Effect of Lipopolysaccharide (LPS) on Oxidative Stress and Apoptosis in Immune Tissues from Schizothorax prenanti
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. LPS Stimulation and Sampling
2.3. Effect of LPS on CAT, Bcl-2, Bax, and Caspase-3 Expression
2.4. Assay for Determining Antioxidant Status
2.5. Hematoxylin–Eosin Detection
2.6. Detection of Apoptotic Cells
2.7. Statistical Analysis
3. Results
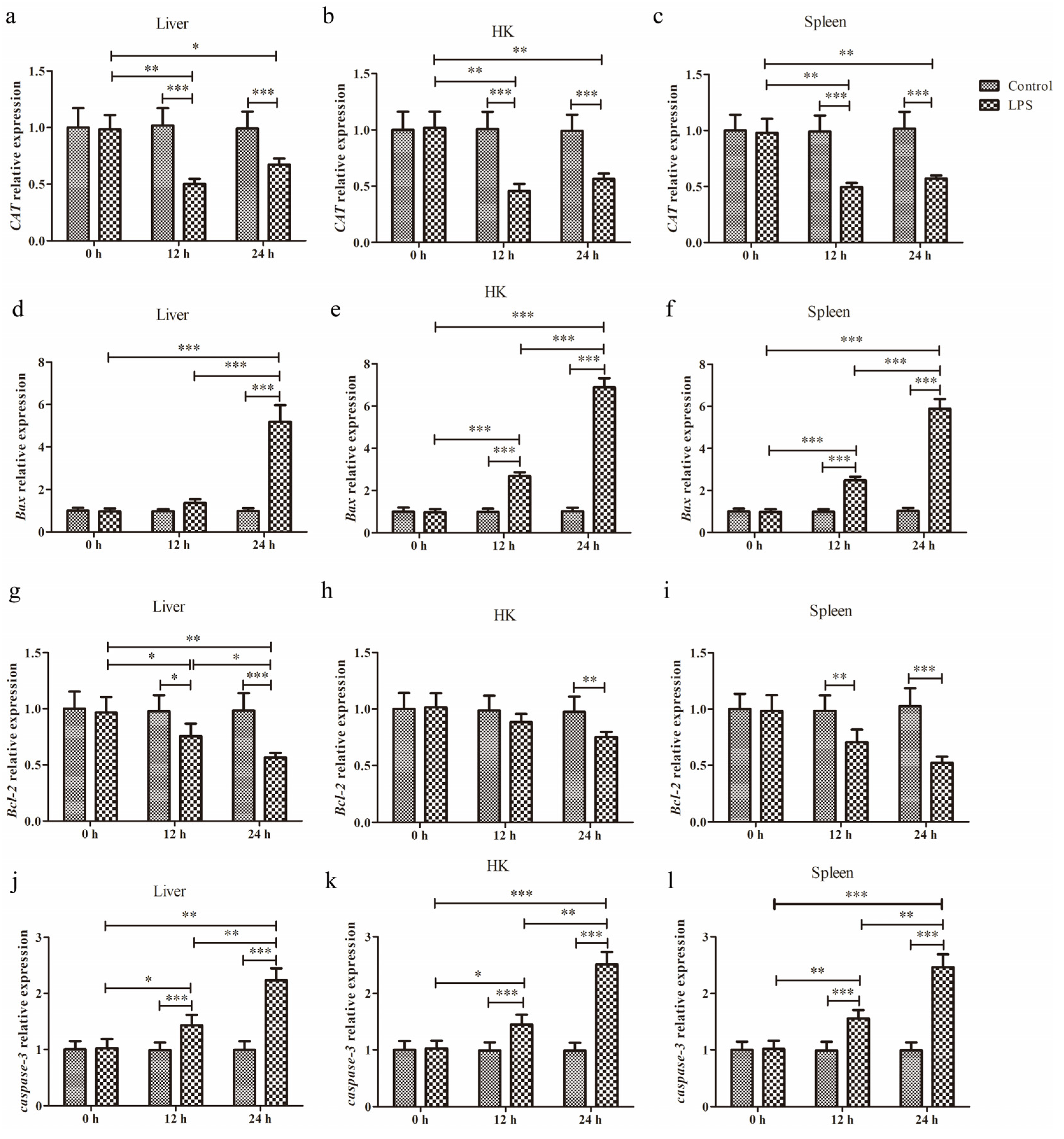
3.1. Expression of CAT, Bax, Bcl-2, and Caspase-3 After LPS Administration
3.1.1. Expression of CAT
3.1.2. Expression of Bax
3.1.3. Expression of Bcl-2
3.1.4. Expression of Caspase-3
3.2. Antioxidant Status Under LPS-Induced Stress
3.2.1. T-SOD Activity
3.2.2. CAT Activity
3.2.3. MDA Content
3.3. Histopathological Analysis
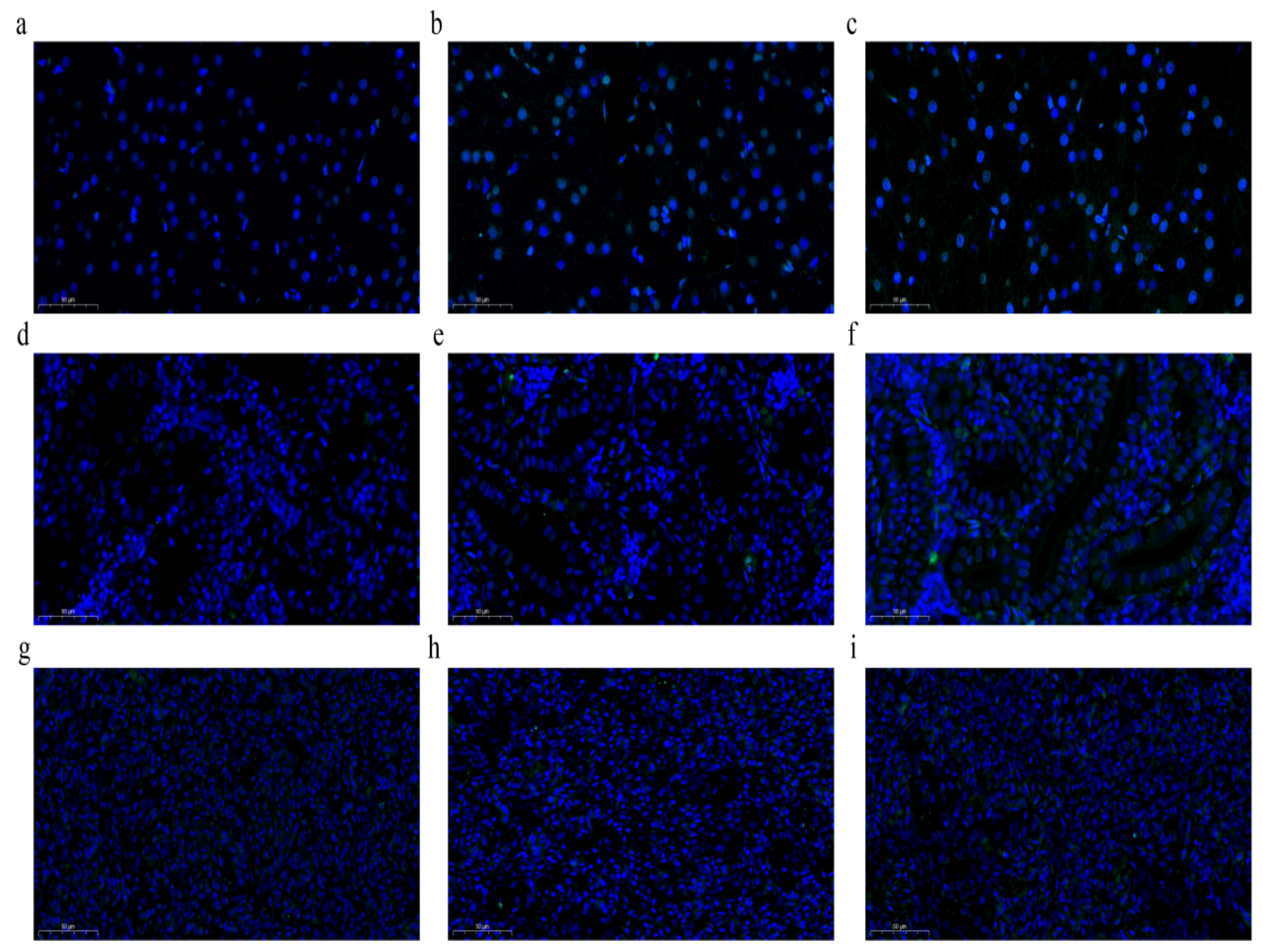
3.4. Induction of Apoptosis by LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bcl-2 | B-cell lymphoma/Leukemia-2 |
| CAT | catalase |
| GPx | glutathione peroxidase |
| HK | head kidney |
| LPS | lipopolysaccharide |
| MDA | malondialdehyde |
| OS | oxidative stress |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TUNEL | terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling |
References
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Di, D.F.; Elizabeth, H.; Allan, B.D.; Marzia, P. Oxidative stress and proteostasis network: Culprit and casualty of Alzheimer’s-Like neurodegeneration. Adv. Geriatr. 2014, 2014, 527518. [Google Scholar] [CrossRef]
- Payton, F.; Bose, R.; Alworth, W.L.; Kumar, A.P.; Ghosh, R. 4-Methylcatechol-induced oxidative stress induces intrinsic apoptotic pathway in metastatic melanoma cells. Biochem. Pharmacol. 2011, 81, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.Y.; Stovall, K.; Franklin, J.L. The intrinsic apoptotic pathway lies upstream of oxidative stress in multiple organs. Free Radic. Biol. Med. 2020, 158, 13–19. [Google Scholar] [CrossRef]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Eberle, J.; Hossini, A.M. Expression and function of bcl-2 proteins in melanoma. Curr. Genom. 2008, 9, 409–419. [Google Scholar] [CrossRef]
- Eskes, R.; Antonsson, B.; Osen-Sand, A.; Montessuit, S.; Richter, C.; Sadoul, R.; Mazzei, G.; Nichols, A.; Martinou, J.C. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J. Cell Biol. 1998, 143, 217–224. [Google Scholar] [CrossRef]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Lavrik, I.N.; Golks, A.; Krammer, P.H. Caspases: Pharmacological manipulation of cell death. J. Clin. Investig. 2005, 115, 2665e72. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, S.; Yamamoto, S.; Tin-Tin-Win-Shwe; Ahmed, S.; Kunugita, N.; Arashidani, K.; Fujimaki, H. Inhalation of low-level formaldehyde increases the Bcl-2/Bax expression ratio in the hippocampus of immunologically sensitized mice. Neuroimmunomodulation 2006, 13, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Walensky, L.D. BCL-2 in the crosshairs: Tipping the balance of life and death. Cell Death Differ. 2006, 13, 1339–1350. [Google Scholar] [CrossRef]
- Kabanov, D.S.; Prokhorenko, I.R. Structural analysis of lipopolysaccharides from Gram-negative bacteria. Biochemistry 2010, 75, 383–404. [Google Scholar] [CrossRef]
- Li, L.; Wei, X.F.; Yang, Z.Y.; Zhu, R.; Li, D.L.; Shang, G.J.; Wang, H.T.; Meng, S.T.; Wang, Y.T.; Liu, S.Y.; et al. Alleviative effect of poly-β-hydroxybutyrate on lipopolysaccharide-induced oxidative stress, inflammation and cell apoptosis in Cyprinus carpio. Int. J. Biol. Macromol. 2023, 253, 126784. [Google Scholar] [CrossRef]
- Luo, S.W.; Xiong, N.X.; Luo, Z.Y.; Luo, K.K.; Liu, S.J.; Wu, C.; Wang, S.; Wen, M. Effect of Lipopolysaccharide (LPS) stimulation on apoptotic process and oxidative stress in fibroblast cell of hybrid crucian carp compared with those of Carassius cuvieri and Carassius auratus red var. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 248, 109085. [Google Scholar] [CrossRef]
- Zhi, S.; Wang, J.; Wang, Y.; Yang, L.; Qin, C.; Yan, X.; Zhao, M.; Liu, M.; Qu, L.; Nie, G. Establishment and characterization of Yellow River carp (Cyprinus carpio haematopterus) muscle cell line and its application to fish virology and immunology. Fish Shellfish Immunol. 2023, 139, 108859. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Fang, C.; Li, W.; Zhao, H.; Kong, F.; Zhang, H.; Zhang, H.; Wang, Q. Molecular cloning of heat shock protein 60 (SpHSP60) from Schizothorax prenanti and the gene expressions of four SpHSPs during lipopolysaccharide (LPS) infection. Fishes 2022, 7, 139. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zheng, Q.R.; Wu, Y.L.; Xu, H.L.; Wang, H.J.; Tang, H.L.; Xia, X.J.; Feng, J. Immune responses to Aeromonas hydrophila infection in Schizothorax prenanti fed with oxidized konjac glucomannan and its acidolysis products. Fish Shellfish Immunol. 2016, 49, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, K.Y.; Huang, X.L.; Chen, D.F.; Li, C.W.; Ren, S.Y.; Liao, Y.T.; Zhou, Z.Y.; Liu, Q.F.; Du, Z.J.; et al. Streptococcus agalactiae, an emerging pathogen for cultured Ya-fish, Schizothorax prenanti, in China. Transbound. Emerg. Dis. 2012, 59, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, G.; Adorian, T.J.; Rafiee, G. Beneficial effects of Bacillus subtilis on water quality, growth, immune responses, endotoxemia and protection against lipopolysaccharide-induced damages in Oreochromis niloticus under biofloc technology system. Aquacult. Nutr. 2020, 26, 1476–1492. [Google Scholar] [CrossRef]
- Caparkaya, D.; Cengiz, S.; Dincel, B.; Demir, S.; Cavas, L. The effects of UV exposure on the antioxidant enzyme systems of anemones. Mediterr. Mar. Sci. 2010, 11, 259–275. [Google Scholar] [CrossRef]
- Radice, S.; Ferraris, M.; Marabini, L.; Grande, S.; Chiesara, E. Effect of iprodione; a dicarboximide fungicide, on primary cultured rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2001, 54, 51–58. [Google Scholar] [CrossRef]
- Mohamadin, A.M.; Elberry, A.A.; Elkablawy, M.A.; Gawad, H.S.; Al-Abbasi, F.A. Montelukast, a leukotriene receptor antagonist abrogates lipopolysaccharide-induced toxicity and oxidative stress in rat liver. Pathophysiology 2011, 18, 235–242. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, K.; Xi, B.; Xie, J.; Bing, X. Protective effects of paeonol against lipopolysaccharide-induced liver oxidative stress and inflammation in gibel carp (Carassius auratus gibelio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 257, 109339. [Google Scholar] [CrossRef]
- Björkholm, B.; Bok, C.M.; Lundin, A.; Rafter, J.; Hibberd, M.L.; Pettersson, S. Intestinal microbiota regulate xenobiotic metabolism in the liver. PLoS ONE 2009, 4, e6958. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Elshopakey, G.E.; Aziza, A.E. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 2020, 96, 213–222. [Google Scholar] [CrossRef]
- Mitchell, P.; Tollervey, D. mRNA turnover. Curr. Opin. Cell Biol. 2001, 13, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Winston, G.W.; Giulio, R.T.D. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Liu, J.D.; Liu, W.B.; Zhang, C.Y.; Xu, C.Y.; Zheng, X.C.; Zhang, D.D.; Chi, C. Dietary glutathione supplementation enhances antioxidant activity and protects against lipopolysaccharide-induced acute hepatopancreatic injury and cell apoptosis in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2020, 97, 440–454. [Google Scholar] [CrossRef]
- Banerjee, P.; Gaddam, N.; Chandler, V.; Chakraborty, S. Oxidative stress-induced liver damage and remodeling of the liver vasculature. Am. J. Pathol. 2023, 193, 1400–1414. [Google Scholar] [CrossRef]
- Xiang, L.X.; Peng, B.; Dong, W.R.; Yang, Z.F.; Shao, J.Z. Lipopolysaccharide induces apoptosis in Carassius auratus lymphocytes, a possible role in pathogenesis of bacterial infection in fish. Dev. Comp. Immunol. 2008, 32, 992–1001. [Google Scholar] [CrossRef]
- Li, S.; Peng, W.; Li, J.; Hao, G.; Geng, X.; Sun, J. Characterization of Japanese flounder (Paralichthys olivaceus) Caspase1 involved in extracellular ATP-mediated immune signaling in fish. Fish Shellfish Immunol. 2017, 67, 536–545. [Google Scholar] [CrossRef]
- Breckenridge, D.G.; Xue, D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr. Opin. Cell Biol. 2004, 16, 647–652. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Z.; Qiao, P.; Liu, S.; Zhang, L.; He, P.; Zhang, X.; Wang, Y.; Min, W. Cadmium induces liver cell apoptosis through caspase-3A activation in purse red common carp (Cyprinus carpio). PLoS ONE 2013, 8, e83423. [Google Scholar] [CrossRef]
- MacKenzie, S.; Montserrat, N.; Mas, M.; Acerete, L.; Tort, L.; Krasnov, A.; Goetz, F.W.; Planas, J.V. Bacterial lipopolysaccharide induces apoptosis in the trout ovary. Reprod. Biol. Endocrinol. 2006, 4, 46. [Google Scholar] [CrossRef]
- Martins, G.P.; Espe, M.; Zhang, Z.; Guimarães, I.G.; Holen, E. Surplus arginine reduced lipopolysaccharide induced transcription of proinflammatory genes in Atlantic salmon head kidney cells. Fish Shellfish Immunol. 2019, 86, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, X.; Li, L.; Dang, Y.; Fang, Y.; Shen, Y.; Xu, X.; Wang, R.; Li, J. Biological parameters, immune enzymes, and histological alterations in the livers of grass carp infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 70, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Feng, S.; Zhang, A.; Yang, K.; Zhou, H. Identification and functional characterization of grass carp (Ctenopharyngodon idella) tumor necrosis factor receptor 2 and its soluble form with potentiality for targeting inflammation. Fish Shellfish Immunol. 2019, 86, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, Z.; Zhang, D.; Guo, F.; Gao, F.; Wang, M.; Yi, M.; Lu, M. Distribution and localization of Streptococcus agalactiae in different tissues of artificially infected tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737370. [Google Scholar] [CrossRef]
- Ke, X.; Liu, Z.; Chen, S.; Chen, Z.; Zhang, D.; Gao, F.; Lu, M. The immune efficacy of a Streptococcus agalactiae immersion vaccine for different sizes of young tilapia. Aquaculture 2021, 534, 736289. [Google Scholar] [CrossRef]


| Primer | Accession Number | Sequence (5′-3′) | Annealing Temperature (°C) | PCR Efficiency | Size (bp) |
|---|---|---|---|---|---|
| Bcl-2 | OQ734947 | F: CTGGATGACAGACTACCTGAAC | 62 | 97.5% | 118 |
| R: CGACAATGGGTGGAACATAGA | |||||
| Bax | OQ347970 | F: GACTCCACTCTTCAACCAACTC | 62 | 98.1% | 116 |
| R: AGCCGACATGCAAAGTAGAA | |||||
| CAT | OQ737946 | F: GGAAACAACACTCCCATCTT | 62 | 101.5% | 120 |
| R: CCAGAAGTCCCAAACCATATC | |||||
| caspase-3 | OQ737945 | F: CAGTCACATGCCTTCAGATAC | 62 | 103.5% | 122 |
| R: GCATCTACATCAGTACCATTCC | |||||
| β-actin | MK439425 | F: GACCACCTTCAACTCCATCAT | 62 | 99.5% | 126 |
| R: GTGATCTCCTTCTGCATCCTATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Jiang, W.; Ma, H.; Zhang, H.; Zhao, H.; Wang, Q.; Zhang, J. Effect of Lipopolysaccharide (LPS) on Oxidative Stress and Apoptosis in Immune Tissues from Schizothorax prenanti. Animals 2025, 15, 1298. https://doi.org/10.3390/ani15091298
Huang J, Jiang W, Ma H, Zhang H, Zhao H, Wang Q, Zhang J. Effect of Lipopolysaccharide (LPS) on Oxidative Stress and Apoptosis in Immune Tissues from Schizothorax prenanti. Animals. 2025; 15(9):1298. https://doi.org/10.3390/ani15091298
Chicago/Turabian StyleHuang, Jiqin, Wei Jiang, Hongying Ma, Han Zhang, Hu Zhao, Qijun Wang, and Jianlu Zhang. 2025. "Effect of Lipopolysaccharide (LPS) on Oxidative Stress and Apoptosis in Immune Tissues from Schizothorax prenanti" Animals 15, no. 9: 1298. https://doi.org/10.3390/ani15091298
APA StyleHuang, J., Jiang, W., Ma, H., Zhang, H., Zhao, H., Wang, Q., & Zhang, J. (2025). Effect of Lipopolysaccharide (LPS) on Oxidative Stress and Apoptosis in Immune Tissues from Schizothorax prenanti. Animals, 15(9), 1298. https://doi.org/10.3390/ani15091298








