Use of Ultrasonography for the Evaluation of Lung Lesions in Lambs with Respiratory Complex
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Facilities and Management
2.2. Diagnostic Methods Employed, Corresponding Animal Procedures and Scores
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacasta, D.; Ferrer, L.M.; Ramos, J.J.; González, J.M.; De las Heras, M. Influence of Climatic Factors on the Development of Pneumonia in Lambs. Small Rumin. Res. 2008, 80, 28–32. [Google Scholar] [CrossRef]
- Hervás, J.; Méndez, A.; Gómez-Villamandos, J.C.; Villalva, E.; Díaz, E.; Cano, T.; Carrasco, L.; Padró, J.M.; Fernández, A.; Sierra, M.A. Etiological and Lesion Study of Respiratory Diseases in Intensively Reared Lambs in Southern Spain. J. Vet. Med. B 1996, 43, 321–330. [Google Scholar] [CrossRef]
- Fernández Delgado, S. Bacterial Characterization of the Ovine Respiratory Complex in Feedlot Lambs in Extremadura: Risk Factors and Measures for Its Prevention and Productivity Improvement. Ph.D. Thesis, University of Extremadura, Badajoz, Spain, 2018. [Google Scholar]
- Navarro, T.; González, J.M.; Ramos, J.J.; Marca, M.C.; Figliola, L.; Ruiz de Arcaute, M.; Borobia, M.; Ortín, A. Impact of Stress on Health and Final Weight in Fattening Lambs. Animals 2020, 10, 1274. [Google Scholar] [CrossRef]
- Navarro, T.; Ramos, J.J.; Figueras, L.; González, J.M. Epidemiology of Ovine Respiratory Complex in Lambs. Small Rumin. Res. 2019, 179, 70–74. [Google Scholar] [CrossRef]
- Love, W.J.; Lehenbauer, T.W.; Kass, P.H.; Van Eenennaam, A.L.; Aly, S.S. Development of a Novel Clinical Scoring System for On-Farm Diagnosis of Bovine Respiratory Disease in Pre-Weaned Dairy Calves. PeerJ 2014, 2, e238. [Google Scholar] [CrossRef]
- Berman, J. Literature Review of the Principal Diagnostic Tests to Detect Bovine Respiratory Disease in Pre-Weaned Dairy and Veal Calves. Animals 2024, 14, 329. [Google Scholar] [CrossRef]
- Hoffelner, J.; Peinhopf-Petz, W.; Wittek, T. Diagnostic and Prognostic Value of Clinical Scoring and Lung Ultrasonography to Assess Pulmonary Lesions in Veal Calves. Animals 2023, 13, 3464. [Google Scholar] [CrossRef]
- Maier, G.U.; Rowe, J.D.; Lehenbauer, T.W.; Karle, B.M.; Williams, D.R.; Champagne, J.D.; Aly, S.S. Development of a clinical scoring system for bovine respiratory disease in weaned dairy calves. J. Dairy Sci. 2019, 102, 7329–7344. [Google Scholar] [CrossRef]
- Berman, J.; Francoz, D.; Abdallah, A.; Dufour, S.; Buczinski, S. Development and Validation of a Clinical Respiratory Disease Scoring System for Guiding Treatment Decisions in Veal Calves Using a Bayesian Framework. J. Dairy Sci. 2022, 105, 9917–9933. [Google Scholar] [CrossRef]
- Buczinski, S.; Faure, C.; Jolivet, S.; Abdallah, A. Evaluation of Inter-Observer Agreement When Using a Clinical Respiratory Scoring System in Pre-Weaned Dairy Calves. N. Z. Vet. J. 2016, 64, 243–247. [Google Scholar] [CrossRef]
- Donlon, J.D.; McAloon, C.G.; Mee, J.F. Performance of Various Interpretations of Clinical Scoring Systems for Diagnosis of Respiratory Disease in Dairy Calves in a Temperate Climate Using Bayesian Latent Class Analysis. J. Dairy Sci. 2024, 107, 7138–7152. [Google Scholar] [CrossRef]
- Ruffin, D.C. Mycoplasma Infections in Small Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 315–332. [Google Scholar] [CrossRef]
- Bell, S. Respiratory Disease in Sheep: Differential Diagnosis and Epidemiology. Practice 2008, 30, 200–207. [Google Scholar] [CrossRef]
- González, J.M.; Lacasta, D.; Ferrer, L.M.; Figueras, L.; Abadie, G.; De las Heras, M. Mannheimia Haemolytica and Bibersteinia Trehalosi Serotypes Isolated from Lambs with Ovine Respiratory Complex in Spain. J. Hell. Vet. Med. Soc. 2013, 64, 177–182. [Google Scholar] [CrossRef][Green Version]
- Nicholas, R.; Ayling, R.; McAuliffe, L. Respiratory Diseases of Small Ruminants. In Mycoplasma Diseases of Ruminants; Nicholas, R., Ayling, R., McAuliffe, L., Eds.; CABI: Wallingford, UK, 2008; pp. 171–179. [Google Scholar]
- Scott, P.R. Lung Auscultation Recordings from Normal Sheep and from Sheep with Well-Defined Respiratory Tract Pathology. Small Rumin. Res. 2010, 92, 104–107. [Google Scholar] [CrossRef]
- Scott, P.; Collie, D.; McGorum, B.; Sargison, N. Relationship Between Thoracic Auscultation and Lung Pathology Detected by Ultrasonography in Sheep. Vet. J. 2010, 186, 53–57. [Google Scholar] [CrossRef]
- Baird, H.J.; Clarke, S.M.; Johnson, P.L. Brief Communication: Development of a Visual Scoring System for Ovine Pneumonia at the Processing Plant. Proc. N. Z. Soc. Anim. Prod. 2017, 72, 169–171. [Google Scholar]
- Korelidou, V.; Grbovic, Z.; Pavlovic, D.; Simovic, I.; Panic, M.; Temenos, A.; Gelasakis, A.I. Clinical Assessment of Dairy Goats’ Udder Health Using Infrared Thermography. Animals 2025, 15, 658. [Google Scholar] [CrossRef]
- Ayuso, D.; González, A.; Hernández, F.; Corral, J.M.; Izquierdo, M. Prediction of Carcass Composition, Ham and Foreleg Weights, and Lean Meat Yields of Iberian Pigs Using Ultrasound Measurements in Live Animals. J. Anim. Sci. 2013, 91, 1884–1892. [Google Scholar] [CrossRef]
- Barrón-Bravo, O.; Avilés-Ruiz, R.; Fraga-Escamilla, E.; Bautista-Martínez, Y. Los Procesos Reproductivos en Vacas y el Uso de la Ultrasonografía. Abanico Vet. 2023, 13, e2022-81. [Google Scholar] [CrossRef]
- Baxter-Smith, K.; More, J.; Hyde, R. Use of Thoracic Ultrasound on Scottish Dairy Cattle Farms to Support the Diagnosis and Treatment of Bovine Respiratory Disease in Calves. Vet. Rec. 2022, 190, e93. [Google Scholar] [CrossRef]
- Masset, N.; Assié, S.; Herman, N.; Jozan, T.; Herry, V. Ultrasonography of the Cranial Part of the Thorax is a Quick and Sensitive Technique to Detect Lung Consolidation in Veal Calves. Vet. Med. Sci. 2022, 8, 1229–1239. [Google Scholar] [CrossRef]
- Feitoza, L.F.B.B.; White, B.J.; Larson, R.L. Thoracic Ultrasound in Cattle: Methods, Diagnostics, and Prognostics. Vet. Sci. 2025, 12, 16. [Google Scholar] [CrossRef]
- Rademacher, R.D.; Buczinski, S.; Tripp, H.M.; Edmonds, M.D.; Johnson, E.G. Systematic Thoracic Ultrasonography in Acute Bovine Respiratory Disease of Feedlot Steers: Impact of Lung Consolidation on Diagnosis and Prognosis in a Case-Control Study. Bov. Pract. 2014, 48, 1–10. [Google Scholar] [CrossRef]
- Scott, P.R.; Gessert, M.E. Ultrasonographic Examination of the Ovine Thorax. Vet. J. 1998, 155, 305–310. [Google Scholar] [CrossRef]
- Scott, P. Practical Use of Ultrasound Scan in Small Ruminant Medicine and Surgery. Vet. Clin. Food Anim. Pract. 2016, 32, 181–205. [Google Scholar] [CrossRef]
- Hussein, H.A.; Binici, C.; Staufenbiel, R. Comparative Evaluation of Ultrasonography with Clinical Respiratory Score in Diagnosis and Prognosis of Respiratory Diseases in Weaned Dairy Buffalo and Cattle Calves. J. Anim. Sci. Technol. 2018, 60, 29. [Google Scholar] [CrossRef]
- Timsit, E.; Tison, N.; Booker, C.W.; Buczinski, S. Association of Lung Lesions Measured by Thoracic Ultrasonography at First Diagnosis of Bronchopneumonia with Relapse Rate and Growth Performance in Feedlot Cattle. J. Vet. Intern. Med. 2019, 33, 1540–1546. [Google Scholar] [CrossRef]
- Gargani, L.; Volpicelli, G. How I Do It: Lung Ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef]
- Scott, P.R. Use of Ultrasonographic Examination in Sheep Health Management—A General Appraisal. Small Rumin. Res. 2017, 152, 2–9. [Google Scholar] [CrossRef]
- Bøysen, S.; Gommeren, K.; Chalhoub, S. The Essentials of Veterinary Point-of-Care Ultrasound: Pleural Space and Lung, 1st ed.; Edra: Zaragoza, Spain, 2022. [Google Scholar]
- Jung, C.; Bostedt, H. Thoracic Ultrasonography Technique in Newborn Calves and Description of Normal and Pathological Findings. Vet. Radiol. Ultrasound 2004, 45, 331–335. [Google Scholar] [CrossRef]
- Babkine, M.; Blond, L. Ultrasonography of the Bovine Respiratory System and Its Practical Application. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 633–649. [Google Scholar] [CrossRef]
- Jourquin, S.; Bokma, J.; De Cremer, L.; van Leenen, K.; Vereecke, N.; Pardon, B. Randomized Field Trial Comparing the Efficacy of Florfenicol and Oxytetracycline in a Natural Outbreak of Calf Pneumonia Using Lung Reaeration as a Cure Criterion. J. Vet. Intern. Med. 2022, 36, 820–828. [Google Scholar] [CrossRef]
- Ollivett, T.L.; Buczinski, S. On-Farm Use of Ultrasonography for Bovine Respiratory Disease. Vet. Clin. Food Anim. Pract. 2016, 32, 19–35. [Google Scholar] [CrossRef]
- McGuirk, S.M.; Peek, S.F. Timely Diagnosis of Dairy Calf Respiratory Disease Using a Standardized Scoring System. Anim. Health Res. Rev. 2014, 15, 145–147. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kumar, A.; Tiwari, R.; Rahal, A.; Malik, Y.; Dhama, K.; Pal, A.; Prasad, M. Advances in Diagnosis of Respiratory Diseases of Small Ruminants. Vet. Med. Int. 2014, 2014, 508304. [Google Scholar] [CrossRef]
- Simões, J.; Abecia, J.A.; Cannas, A.; Delgadillo, J.A.; Lacasta, D.; Voigt, K.; Chemineau, P. Review: Managing Sheep and Goats for Sustainable High Yield Production. Animal 2021, 15, 100293. [Google Scholar] [CrossRef]
- Nisha, A.R. Antibiotic Residues—A Global Health Hazard. Vet. World 2008, 1, 375–377. [Google Scholar] [CrossRef]
- Levy, J. Probiotics and Antibiotic-Associated Diarrhea: The Effects of Antibiotic Use on Gastrointestinal Function. Am. J. Med. 2000, 108, 76S–84S. [Google Scholar] [CrossRef]
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic Residues in Food Animals: Public Health Concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Lindström, L.; Tauni, F.A.; Vargmar, K. Bronchopneumonia in Swedish Lambs: A Study of Pathological Changes and Bacteriological Agents. Acta Vet. Scand. 2018, 60, 54. [Google Scholar] [CrossRef]
- Curtis, R.A.; Viel, L.; McGuirk, S.M.; Radostits, O.M.; Harris, F.W. Lung Sounds in Cattle, Horses, Sheep and Goats. Can. Vet. J. 1986, 27, 170–172. [Google Scholar]
- Aly, S.S.; Love, W.J.; Williams, D.R.; Lehenbauer, T.W.; Van Eenennaam, A.; Drake, C.; Kass, P.H.; Farver, T.B. Agreement Between Bovine Respiratory Disease Scoring Systems for Pre-Weaned Dairy Calves. Anim. Health Res. Rev. 2014, 15, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Breuer, R.M.; Riedesel, E.A.; Fowler, J.; Yaeger, M.J.; Smith, J.S.; Kreuder, A.J. Ultrasonography and Digital Radiography Findings in Sheep with Clinical Disease Associated with Small Ruminant Lentivirus Infection. Can. Vet. J. 2022, 63, 429–434. [Google Scholar]
- Lisuzzo, A.; Achard, D.; Valenza, A.; Contiero, B.; Cozza, L.; Schiavon, E.; Catarin, G.; Conte, F.; Fiore, E. Bovine Respiratory Disease in Veal Calves: Benefits Associated with Its Early Detection by Lung Ultrasonography and Its Prompt Treatment with a Single Dose of a Fixed Combination of Florfenicol and Meloxicam. Animals 2024, 14, 3499. [Google Scholar] [CrossRef]
- Baruch, J.; Cernicchiaro, N.; Cull, C.A.; Lechtenberg, K.F.; Nickell, J.S.; Renter, D.G. Performance of Multiple Diagnostic Methods in Assessing the Progression of Bovine Respiratory Disease in Calves Challenged with Infectious Bovine Rhinotracheitis Virus and Mannheimia Haemolytica. J. Anim. Sci. 2022, 97, 2357–2371. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.; Carvallo Chaigneau, F.R.; Lebedev, M.; Mutua, V.; McEligot, H.; Lam, S.H.F.; Hwang, B.; Bang, H.; Gershwin, L.J. Validating a Bovine Model for Lung Ultrasound of Bronchiolitis. J. Ultrasound 2022, 25, 49–58. [Google Scholar] [CrossRef]
- Tharwat, M.; Al-Sobayil, F. Ultrasonographic Findings in Goats with Contagious Caprine Pleuropneumonia Caused by Mycoplasma Capricolum Subsp. Capripneumoniae. BMC Vet. Res. 2017, 13, 263. [Google Scholar] [CrossRef]
- Ferrer, L.M.; Lacasta, D.; Chacón, G.; Ramos, J.J.; Villa, A.; Gómez, P.; Latre, M.V. Clinical Diagnosis of Visceral Caseous Lymphadenitis in a Salz Ewe. Small Rumin. Res. 2009, 87, 126–127. [Google Scholar] [CrossRef]
- Corda, A.; Corda, F.; Secchi, V.; Pentcheva, P.; Tamponi, C.; Tilocca, L.; Varcasia, A.; Scala, A. Ultrasonography of Parasitic Diseases in Domestic Animals: A Systematic Review. Animals 2022, 12, 1252. [Google Scholar] [CrossRef]
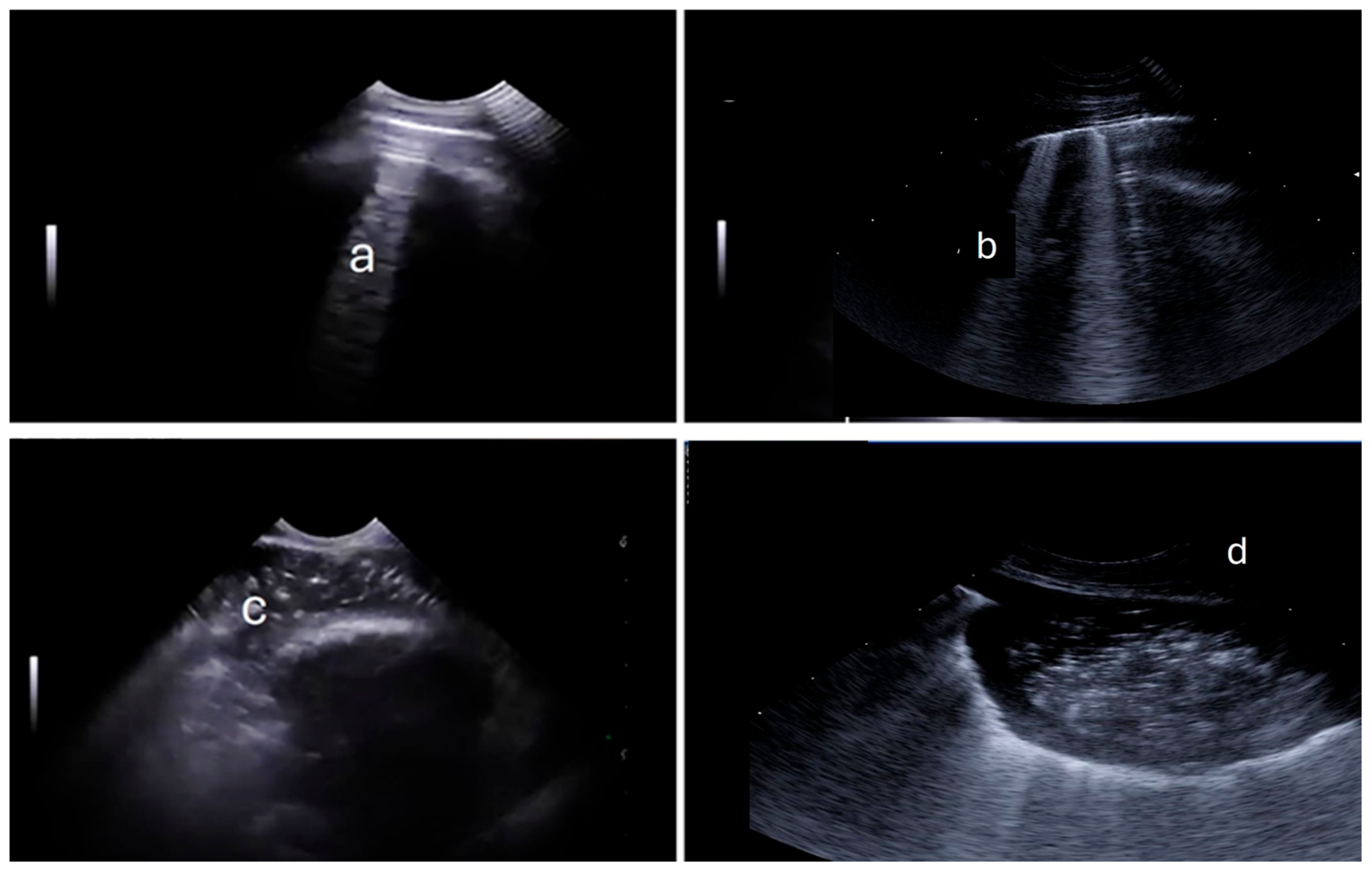
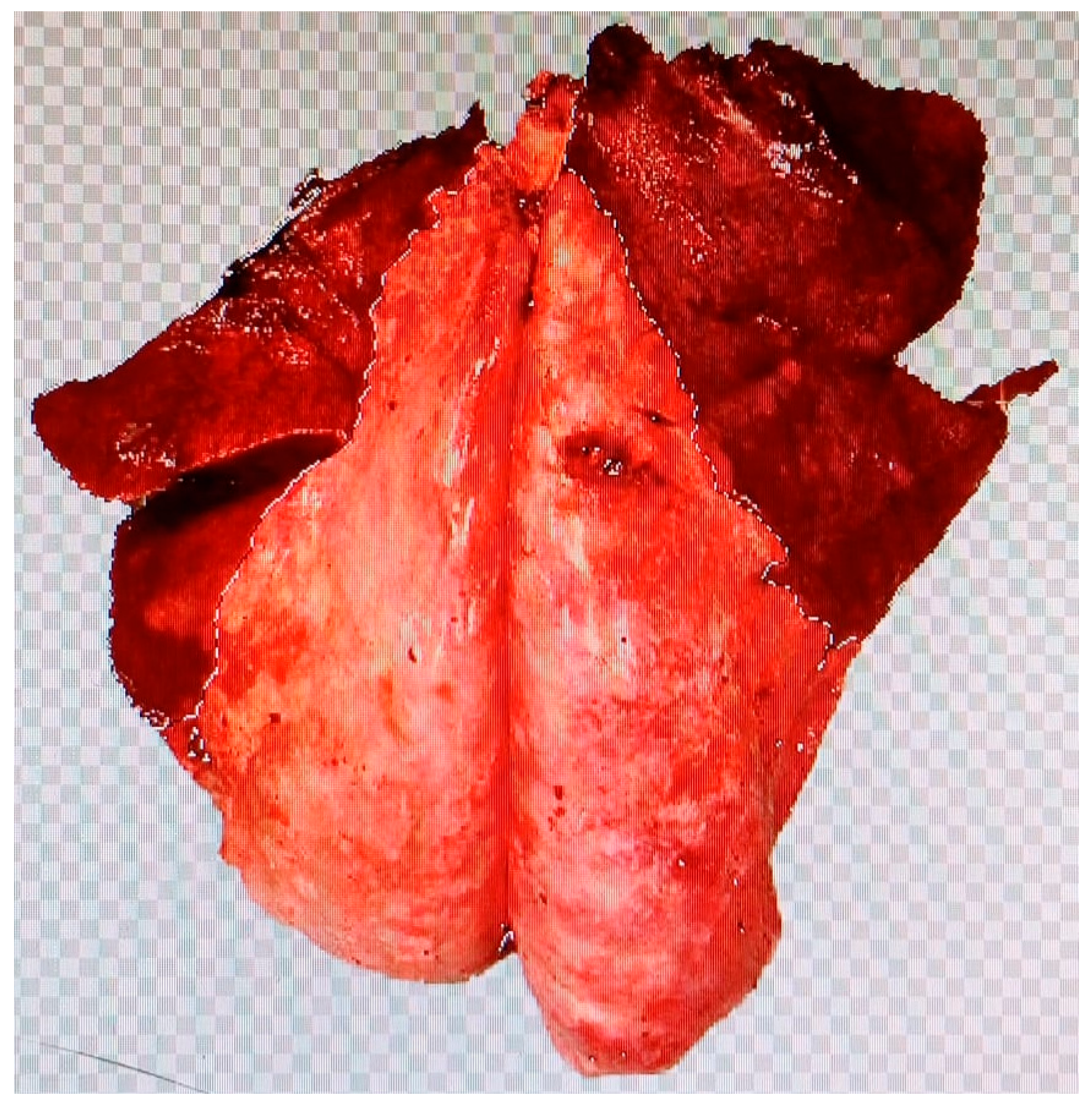
| Diagnosis | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| OD | No | Mild unilateral serous discharge | Bilateral serous discharge | Bilateral mucosal discharge |
| ND | No | Mild unilateral serous discharge | Unilateral serous discharge | Bilateral mucosal discharge |
| HT | No | Head movement | Unilateral head drooping | Bilateral head drooping |
| COUGH | No | Induced and single cough | Induced and multiple or Spontaneous and occasional | Spontaneous and multiple |
| RT | <39.49 °C | 39.5–39.89 °C | 39.9–40.49 °C | ≥40.5 °C |
| SAusc | SClinic | SUlt1 | SPost | |
|---|---|---|---|---|
| 0 | Normal lung | Sumatory between 0–2 | Normal lung | <10% |
| 1 | Bronquial breath sounds | Sumatory between 3–7 | >5 B-lines, no CON | 10–20% |
| 2 | Crepitations, wheezing and rales | Sumatory between 8–11 | >5 B-lines, <5 CON | 20–30% |
| 3 | Absence of lungs sounds | Sumatory between 12–15 | >5 CON, PF o ABS | >30% |
| Evaluation | Score 0 | Score 1 | Score 2 | Score 3 | Total |
|---|---|---|---|---|---|
| SAusc | 34 | 34 | 34 | 9 | 111 |
| SClinic | 36 | 38 | 26 | 11 | 111 |
| SUlt | 27 | 22 | 26 | 36 | 111 |
| SPost | 38 | 22 | 20 | 31 | 111 |
| SAusc | SClinic | SUlt | SPost | |
|---|---|---|---|---|
| SAusc | 1.00 | 0.500–0.710 | 0.500–0.710 | 0.246–0.546 |
| SClinic | 0.634 | 1.00 | 0.497–0.722 | 0.331–0.617 |
| SUlt | 0.611 | 0.611 | 1.00 | 0.497–0.722 |
| SPost | 0.407 | 0.480 | 0.608 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fernández, A.; Gardón, J.C.; Ibáñez, C.; Bueso-Ródenas, J. Use of Ultrasonography for the Evaluation of Lung Lesions in Lambs with Respiratory Complex. Animals 2025, 15, 1153. https://doi.org/10.3390/ani15081153
Sánchez-Fernández A, Gardón JC, Ibáñez C, Bueso-Ródenas J. Use of Ultrasonography for the Evaluation of Lung Lesions in Lambs with Respiratory Complex. Animals. 2025; 15(8):1153. https://doi.org/10.3390/ani15081153
Chicago/Turabian StyleSánchez-Fernández, Alejandro, Juan Carlos Gardón, Carla Ibáñez, and Joel Bueso-Ródenas. 2025. "Use of Ultrasonography for the Evaluation of Lung Lesions in Lambs with Respiratory Complex" Animals 15, no. 8: 1153. https://doi.org/10.3390/ani15081153
APA StyleSánchez-Fernández, A., Gardón, J. C., Ibáñez, C., & Bueso-Ródenas, J. (2025). Use of Ultrasonography for the Evaluation of Lung Lesions in Lambs with Respiratory Complex. Animals, 15(8), 1153. https://doi.org/10.3390/ani15081153





