 (29/70) | 29.4 (25/85) |
| Body abscesses | 30 | 9 | 8 | 8 | 16 | 11 | 51.1 (47/92) | 48.6 (35/72) |
| Swollen body lymph nodes (at least one) | NA | 13 | 9 | NA | 8 | 10 | 25.9 (22/85) | 24.7 (18/73) |
| Farm B (Extensive Farm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| New Cases (n) | Cumulative Incidence (%) | |||||||
| Year 1 | Year 2 | Year 1 | Year 2 | |||||
| RS2 | RS3 | RS4 | RS2 | RS3 | RS4 | |||
| Lameness | 1 | 1 | 0 | 0 | 0 | 0 | 1.7 (2/115) | 0.0 (0/81) |
| Overgrown hooves | 3 | 2 | 0 | 2 | 2 | 0 | 4.3 (5/115) | 4.9 (4/81) |
| Anemia | NA | 40 | 15 | 18 | 8 | 5 | 50.5 (55/109) | 67.4 (31/46) |
| Jaw swelling | 0 | 1 | 2 | 1 | 0 | 0 | 2.6 (3/115) | 1.2 (1/81) |
| Nasal discharge | 0 | 1 | 16 | 3 | 4 | 20 | 14.8 (17/115) | 47.4 (27/57) |
| Teat cysts | 1 | 4 | 1 | 2 | 1 | 0 | 5.8 (6/104) | 3.9 (3/76) |
| Udder abscesses | 3 | 1 | 2 | 0 | 3 | 0 | 5.6 (6/108) | 4.1 (3/73) |
| Udder cysts | 4 | 6 | 0 | 0 | 0 | 0 | 8.8 (10/114) | 0.0 (0/80) |
| Udder fibrosis | 26 | 28 | 22 | 29 | 13 | 13 | 67.9 (76/112) | 76.4 (55/72) |
| Udder asymmetry | 46 | 17 | 31 | 18 | 12 | 7 | 91.3 (94/103) | 50.7 (37/73) |
| Swollen supra-mammary lymph nodes | 3 | 5 | 6 | 18 | 7 | 9 | 14.3 (14/98) | 54.8 (34/62) |
| Fecal soiling | 4 | 4 | 3 | 0 | 0 | 1 | 9.6 (11/114) | 1.2 (1/81) |
| Poor hair coat quality | 9 | 11 | 6 | 5 | 3 | 1 | 44.1 (26/59) | 18.4 (9/49) |
| Body abscesses | 17 | 12 | 9 | 6 | 9 | 9 | 43.7 (38/87) | 35.8 (24/67) |
| Swollen body lymph nodes (at least one) | NA | 14 | 11 | NA | 16 | 7 | 32.1 (25/78) | 51.1 (23/45) |
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| EMM | B-Coefficient † | SE | Significance | Lower | Upper | |
| Lameness | 1.35 | −0.25 | 0.123 | 0.040 | −0.50 | −0.01 |
| Arthritis | 1.34 | −0.13 | 0.220 | 0.562 | −0.56 | 0.30 |
| Overgrown hooves | 1.35 | −0.09 | 0.046 | 0.059 | −0.18 | 0.00 |
| Anemia | 1.33 | −0.03 | 0.031 | 0.347 | −0.09 | 0.03 |
| Mouth scabs, papules | 1.35 | −0.19 | 0.081 | 0.020 | −0.35 | −0.03 |
| Jaw swelling | 1.34 | −0.13 | 0.084 | 0.115 | −0.03 | 0.30 |
| Ocular discharge | 1.34 | 0.16 | 0.163 | 0.340 | −0.16 | 0.48 |
| Cough | 1.35 | −0.35 | 0.228 | 0.127 | −0.79 | 0.10 |
| Nasal discharge | 1.34 | 0.02 | 0.043 | 0.690 | −0.07 | 0.10 |
| Teat cysts | 1.35 | 0.05 | 0.084 | 0.573 | −0.12 | 0.21 |
| Teat fibrosis | 1.35 | 0.18 | 0.465 | 0.696 | −0.73 | 1.09 |
| Signs of acute clinical intramammary infection | 1.35 | - | - | - | - | - |
| Udder lesions | 1.35 | 0.05 | 0.164 | 0.779 | −0.28 | 0.37 |
| Udder abscesses | 1.34 | 0.09 | 0.074 | 0.230 | −0.06 | 0.23 |
| Udder cysts | 1.34 | 0.18 | 0.111 | 0.107 | −0.04 | 0.40 |
| Udder fibrosis | 1.38 | −0.13 | 0.025 | <0.001 | −0.18 | −0.08 |
| Udder asymmetry | 1.38 | −0.09 | 0.024 | <0.001 | −0.13 | −0.04 |
| Swollen supra-mammary lymph nodes | 1.35 | 0.00 | 0.033 | 0.959 | −0.06 | 0.07 |
| Fecal soiling | 1.35 | 0.00 | 0.086 | 0.999 | −0.17 | 0.17 |
| Poor hair coat quality | 1.34 | 0.03 | 0.029 | 0.286 | −0.03 | 0.09 |
| Vaginitis | 1.35 | −0.03 | 0.304 | 0.918 | −0.63 | 0.57 |
| Meteorism | 1.35 | −0.08 | 0.132 | 0.530 | −0.34 | 0.18 |
| Body abscesses | 1.35 | −0.04 | 0.028 | 0.145 | −0.10 | 0.01 |
| Swollen body lymph nodes (at least one) | 1.29 | −0.04 | 0.032 | 0.247 | −0.10 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korelidou, V.; Kalogianni, A.I.; Arsenos, G.; Gelasakis, A.I. Health Status of Skopelos Goats and Its Impact on Milk Yield Under Intensive and Extensive Farming Systems. Animals 2025, 15, 1328. https://doi.org/10.3390/ani15091328
Korelidou V, Kalogianni AI, Arsenos G, Gelasakis AI. Health Status of Skopelos Goats and Its Impact on Milk Yield Under Intensive and Extensive Farming Systems. Animals. 2025; 15(9):1328. https://doi.org/10.3390/ani15091328
Chicago/Turabian StyleKorelidou, Vera, Aphrodite I. Kalogianni, Georgios Arsenos, and Athanasios I. Gelasakis. 2025. "Health Status of Skopelos Goats and Its Impact on Milk Yield Under Intensive and Extensive Farming Systems" Animals 15, no. 9: 1328. https://doi.org/10.3390/ani15091328
APA StyleKorelidou, V., Kalogianni, A. I., Arsenos, G., & Gelasakis, A. I. (2025). Health Status of Skopelos Goats and Its Impact on Milk Yield Under Intensive and Extensive Farming Systems. Animals, 15(9), 1328. https://doi.org/10.3390/ani15091328







