Effects of Postpartal Relative Body Weight Change on Production Performance, Serum Biomarkers, and Fecal Microbiota in Multiparous Holstein Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experimental Design
2.3. Diets and Feeding Management
2.4. Sampling and Measurement
2.5. DNA Extraction, PCR Amplification, and Sequencing
2.6. Sequencing Data Processing and Analysis
2.7. Statistical Analysis
3. Results
3.1. Production Performance
3.2. Serum Biomarkers
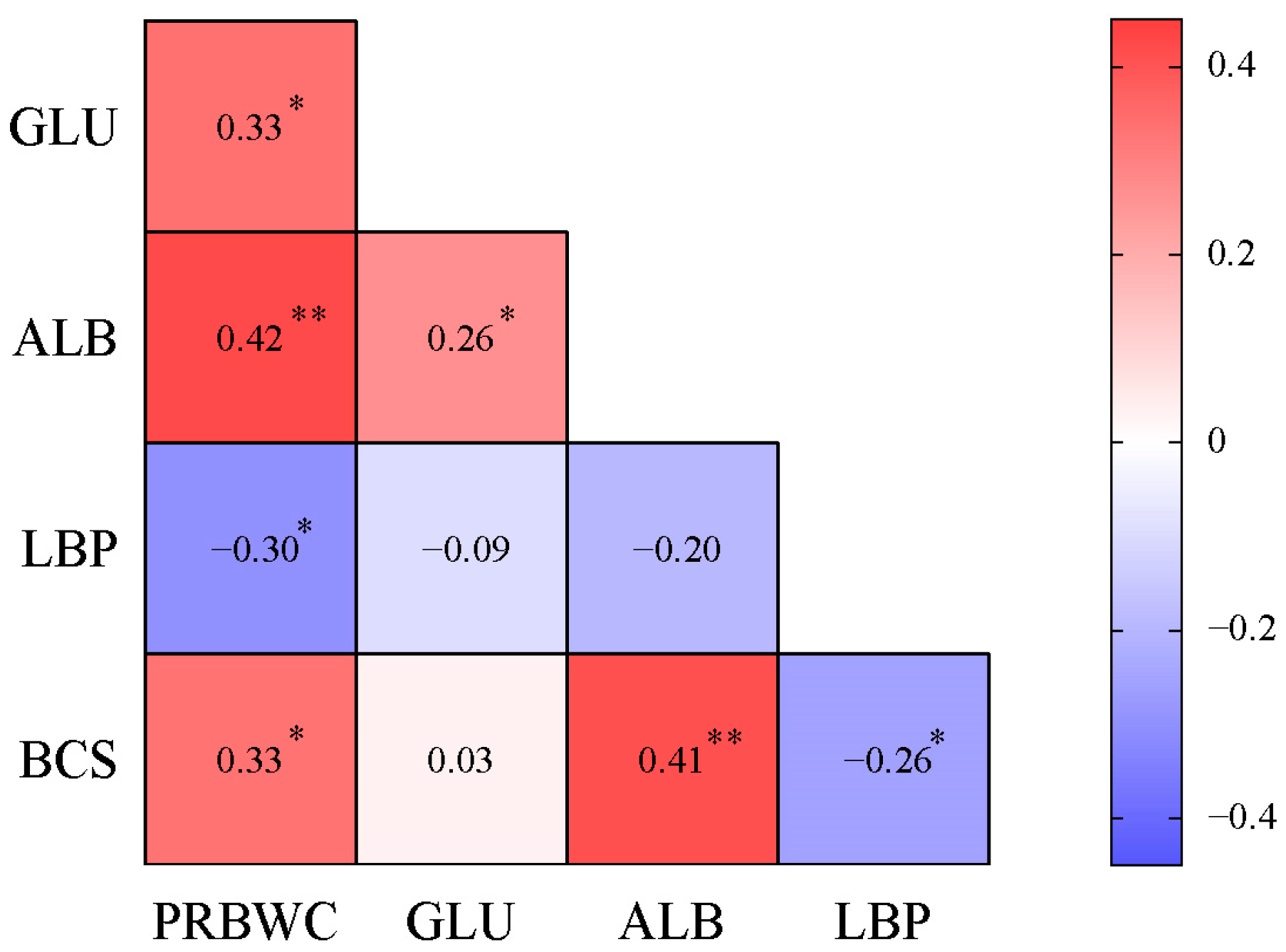
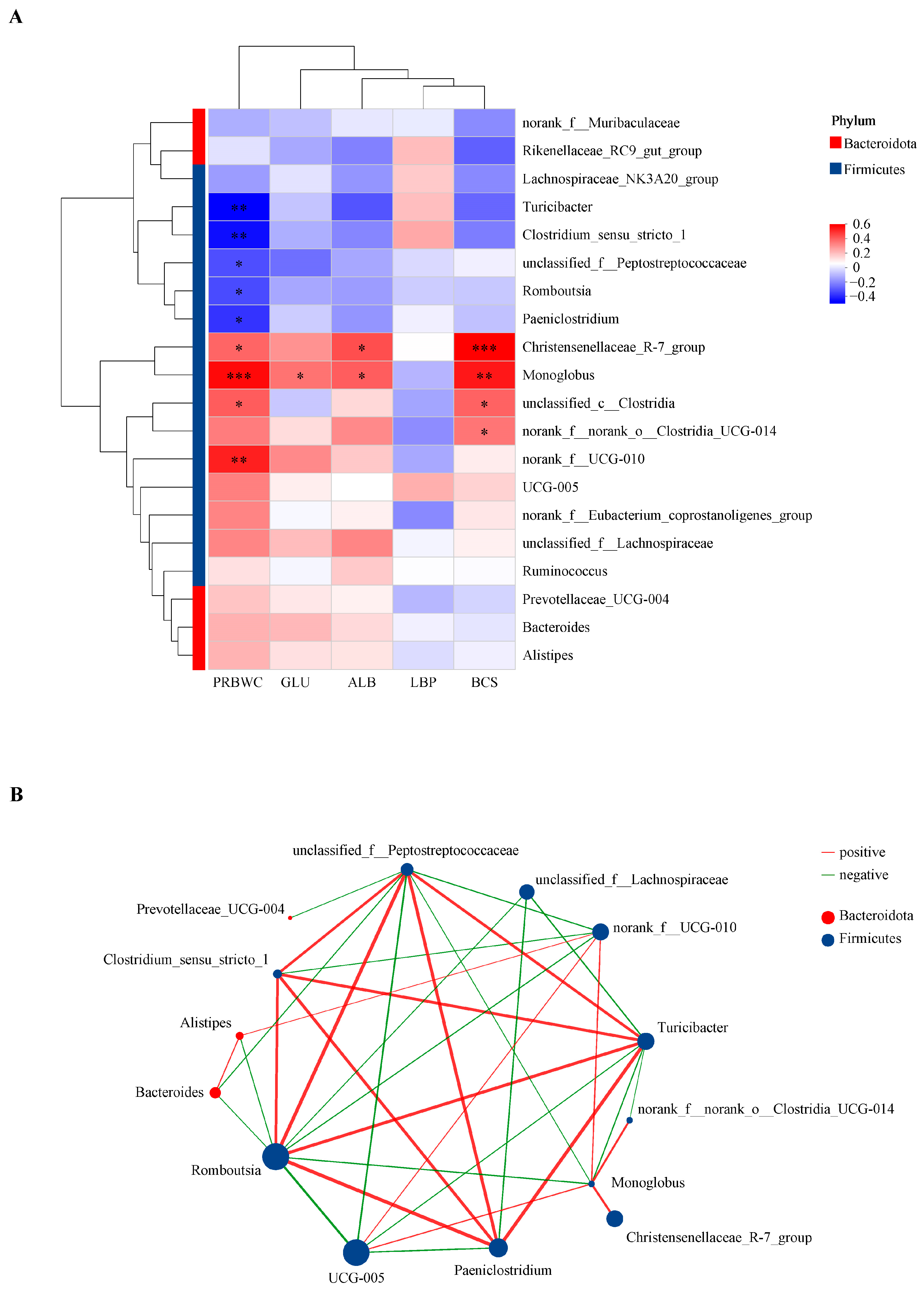
3.3. Correlation Matrix of PRBWC and Intergroup Differential Indicators on Day 21
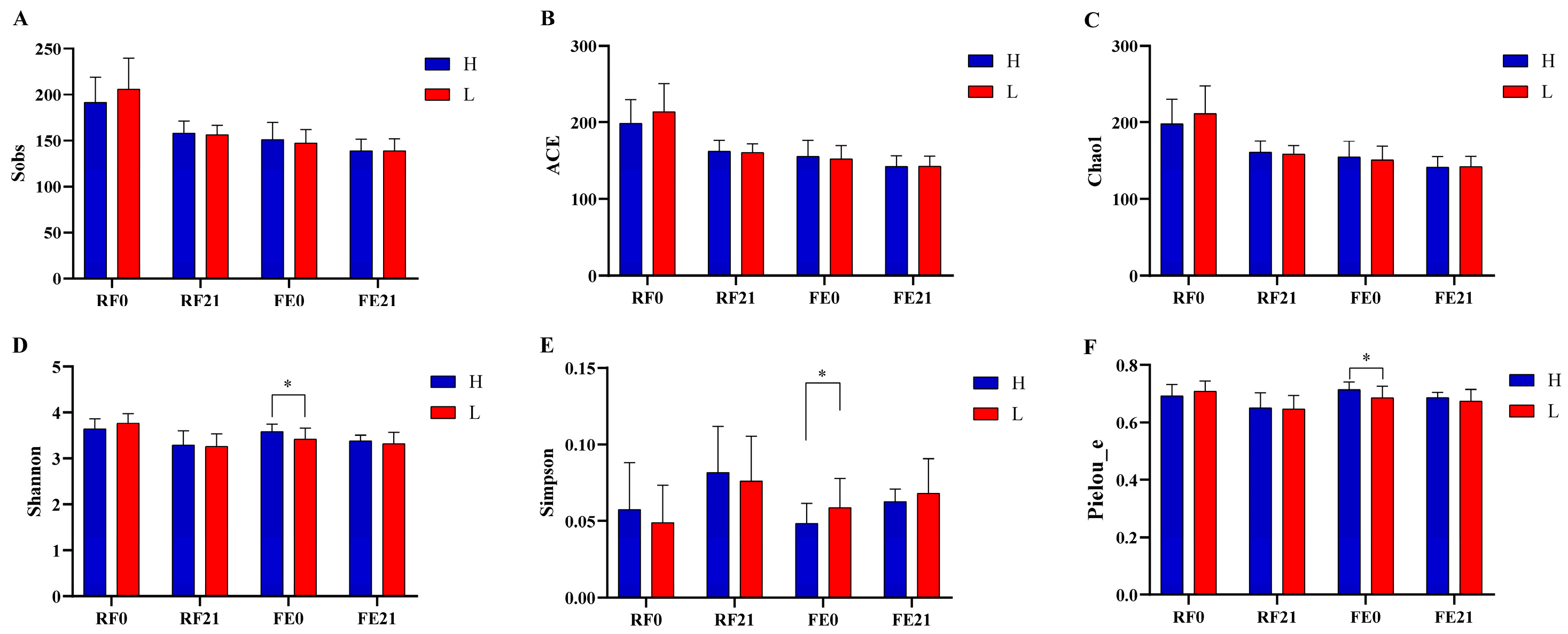
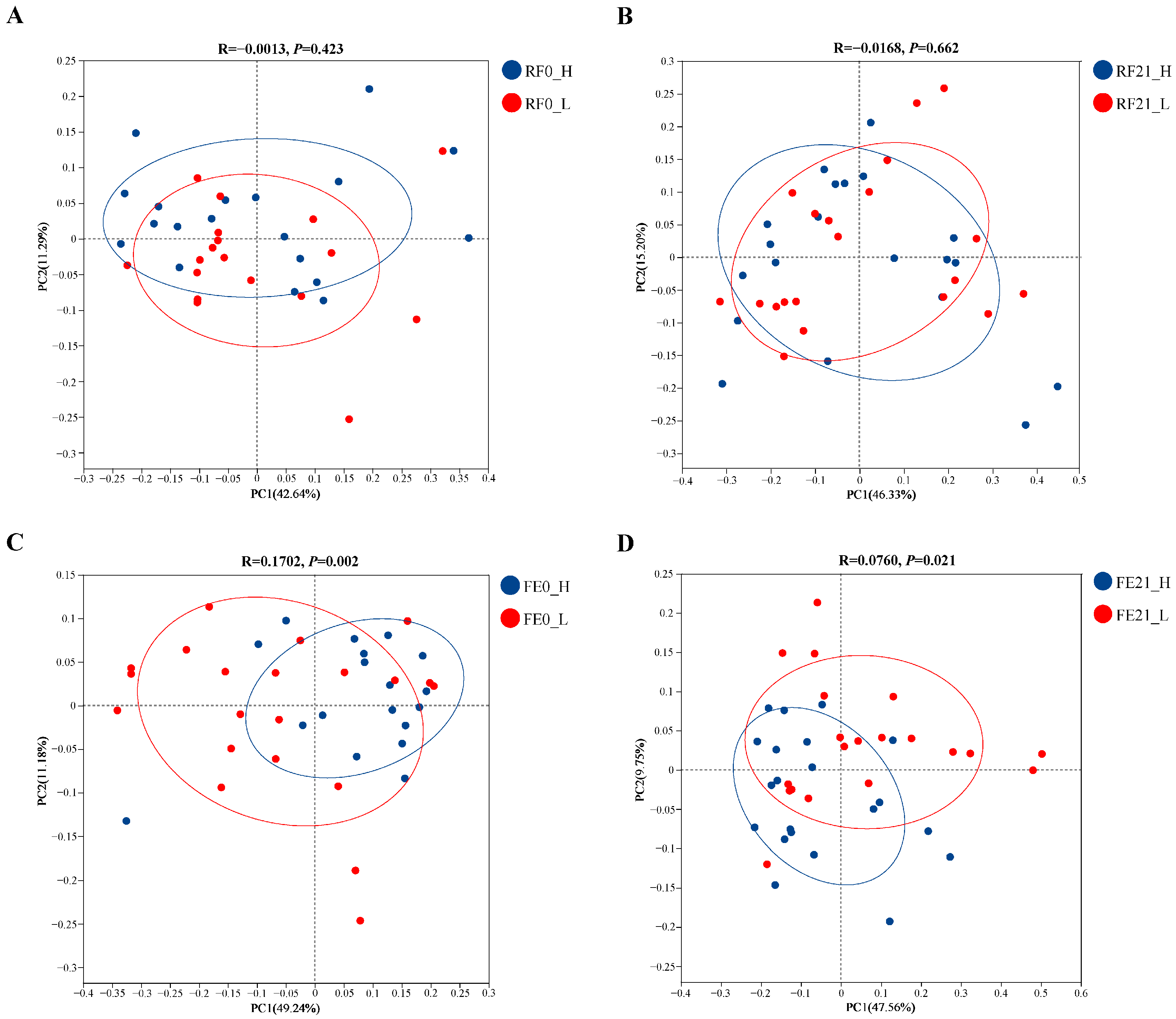
3.4. Intergroup Diversity Analyses at Diverse Gastrointestinal Sites on Different Days
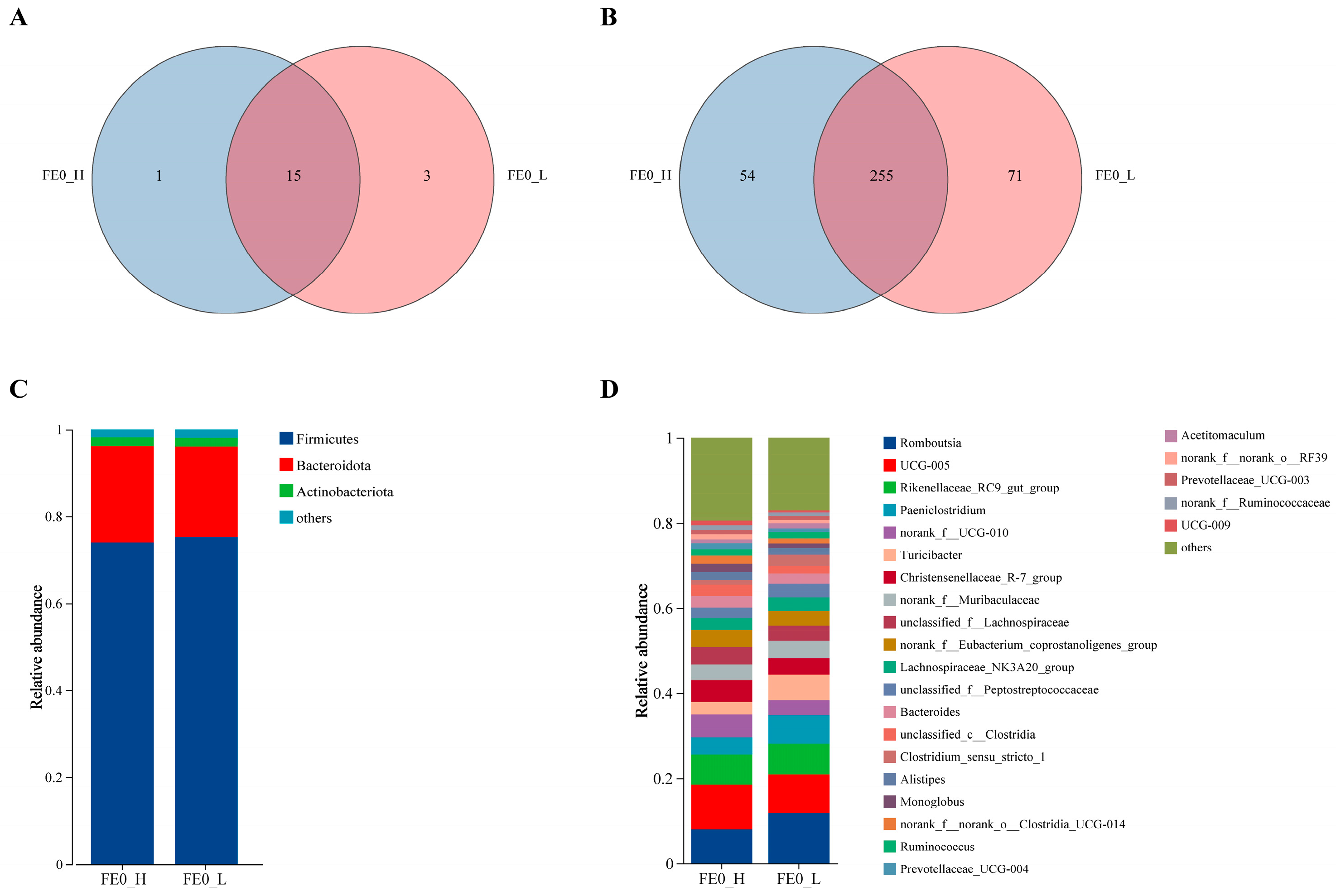
3.5. Bacterial Richness and Structural Composition of FE0
3.6. Intergroup Differential Bacterial Genera of FE0
3.7. Correlations Between FE0 Bacterial Genera and Intergroup Differential Indicators on Day 21
3.8. Functional Profiling Toward Bacteria of FE0
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martens, H. Invited Review: Increasing Milk Yield and Negative Energy Balance: A Gordian Knot for Dairy Cows? Animals 2023, 13, 3097. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Erdmann, S.; Derno, M.; Schäff, C.T.; Börner, S.; Kautzsch, U.; Kuhla, B.; Hammon, H.M.; Tuchscherer, A.; Röntgen, M. Comparative analyses of estimated and calorimetrically determined energy balance in high-yielding dairy cows. J. Dairy Sci. 2019, 102, 4002–4013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, G.; Wang, H.; Li, X.; Wang, Z. Detection of Subclinical Ketosis in Dairy Cows. Pak. Vet. J. 2012, 32, 156–160. [Google Scholar]
- Hady, P.J.; Domecq, J.J.; Kaneene, J.B. Frequency and Precision of Body Condition Scoring in Dairy Cattle. J. Dairy Sci. 1994, 77, 1543–1547. [Google Scholar] [CrossRef]
- Ghaffari, M.H.; Sadri, H.; Sauerwein, H. Invited review: Assessment of body condition score and body fat reserves in relation to insulin sensitivity and metabolic phenotyping in dairy cows. J. Dairy Sci. 2023, 106, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.A.; Dolgov, E.P.; Yakusheva, L.I.; Kuzminova, E.V.; Semenenko, M.P.; Kovaluk, N.V.; Semenenko, K.A. Features of free radical oxidation of lipids in cows with fat liver dystrophy. IOP Conf. Ser. Earth Environ. Sci. 2020, 421, 52037. [Google Scholar] [CrossRef]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef]
- Portune, K.J.; Benítez-Páez, A.; Del Pulgar, E.M.G.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet, and obesity-related disorders—The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef]
- Byndloss, M.; Devkota, S.; Duca, F.; Niess, J.H.; Nieuwdorp, M.; Orho-Melander, M.; Sanz, Y.; Tremaroli, V.; Zhao, L. The Gut Microbiota and Diabetes: Research, Translation, and Clinical Applications—2023 Diabetes, Diabetes Care, and Diabetologia Expert Forum. Diabetes Care 2024, 47, 1491. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, S.F.; Smith, B.J.; Hein, R.; Roller, B.R.K.; Schmidt, T.M. rrnDB: Improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 2015, 43, D593–D598. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2014, 8, 952. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Steen, A.; Grønstøl, H.; Torjesen, P.A. Glucose and Insulin Responses to Glucagon Injection in Dairy Cows with Ketotis and Fatty Live. J. Vet. Med. Ser. A 1997, 44, 521–530. [Google Scholar] [CrossRef]
- Borchardt, S.; Staufenbiel, R. Evaluation of the use of nonesterified fatty acids and β-hydroxybutyrate concentrations in pooled serum samples for herd-based detection of subclinical ketosis in dairy cows during the first week after parturition. J. Am. Vet. Med. Assoc. 2012, 240, 1003–1011. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; McBride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, P.; VandeHaar, M.J. Symposium review: The impact of absorbed nutrients on energy partitioning throughout lactation. J. Dairy Sci. 2023, 106, 2167–2180. [Google Scholar] [CrossRef]
- Lefebvre, R.; Faverdin, P.; Barbey, S.; Jurquet, J.; Tribout, T.; Boichard, D.; Martin, P. Association between body condition genomic values and feed intake, milk production, and body weight in French Holstein cows. J. Dairy Sci. 2023, 106, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Delelesse, G.D.; Lu, M.; Fang, W.; Todd, R.C.; Dengpan, B. Pre-calving energy density and rumen protected lysine impacted blood metabolites and biomarkers of liver functions in dairy cows during the transition period. Trop. Anim. Health Prod. 2023, 55, 273. [Google Scholar] [CrossRef]
- Bobbo, T.; Fiore, E.; Gianesella, M.; Morgante, M.; Gallo, L.; Ruegg, P.L.; Bittante, G.; Cecchinato, A. Variation in blood serum proteins and association with somatic cell count in dairy cattle from multi-breed herds. Animal 2017, 11, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Grindler, S.; Kenéz, Á.; Dänicke, S.; Frahm, J.; Huber, K. Changes of the liver metabolome following an intravenous lipopolysaccharide injection in Holstein cows supplemented with dietary carnitine. J. Anim. Sci. Biotechnol. 2022, 13, 94. [Google Scholar] [CrossRef]
- Osorio, J.S.; Trevisi, E.; Ji, P.; Drackley, J.K.; Luchini, D.; Bertoni, G.; Loor, J.J. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 2014, 97, 7437–7450. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Han, X.; Bionaz, M. Effects of Inflammatory Conditions on Liver Activity in Puerperium Period and Consequences for Performance in Dairy Cows. J. Dairy Sci. 2008, 91, 3300–3310. [Google Scholar] [CrossRef]
- Bionaz, M.; Trevisi, E.; Calamari, L.; Librandi, F.; Ferrari, A.; Bertoni, G. Plasma Paraoxonase, Health, Inflammatory Conditions, and Liver Function in Transition Dairy Cows. J. Dairy Sci. 2007, 90, 1740–1750. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Contreras, G.A. Symposium review: Mechanistic insights into adipose tissue inflammation and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2022, 105, 3670–3686. [Google Scholar] [CrossRef]
- Costa-Roura, S.; Villalba, D.; Balcells, J.; De la Fuente, G. First Steps into Ruminal Microbiota Robustness. Animals 2022, 12, 2366. [Google Scholar] [CrossRef] [PubMed]
- Tardón, D.C.; Hoffmann, C.; Santos, F.C.R.; Decaris, N.; Pinheiro, F.A.; Queiroz, L.L.; Hurley, D.J.; Gomes, V. Relationships among Indicators of Metabolism, Mammary Health and the Microbiomes of Periparturient Holstein Cows. Animals 2021, 12, 3. [Google Scholar] [CrossRef]
- Wang, S.; Tang, W.; Jiang, T.; Wang, R.; Zhang, R.; Ou, J.; Wang, Q.; Cheng, X.; Ren, C.; Chen, J.; et al. Effect of Dietary Concentrate-to-Forage Ratios During the Cold Season on Slaughter Performance, Meat Quality, Rumen Fermentation and Gut Microbiota of Tibetan Sheep. Animals 2024, 14, 3305. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, W.; Zhou, S.; Ma, N.; Wang, Y.; Huang, J.; Shen, X.; Chang, G. A high-concentrate diet induces colonic inflammation and barrier damage in Hu sheep. J. Dairy Sci. 2023, 106, 9644–9662. [Google Scholar] [CrossRef]
- Wang, K.; Ren, A.; Zheng, M.; Jiao, J.; Yan, Q.; Zhou, C.; Tan, Z. Diet with a High Proportion of Rice Alters Profiles and Potential Function of Digesta-Associated Microbiota in the Ileum of Goats. Animals 2020, 10, 1261. [Google Scholar] [CrossRef]
- Tao, S.; Tian, P.; Luo, Y.; Tian, J.; Hua, C.; Geng, Y.; Cong, R.; Ni, Y.; Zhao, R. Microbiome-Metabolome Responses to a High-Grain Diet Associated with the Hind-Gut Health of Goats. Front. Microbiol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Liu, J.; Xu, T.; Zhu, W.; Mao, S. High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats. Br. J. Nutr. 2014, 112, 416–427. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, Y.; Liu, G.; Yi, G.; Liu, H.; Zhang, T.; Tong, M. Protective effect of a newly probiotic Lactobacillus reuteri LY2-2 on DSS-induced colitis. Eur. J. Nutr. 2025, 64, 5. [Google Scholar] [CrossRef]
- Sun, M.; Ji, W.; Ye, H.; Cai, Y.; Yun, Y.; Wei, X.; Wang, C.; Mao, H. Sodium butyrate administration improves intestinal development of suckling lambs. J. Anim. Sci. 2024, 102, skae028. [Google Scholar] [CrossRef] [PubMed]


| Item 1 | Group 2 | SEM | p-Value | |
|---|---|---|---|---|
| H | L | |||
| BW0 (kg) | 712.0 | 735.5 | 10.60 | 0.27 |
| BW21 (kg) | 689.9 | 631.1 | 10.76 | <0.01 |
| BCS0 (point) | 3.32 | 3.18 | 0.04 | 0.09 |
| BCS21 (point) | 3.18 | 2.98 | 0.04 | <0.01 |
| ADMY21 (kg/d) | 34.82 | 34.97 | 0.91 | 0.93 |
| ADMY147 (kg/d) | 40.31 | 43.35 | 0.94 | 0.11 |
| Item 1 | Group 2 | SEM | p-Value | |
|---|---|---|---|---|
| H | L | |||
| BHBA (μmol/L) | 607.9 | 651.8 | 19.03 | 0.25 |
| BUN (mmol/L) | 5.05 | 4.51 | 0.15 | 0.07 |
| GLU (mmol/L) | 3.29 | 2.53 | 0.19 | 0.05 |
| IGF-1 (pg/mL) | 150.8 | 156.1 | 2.81 | 0.35 |
| NEFA (mmol/L) | 0.93 | 1.02 | 0.03 | 0.18 |
| TC (mmol/L) | 3.83 | 3.53 | 0.13 | 0.23 |
| ALB (g/L) | 33.95 | 32.07 | 0.34 | <0.01 |
| HPT (μg/mL) | 312.8 | 335.8 | 7.28 | 0.11 |
| TBIL (umol/L) | 3.41 | 4.02 | 0.15 | 0.45 |
| TP (g/L) | 73.74 | 75.31 | 0.56 | 0.17 |
| GSH-Px (U/mL) | 189.6 | 185.7 | 6.18 | 0.76 |
| MDA (nmol/mL) | 2.99 | 2.72 | 0.15 | 0.38 |
| SOD (U/mL) | 158.4 | 165.3 | 2.31 | 0.14 |
| T-AOC (mmol/L) | 0.26 | 0.27 | 0.01 | 0.63 |
| IL-1β (pg/mL) | 32.42 | 44.7 | 4.46 | 0.17 |
| IL-6 (pg/mL) | 94.64 | 69.21 | 10.87 | 0.25 |
| LBP (μg/mL) | 126.2 | 157 | 7.12 | 0.03 |
| SAA (μg/mL) | 208.2 | 208.8 | 1.79 | 0.87 |
| TNF-α (pg/mL) | 136.8 | 89.55 | 14.50 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xu, Y.; Chen, T.; Gao, D.; Wang, J.; Zhuang, Y.; Jiang, W.; Hou, G.; Liu, S.; Li, S.; et al. Effects of Postpartal Relative Body Weight Change on Production Performance, Serum Biomarkers, and Fecal Microbiota in Multiparous Holstein Cows. Animals 2025, 15, 1252. https://doi.org/10.3390/ani15091252
Zhang S, Xu Y, Chen T, Gao D, Wang J, Zhuang Y, Jiang W, Hou G, Liu S, Li S, et al. Effects of Postpartal Relative Body Weight Change on Production Performance, Serum Biomarkers, and Fecal Microbiota in Multiparous Holstein Cows. Animals. 2025; 15(9):1252. https://doi.org/10.3390/ani15091252
Chicago/Turabian StyleZhang, Siyuan, Yiming Xu, Tianyu Chen, Duo Gao, Jingjun Wang, Yimin Zhuang, Wen Jiang, Guobin Hou, Shuai Liu, Shengli Li, and et al. 2025. "Effects of Postpartal Relative Body Weight Change on Production Performance, Serum Biomarkers, and Fecal Microbiota in Multiparous Holstein Cows" Animals 15, no. 9: 1252. https://doi.org/10.3390/ani15091252
APA StyleZhang, S., Xu, Y., Chen, T., Gao, D., Wang, J., Zhuang, Y., Jiang, W., Hou, G., Liu, S., Li, S., Shao, W., & Cao, Z. (2025). Effects of Postpartal Relative Body Weight Change on Production Performance, Serum Biomarkers, and Fecal Microbiota in Multiparous Holstein Cows. Animals, 15(9), 1252. https://doi.org/10.3390/ani15091252






