Integrated Multi-Tissue Lipidomics and Transcriptomics Reveal Differences in Lipid Composition Between Mashen and Duroc × (Landrace × Yorkshire) Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Hematoxylin–Eosin Staining
2.3. Intramuscular Fat Content Detection
2.4. Lipid Extraction and Lipidomics Analysis
2.5. Receiver Operating Characteristic Analysis
2.6. RNA-Seq Analysis
2.7. Detection of Serum Biochemical Indexes
2.8. Weighted Gene Co-Expression Network Analysis
2.9. Association Analysis
2.10. Statistical Analysis
3. Results
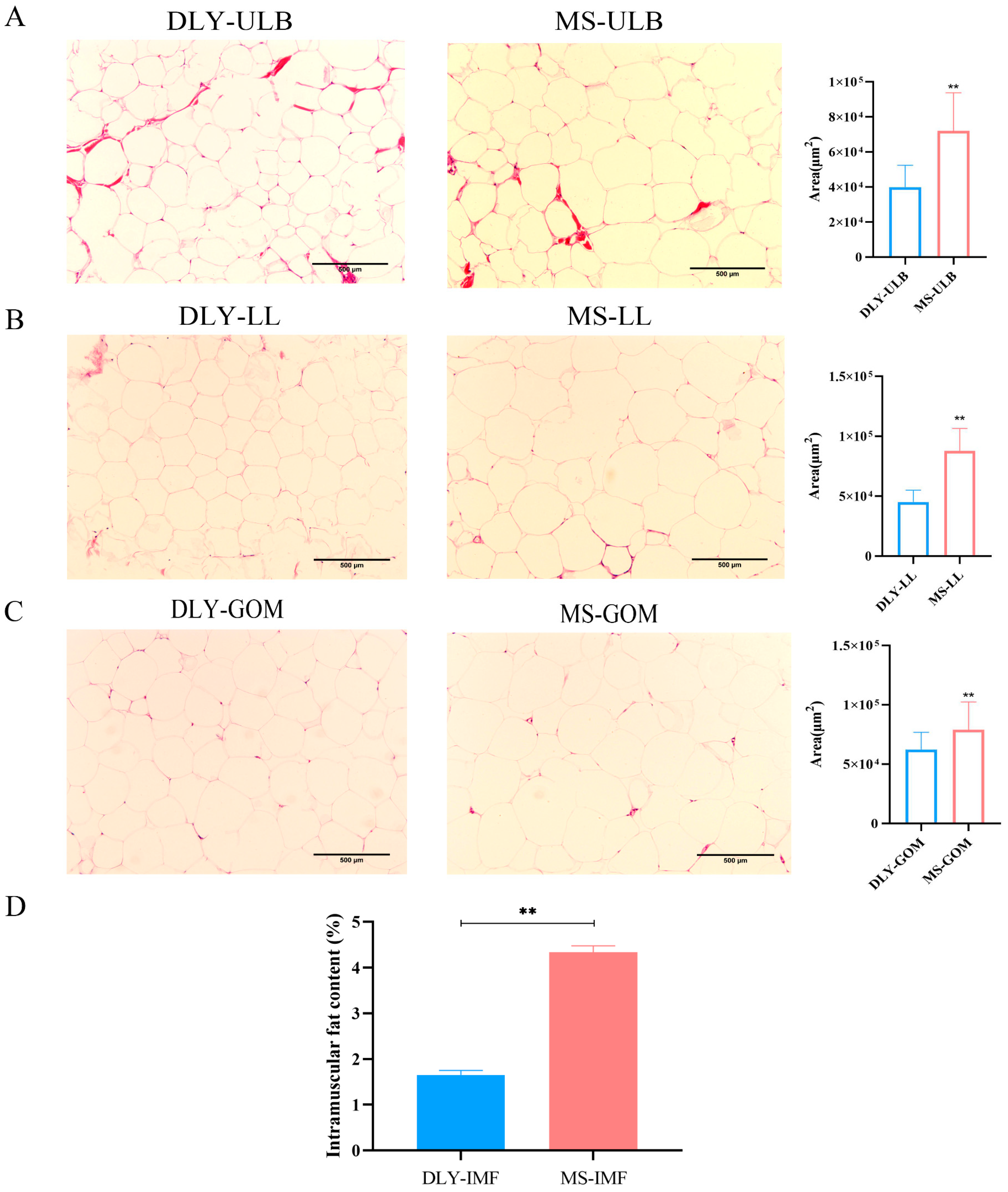
3.1. Hematoxylin–Eosin Staining and Intramuscular Fat Content Detection
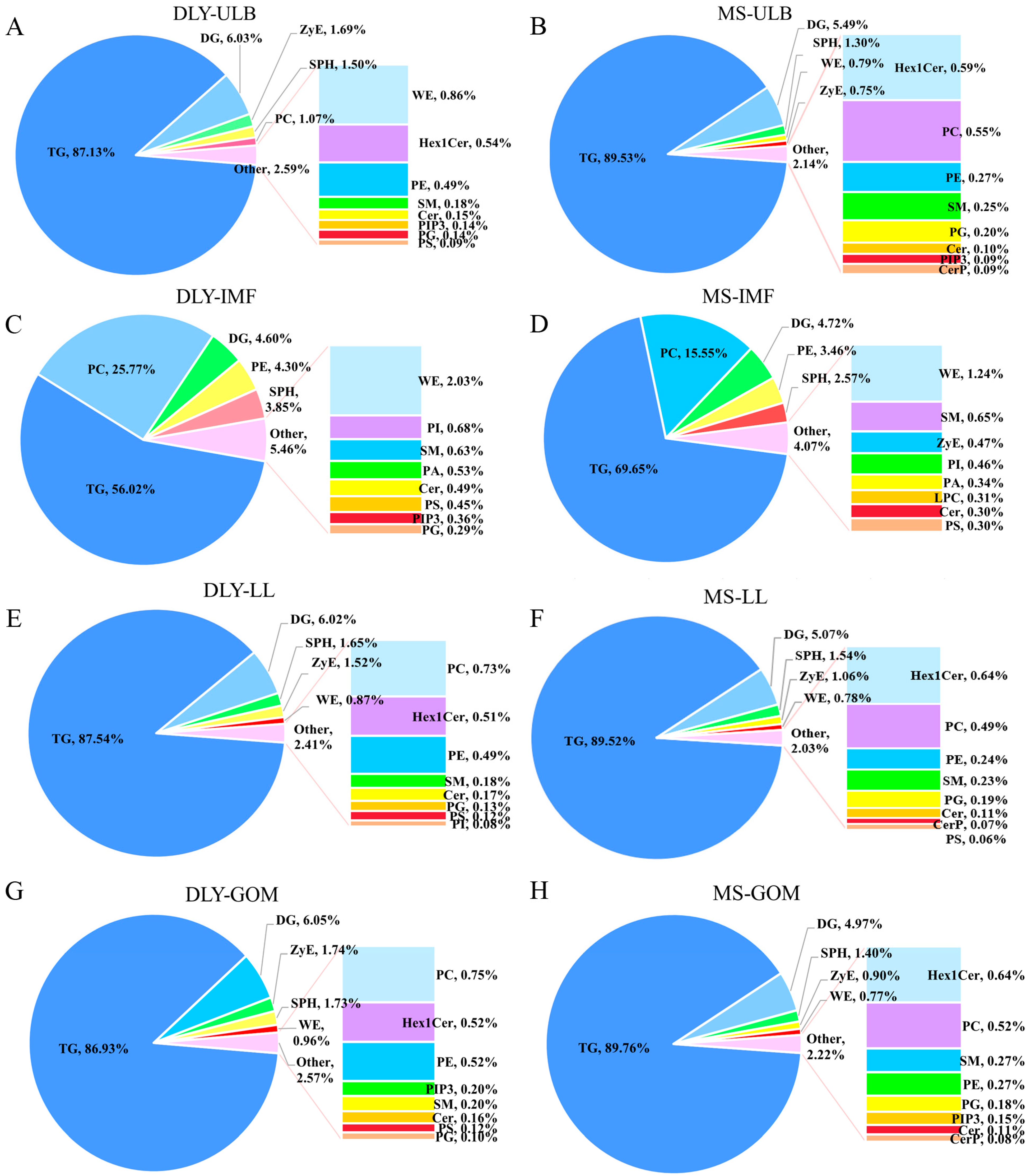
3.2. Lipid Profiles of Tissue Samples
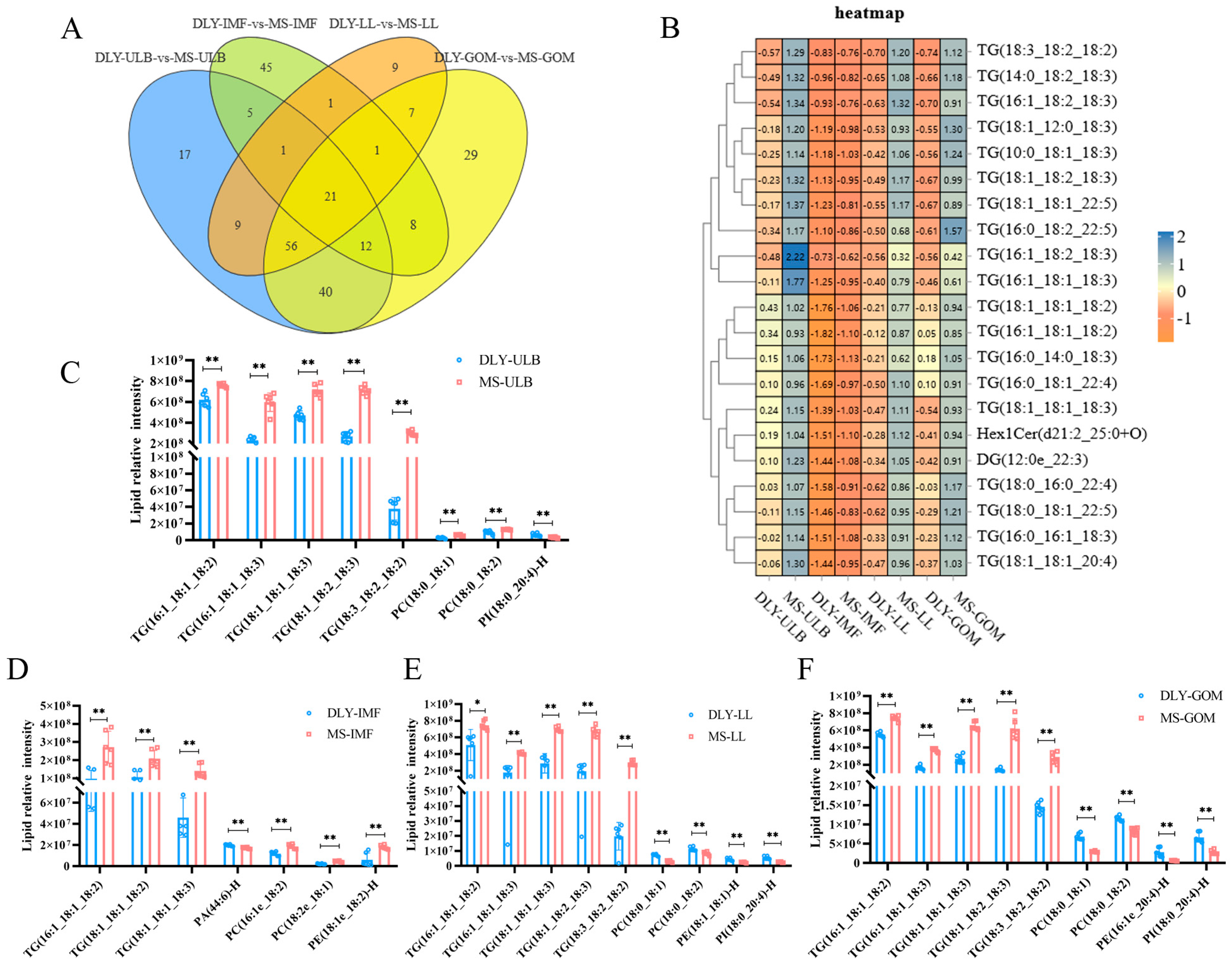
3.3. Differential Lipid Analysis
3.4. Fatty Acid Composition Analysis
3.5. Differential Lipid Molecules and Potential Lipid Markers
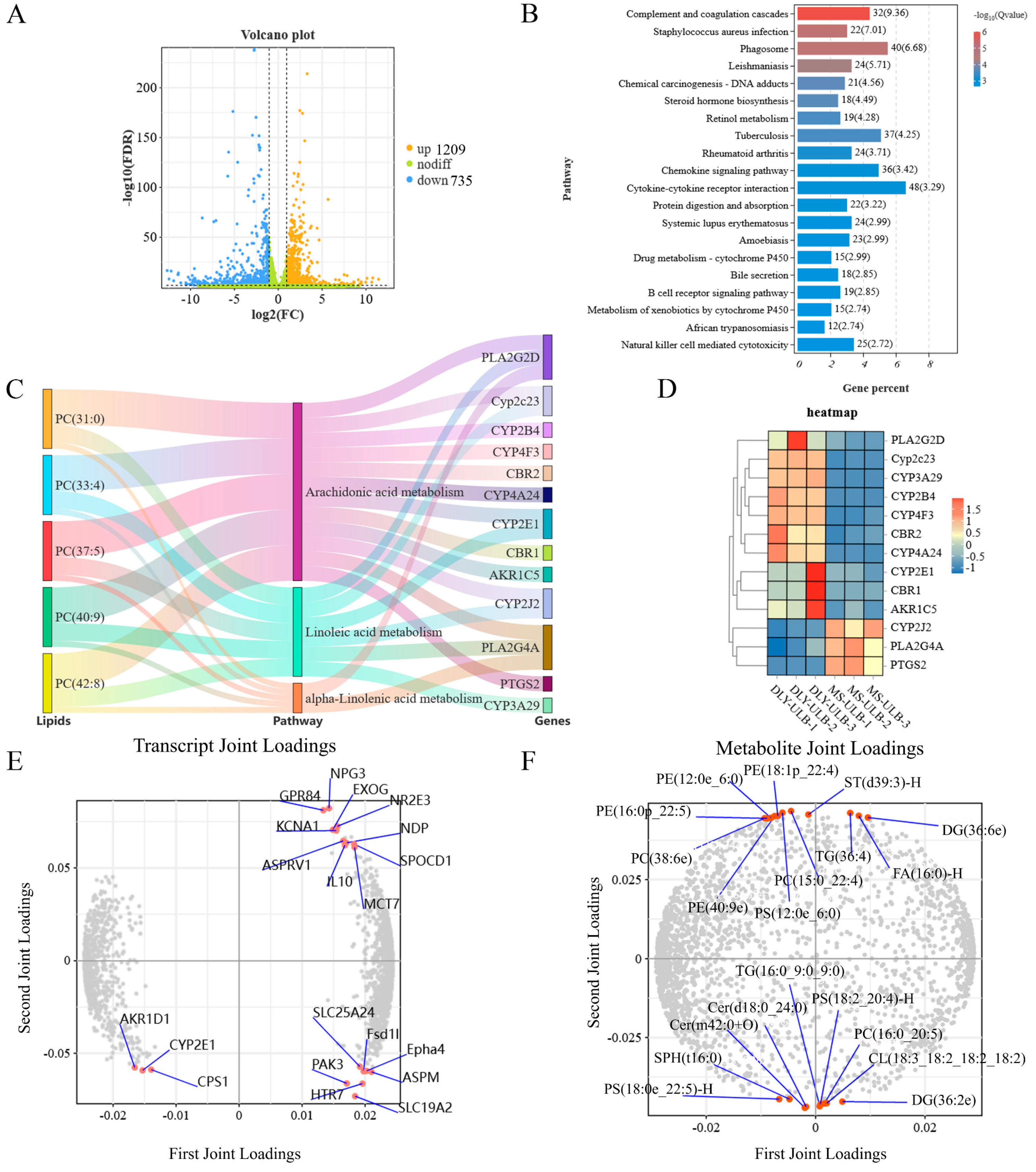
3.6. WGCNA Analysis of Lipid Molecules
3.7. Association Analysis Between Genes and Lipid Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Mashen |
| DLY | Duroc × (Landrace × Yorkshire) |
| ULB | Upper layer of backfat |
| LDM | Linear dichroism |
| LL | Leaf lard |
| GOM | Greater omentum |
| TG | Triglyceride |
| DG | Diglyceride |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| Hex1Cer | Hexosylceramide |
| SM | Sphingomyelin |
| SPH | Sphingosine |
| WE | Wax ester |
| PA | Phosphatidic acid |
| SFAs | Saturated fatty acids |
| MUFAs | Monounsaturated fatty acids |
| PUFAs | Polyunsaturated fatty acids |
| KEGG | Kyoto encyclopedia of genes and genomes |
| WGCNA | Weighted gene co-expression network analysis |
References
- Alfaia, C.M.; Lopes, P.A.; Madeira, M.S.; Pestana, J.M.; Coelho, D.; Toldrá, F.; Prates, J.A.M. Current feeding strategies to improve pork intramuscular fat content and its nutritional quality. Adv. Food Nutr. Res. 2019, 89, 53–94. [Google Scholar] [PubMed]
- Nolan-Clark, D.J.; Neale, E.P.; Charlton, K.E. Processed pork is the most frequently consumed type of pork in a survey of Australian children. Nutr. Res. 2013, 33, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Nute, G.R.; Richardson, R.I.; Whittington, F.M.; Southwood, O.; Plastow, G.; Mansbridge, R.; da Costa, N.; Chang, K.C. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004, 67, 651–667. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Kikuchi, Y.; Kusakabe, T.; Takano, H.; Sakurai, K.; Furui, S.; Oba, H. Imaging spectrum of abnormal subcutaneous and visceral fat distribution. Insights Imaging 2020, 11, 24. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Wu, Y.; Zhang, Y.; Zhang, B.; Zhang, H. Identification of candidate genes that specifically regulate subcutaneous and intramuscular fat deposition using transcriptomic and proteomic profiles in Dingyuan pigs. Sci. Rep. 2022, 12, 2844. [Google Scholar] [CrossRef]
- Li, M.; Wu, H.; Luo, Z.; Xia, Y.; Guan, J.; Wang, T.; Gu, Y.; Chen, L.; Zhang, K.; Ma, J.; et al. An atlas of DNA methylomes in porcine adipose and muscle tissues. Nat. Commun. 2012, 3, 850. [Google Scholar] [CrossRef]
- Monziols, M.; Bonneau, M.; Davenel, A.; Kouba, M. Comparison of the lipid content and fatty acid composition of intermuscular and subcutaneous adipose tissues in pig carcasses. Meat Sci. 2007, 76, 54–60. [Google Scholar] [CrossRef]
- Garrido, N.; Izquierdo, M.; Hernández-García, F.I.; Núñez, Y.; García-Torres, S. Differences in muscle lipogenic gene expression, carcass traits and fat deposition among three Iberian pig strains finished in two different feeding systems. Animals 2023, 13, 1138. [Google Scholar] [CrossRef]
- Gao, K.; Han, S.; Li, Z.; Luo, Z.; Lv, S.; Choe, H.M.; Paek, H.J.; Quan, B.; Kang, J.; Yin, X. Analysis of metabolome and transcriptome of longissimus thoracis and subcutaneous adipose tissues reveals the regulatory mechanism of meat quality in MSTN mutant castrated male finishing pigs. Meat Sci. 2024, 207, 109370. [Google Scholar] [CrossRef]
- Yi, X.; Feng, M.; Zhu, J.; Yu, H.; He, Z.; Zhang, Z.; Zhao, T.; Zhang, Q.; Pang, W. Adipocyte progenitor pools composition and cellular Niches affect Aadipogenesis divergence in porcine subcutaneous and intramuscular fat. J. Agric. Food Chem. 2024, 72, 14044–14056. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qi, L.; Zhang, S.; Peng, H.; Lin, Z.; Zhang, X.; Nie, Q.; Luo, W. Metabolomic, lipidomic, and proteomic profiles provide insights on meat quality differences between Shitou and Wuzong geese. Food Chem. 2024, 438, 137967. [Google Scholar] [PubMed]
- Hou, X.; Zhang, R.; Yang, M.; Niu, N.; Wu, J.; Shu, Z.; Zhang, P.; Shi, L.; Zhao, F.; Wang, L.; et al. Metabolomics and lipidomics profiles related to intramuscular fat content and flavor precursors between Laiwu and Yorkshire pigs. Food Chem. 2023, 404, 134699. [Google Scholar]
- Xie, C.; Zhu, X.; Xu, B.; Niu, Y.; Zhang, X.; Ma, L.; Yan, X. Integrated analysis of multi-tissues lipidome and gut microbiome reveals microbiota-induced shifts on lipid metabolism in pigs. Anim. Nutr. 2022, 10, 280–293. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar]
- Liu, R.; Kong, F.; Xing, S.; He, Z.; Bai, L.; Sun, J.; Tan, X.; Zhao, D.; Zhao, G.; Wen, J. Dominant changes in the breast muscle lipid profiles of broiler chickens with wooden breast syndrome revealed by lipidomics analyses. J. Anim. Sci. Biotechnol. 2022, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, X.; Cong, P.; Xu, J. Mass spectrometry-based lipidomics in food science and nutritional health: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2530–2558. [Google Scholar] [CrossRef]
- Harlina, P.W.; Maritha, V. Possibilities of liquid chromatography mass spectrometry (LC-MS)-based metabolomics and lipidomics in the authentication of meat products: A mini review. Food Sci. Anim. Resour. 2022, 42, 744–761. [Google Scholar]
- Zhang, Z.; Liao, Q.; Sun, Y.; Pan, T.; Liu, S.; Miao, W.; Li, Y.; Zhou, L.; Xu, G. Lipidomic and transcriptomic analysis of the longissimus muscle of Luchuan and Duroc pigs. Front. Nutr. 2021, 8, 667622. [Google Scholar]
- Schermuly, I.I.; Romanet, S.; Klünemann, M.; Mastrototaro, L.; Pieper, R.; Zentek, J.; Whelan, R.A.; Aschenbach, J.R. Dietary methionine source alters the lipidome in the small intestinal epithelium of pigs. Sci. Rep. 2022, 12, 4863. [Google Scholar] [CrossRef]
- Li, M.; Zhu, M.; Chai, W.; Wang, Y.; Song, Y.; Liu, B.; Cai, C.; Song, Y.; Sun, X.; Xue, P.; et al. Determination of the heterogeneity of intramuscular fat and visceral adipose tissue from Dezhou Donkey by lipidomics and transcriptomics profiling. Front. Nutr. 2021, 8, 746684. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xie, F.; Wang, J.; Luo, J.; Chen, T.; Jiang, Q.; Xi, Q.; Liu, G.E.; Zhang, Y. Integrated meta-omics reveals the regulatory landscape involved in lipid metabolism between pig breeds. Microbiome 2024, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xu, Y.; Gu, X.; Zhang, J.; Wang, J.; Geng, F. Divergence of liver lipidomes in Tibetan and Yorkshire pigs living at different altitudes. Molecules 2023, 28, 2991. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Zheng, C.; Wang, L.; Zhou, Y.; Chen, W.; Duan, Y.; Shan, T. Leucine improves the growth performance, carcass traits, and lipid nutritional quality of pork in Shaziling pigs. Meat Sci. 2024, 210, 109435. [Google Scholar]
- Wang, L.; Nong, Q.; Zhou, Y.; Sun, Y.; Chen, W.; Xie, J.; Zhu, X.; Shan, T. Changes in serum fatty acid composition and metabolome-microbiome responses of Heigai pigs induced by dietary N-6/n-3 polyunsaturated fatty acid ratio. Front. Microbiol. 2022, 13, 917558. [Google Scholar] [CrossRef]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of carcass traits, meat quality, and serum metabolome between Shaziling and Yorkshire pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef]
- Wang, Z.; Song, B.; Yao, J.; Li, X.; Zhang, Y.; Tang, Z.; Yi, G. Whole-genome analysis reveals distinct adaptation signatures to diverse environments in Chinese domestic pigs. J. Anim. Sci. Biotechnol. 2024, 15, 97. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, X.; Yang, J.; Zhou, L.; Yang, B.; Ai, H.; Ma, H.; Xie, X.; Huang, Y.; Fang, S.; et al. Genome-wide association analyses for meat quality traits in Chinese Erhualian pigs and a Western Duroc × (Landrace × Yorkshire) commercial population. Genet. Sel. Evol. 2015, 47, 44. [Google Scholar] [CrossRef]
- Zhao, J.; Li, K.; Yang, Q.; Du, M.; Liu, X.; Cao, G. Enhanced adipogenesis in Mashen pigs compared with Large White pigs. Ital. J. Anim. Sci. 2017, 16, 217–225. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lim, B.; Lim, D.; Park, W.; Lee, K.T.; Cho, E.S.; Lim, K.S.; Cheon, S.N.; Choi, B.H.; Park, J.E.; et al. Integrative methylome and transcriptome analysis of porcine abdominal fat indicates changes in fat metabolism and immune responses during different development. J. Anim. Sci. 2022, 100, skac302. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. BMC Bioinform. 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, M.; Huang, Y.; Liu, X.; Zhong, L.; Ji, J.; Zhou, L.; Zeng, Q.; Ma, J.; Huang, L. The effect of purine content on sensory quality of pork. Meat Sci. 2021, 172, 108346. [Google Scholar] [CrossRef]
- Cui, H.; Karim, N.; Jiang, F.; Hu, H.; Chen, W. Assessment of quality deviation of pork and salmon due to temperature fluctuations during superchilling. J. Zhejiang Univ. Sci. B 2022, 23, 578–586. [Google Scholar] [CrossRef]
- Natalello, A.; Khelil-Arfa, H.; Luciano, G.; Zoon, M.; Menci, R.; Scerra, M.; Blanchard, A.; Mangano, F.; Biondi, L.; Priolo, A. Effect of different levels of organic zinc supplementation on pork quality. Meat Sci. 2022, 186, 108731. [Google Scholar] [CrossRef]
- Martins, J.M.; Charneca, R. Influence of sex on meat and fat quality from Heavy Alentejano pigs finished outdoors on commercial and high fiber diets. Animals 2023, 13, 3099. [Google Scholar] [CrossRef]
- Minelli, G.; D’Ambra, K.; Macchioni, P.; Lo Fiego, D.P. Effects of pig dietary n-6/n-3 polyunsaturated fatty acids ratio and gender on carcass traits, fatty acid profiles, nutritional indices of lipid depots and oxidative stability of meat in medium-heavy pigs. Foods 2023, 12, 4106. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, C.; Zheng, J.; Ma, L.; Ma, X.; Zhong, Y.; Zhao, X.; Li, F.; Guo, Q.; Yin, Y. Profiles of muscular amino acids, fatty acids, and metabolites in Shaziling pigs of different ages and relation to meat quality. Sci. China Life Sci. 2023, 66, 1323–1339. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Duan, Y.; Li, R.; Liang, X.; Li, T.; Huang, X.; Yin, Y.; Yin, J. Gut microbial profiles and the role in lipid metabolism in Shaziling pigs. Anim. Nutr. 2022, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Zhang, Y.; Jiang, W.; He, C. Muscle characteristics comparison analysis reveal differences in the meat quality and nutritional components of three Shanghai local pig breeds. Foods 2025, 14, 569. [Google Scholar] [CrossRef]
- Hoa, V.B.; Song, D.H.; Min, Y.J.; Seol, K.H.; Kang, S.M.; Kim, H.W.; Moon, S.S.; Cho, S.H. Carcass trait, meat yield and quality characteristics of recently-synthesized Woori Heukdon and commercial LYD pigs under identical rearing condition. Anim. Biosci. 2023, 36, 943–952. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X.; et al. Impacts of dietary fiber level on growth performance, apparent digestibility, intestinal development, and colonic microbiota and metabolome of pigs. J. Anim. Sci. 2023, 101, skad174. [Google Scholar] [CrossRef]
- Gao, P.; Cheng, Z.; Li, M.; Zhang, N.; Le, B.; Zhang, W.; Song, P.; Guo, X.; Li, B.; Cao, G. Selection of candidate genes affecting meat quality and preliminary exploration of related molecular mechanisms in the Mashen pig. Asian-Australas. J. Anim. Sci. 2019, 32, 1084–1094. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Zhang, Y.; Zhao, N.; Zhang, W.; Shi, M.; Zhao, Y.; Cai, C.; Lu, C.; Gao, P. Transcriptome analysis revealed potential genes of skeletal muscle thermogenesis in Mashen pigs and Large White pigs under cold stress. Int. J. Mol. Sci. 2023, 24, 15534. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, M.; Hou, X.; Hou, R.; Wang, L.; Shi, L.; Zhao, F.; Liu, X.; Meng, Q.; Wang, L.; et al. Characterization and difference of lipids and metabolites from Jianhe White Xiang and Large White pork by high-performance liquid chromatography-tandem mass spectrometry. Food Res. Int. 2022, 162, 111946. [Google Scholar] [CrossRef]
- Qiu, Y.Q.; Yang, X.F.; Ma, X.Y.; Xiong, Y.X.; Tian, Z.M.; Fan, Q.L.; Wang, L.; Jiang, Z.Y. CIDE gene expression in adipose tissue, liver, and skeletal muscle from obese and lean pigs. J. Zhejiang Univ. Sci. B 2017, 18, 492–500. [Google Scholar] [CrossRef]
- Zhou, L.; Ren, Y.; Shi, Y.; Fan, S.; Zhao, L.; Dong, M.; Li, J.; Yang, Y.; Yu, Y.; Zhao, Q.; et al. Comprehensive foodomics analysis reveals key lipids affect aroma generation in beef. Food Chem. 2024, 461, 140954. [Google Scholar] [CrossRef]
- Duan, S.; Tian, Z.; Zheng, X.; Tang, X.; Li, W.; Huang, X. Characterization of flavour components and identification of lipid flavour precursors in different cuts of pork by phospholipidomics. Food Chem. 2024, 458, 139422. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Escudero, R.; de Ávila, M.D.R.; Cambero, M.I.; López-Bote, C.J. Effect of fatty acid composition and positional distribution within the triglyceride on selected physical properties of dry-cured ham subcutaneous fat. Meat Sci. 2015, 103, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Jin, J. Evaluation of fatty acid distributions and triacylglycerol species in sow milk and commercial piglet formulas: A comparative study based on fat sources and lactation Sstages. Animals 2022, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Kildahl-Andersen, G.; Gjerlaug-Enger, E.; Rise, F.; Haug, A.; Egelandsdal, B. Quantification of fatty acids and their regioisomeric distribution in triacylglycerols from porcine and bovine sources using 13 C NMR Spectroscopy. Lipids 2021, 56, 111–122. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Xiao, L.; Sun, Q.; Wu, F.; Liu, G.; Wang, Y.; Pan, Y.; Wang, Q. Multi-omics reveals the effect of crossbreeding on some precursors of flavor and nutritional quality of pork. Foods 2023, 12, 3237. [Google Scholar] [CrossRef]
- Enomoto, H.; Furukawa, T. Unique distribution of diacyl-, alkylacyl-, and alkenylacyl-phosphatidylcholine species visualized in pork chop tissues by Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging. Foods 2020, 9, 205. [Google Scholar] [CrossRef]
- Cikes, D.; Elsayad, K.; Sezgin, E. PCYT2-regulated lipid biosynthesis is critical to muscle health and ageing. Nat. Metab. 2023, 5, 495–515. [Google Scholar] [CrossRef]
- Weir, J.M.; Wong, G.; Barlow, C.K.; Greeve, M.A.; Kowalczyk, A.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Jowett, J.B.; Shaw, J.; et al. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013, 54, 2898–2908. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Weir, J.M.; Greeve, M.A.; MacIntosh, G.L.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Kowalczyk, A.; et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS ONE 2013, 8, e74341. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoon, Y.C. Adipocyte lysoplasmalogenase TMEM86A regulates plasmalogen homeostasis and protein kinase A-dependent energy metabolism. Nat. Commun. 2022, 13, 4084. [Google Scholar] [CrossRef]
- Tang, G.; Ma, C.; Li, L.; Zhang, S.; Li, F.; Wu, J.; Yin, Y.; Zhu, Q.; Liang, Y.; Wang, R.; et al. PITPNC1 promotes the thermogenesis of brown adipose tissue under acute cold exposure. Sci. China Life Sci. 2022, 65, 2287–2300. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, X.C. Identification of important odorants derived from phosphatidylethanolamine species in steamed male Eriocheir sinensis hepatopancreas in model systems. Food Chem. 2019, 286, 491–499. [Google Scholar] [CrossRef]
- M’barek, K.B.; Ajjaji, D.; Chorlay, A.; Vanni, S.; Forêt, L.; Thiam, A.R. ER membrane phospholipids and surface tension control cellular lipid droplet formation. Dev. Cell 2017, 41, 591–604.e7. [Google Scholar]
- Li, J.; Yang, Y.; Zhan, T.; Zhao, Q.; Zhang, J.; Ao, X.; He, J.; Zhou, J.; Tang, C. Effect of slaughter weight on carcass characteristics, meat quality, and lipidomics profiling in longissimus thoracis of finishing pigs. LWT 2021, 140, 110705. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Z.; Lai, C. Significance of increasing n-3 PUFA content in pork on human health. Crit. Rev. Food Sci. Nutr. 2016, 56, 858–870. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, L.; Zhang, J.; Liu, X.; Zhang, Y.; Cai, L.; Zhang, W.; Cui, L.; Yang, J.; Ji, J.; et al. A large-scale comparison of meat quality and intramuscular fatty acid composition among three Chinese indigenous pig breeds. Meat Sci. 2020, 168, 108182. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Zotor, F.B. n-3 Fatty acids and risk for fatal coronary disease. Proc. Nutr. Soc. 2019, 78, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef]
- Kruger, M.C.; Coetzee, M.; Haag, M.; Weiler, H. Long-chain polyunsaturated fatty acids: Selected mechanisms of action on bone. Prog. Lipid Res. 2010, 49, 438–449. [Google Scholar] [CrossRef]
- Xu, H.E.; Lambert, M.H.; Montana, V.G.; Parks, D.J.; Blanchard, S.G.; Brown, P.J.; Sternbach, D.D.; Lehmann, J.M.; Wisely, G.B.; Willson, T.M.; et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell 1999, 3, 397–403. [Google Scholar] [PubMed]
- Casado-Díaz, A.; Santiago-Mora, R.; Dorado, G.; Quesada-Gómez, J.M. The omega-6 arachidonic fatty acid, but not the omega-3 fatty acids, inhibits osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: Potential implication in osteoporosis. Osteoporos. Int. 2013, 24, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, J.; Marnett, L.J.; Chacos, N.; Prough, R.A.; Estabrook, R.W. Cytochrome P-450-dependent oxygenation of arachidonic acid to hydroxyicosatetraenoic acids. Proc. Natl. Acad. Sci. USA 1982, 79, 767–770. [Google Scholar] [PubMed]
- Xu, X.; Zhang, X.A.; Wang, D.W. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv. Drug Deliv. Rev. 2011, 63, 597–609. [Google Scholar] [CrossRef]
- Gao, J.; Sun, L. Characterization of meat metabolites and lipids in Shanghai local pig breeds revealed by LC-MS-Based Method. Foods 2024, 13, 2327. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Li, W.; Yang, S.; Lv, Q.; Yang, J.; Sun, D.; Yang, G.; Zhao, Y.; Zhang, W.; Li, M.; et al. Integrated Multi-Tissue Lipidomics and Transcriptomics Reveal Differences in Lipid Composition Between Mashen and Duroc × (Landrace × Yorkshire) Pigs. Animals 2025, 15, 1280. https://doi.org/10.3390/ani15091280
Shi M, Li W, Yang S, Lv Q, Yang J, Sun D, Yang G, Zhao Y, Zhang W, Li M, et al. Integrated Multi-Tissue Lipidomics and Transcriptomics Reveal Differences in Lipid Composition Between Mashen and Duroc × (Landrace × Yorkshire) Pigs. Animals. 2025; 15(9):1280. https://doi.org/10.3390/ani15091280
Chicago/Turabian StyleShi, Mingyue, Wenxia Li, Shuai Yang, Qipin Lv, Jingxian Yang, Di Sun, Guanqing Yang, Yan Zhao, Wanfeng Zhang, Meng Li, and et al. 2025. "Integrated Multi-Tissue Lipidomics and Transcriptomics Reveal Differences in Lipid Composition Between Mashen and Duroc × (Landrace × Yorkshire) Pigs" Animals 15, no. 9: 1280. https://doi.org/10.3390/ani15091280
APA StyleShi, M., Li, W., Yang, S., Lv, Q., Yang, J., Sun, D., Yang, G., Zhao, Y., Zhang, W., Li, M., Yang, Y., Cai, C., Gao, P., Guo, X., Li, B., & Cao, G. (2025). Integrated Multi-Tissue Lipidomics and Transcriptomics Reveal Differences in Lipid Composition Between Mashen and Duroc × (Landrace × Yorkshire) Pigs. Animals, 15(9), 1280. https://doi.org/10.3390/ani15091280




