Establishment and Maintenance of Feline Pregnancy—A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Endocrinology of Early Pregnancy—What Is Special in Cats
3. Ovarian Activity During Pregnancy
3.1. Follicle Formation and AMH Secretion
3.2. Corpus Luteum Formation and Function
4. Placental Hormone Secretion
5. Prerequisites for Feline Pregnancy Establishment
Effect of Ovariectomy and Hormone Disruptors
6. Physiological Mechanisms in the Oviduct and Uterus—What Is Different from Dogs
7. Physiological Biomolecular Mechanisms at the Uterine Level
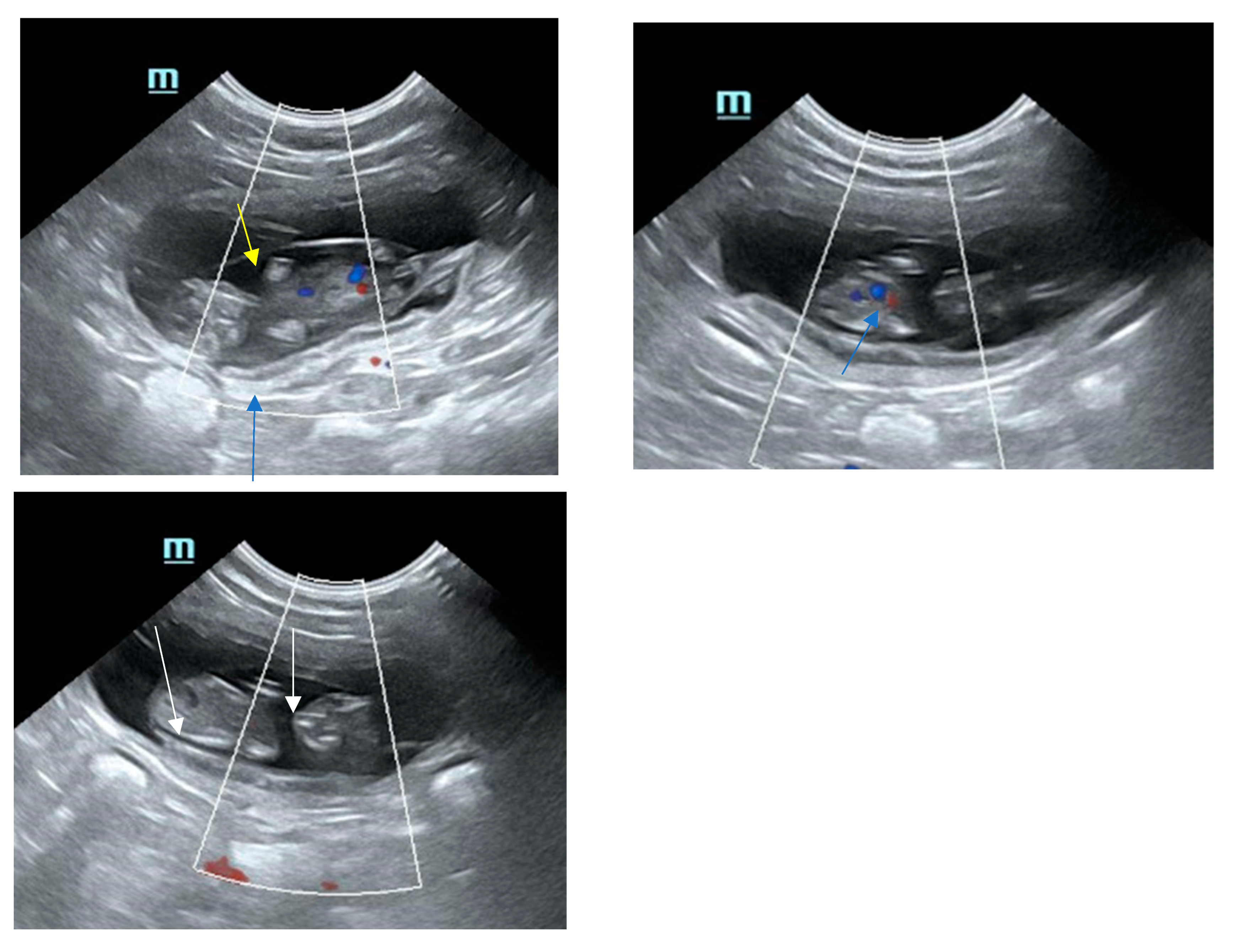
8. Sonographical Monitoring of the Developing Pregnancy
9. Outlook
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, S.D.; Kustritz, M.V.R.; Olson, P.N.S. (Eds.) Canine and Feline Theriogenology: The Feline Estrous Cycle, 1st ed.; Saunders: Philadelphia, PA, USA, 2001. [Google Scholar]
- Verhage, H.G.; Beamer, N.B.; Brenner, R.M. Plasma levels of estradiol and progesterone in the cat during polyestrus, pregnancy and pseudopregnancy. Biol. Reprod. 1976, 14, 579–585. [Google Scholar] [CrossRef]
- Concannon, P.; Hodgson, B.; Lein, D. Reflex LH release in estrous cats following single and multiple copulations. Biol. Reprod. 1980, 23, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Aurich, C.; Reifinger, M.; Aurich, J. Spontaneous ovulation in cats-Uterine findings and correlations with animal weight and age. Anim. Reprod. Sci. 2019, 209, 106167. [Google Scholar] [CrossRef]
- Zambelli, D.; Castagnetti, C.; Belluzzi, S.; Paladini, C. Correlation between fetal age and ultrasonographic measurements during the second half of pregnancy in domestic cats (Felis catus). Theriogenology 2004, 62, 1430–1437. [Google Scholar] [CrossRef]
- Johnston, S.D.; Kustritz, M.V.R.; Olson, P.N.S. (Eds.) Canine and Feline Theriogenology: Feline Pregnancy, 1st ed.; Saunders: Philadelphia, PA, USA, 2001. [Google Scholar]
- Schmidt, P.M.; Chakraborty, P.K.; Wildt, D.E. Ovarian activity, circulating hormones and sexual behavior in the cat. II. Relationships during pregnancy, parturition, lactation and the postpartum estrus. Biol. Reprod. 1983, 28, 657–671. [Google Scholar] [CrossRef]
- Siemieniuch, M.J.; Jursza, E.; Szostek, A.Z.; Skarzynski, D.J.; Boos, A.; Kowalewski, M.P. Steroidogenic capacity of the placenta as a supplemental source of progesterone during pregnancy in domestic cats. Reprod. Biol. Endocrinol. 2012, 10, 89. [Google Scholar] [CrossRef]
- Watt, M.; Mohammadzadeh, P.; Pinsinski, E.; Hollinshead, F.K.; Bouma, G.J. Corticotropin releasing hormone is present in the feline placenta and maternal serum. Front. Endocrinol. 2023, 14, 1132743. [Google Scholar] [CrossRef]
- Papa, P.C.; Kowalewski, M.P. Factors affecting the fate of the canine corpus luteum: Potential contributors to pregnancy and non-pregnancy. Theriogenology 2020, 150, 339–346. [Google Scholar] [CrossRef]
- Amelkina, O.; Braun, B.C.; Dehnhard, M.; Jewgenow, K. The corpus luteum of the domestic cat: Histologic classification and intraluteal hormone profile. Theriogenology 2015, 83, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Amelkina, O.; Zschockelt, L.; Painer, J.; Serra, R.; Villaespesa, F.; Krause, E.; Jewgenow, K.; Braun, B.C. Progesterone, estrogen, and androgen receptors in the corpus luteum of the domestic cat, Iberian lynx (Lynx pardinus) and Eurasian lynx (Lynx lynx). Theriogenology 2016, 86, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.C.; Jewgenow, K. Role of sex steroids and prostaglandins during the luteal life cycle in domestic cats and lynxes. Domest. Anim. Endocrinol. 2022, 78, 106689. [Google Scholar] [CrossRef]
- Roth, T.L.; Munson, L.; Swanson, W.F.; Wildt, D.E. Histological characteristics of the uterine endometrium and corpus luteum during early embryogenesis and the relationship to embryonic mortality in the domestic cat. Biol. Reprod. 1995, 53, 1012–1021. [Google Scholar] [CrossRef]
- Gervasi, M.G.; Soler, A.J.; González-Fernández, L.; Alves, M.G.; Oliveira, P.F.; Martín-Hidalgo, D. Extracellular Vesicles, the Road toward the Improvement of ART Outcomes. Animals 2020, 10, 2171. [Google Scholar] [CrossRef]
- Segura-Benítez, M.; Carbajo-García, M.C.; Quiñonero, A.; de Los Santos, M.J.; Pellicer, A.; Cervelló, I.; Ferrero, H. Endometrial extracellular vesicles regulate processes related to embryo development and implantation in human blastocysts. Hum. Reprod. 2025, 40, 56–68. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, W.; Han, L.; Chen, J. Hormonal Trends During Reproductive Stages and Ultrasonographic Monitoring of Gestational Age in British Shorthair Cats. Vet. J. 2025, 106321. [Google Scholar] [CrossRef]
- Gültiken, N.; Yarim, M.; Aslan, S.; Gürler, H.; Yarim, G.F.; Tuncay, M.; İnal, S.; Schäfer-Somi, S. Expression of Anti-Müllerian Hormone and Its Type 2 Receptor in the Ovary of Pregnant and Cyclic Domestic Cats. Animals 2022, 12, 877. [Google Scholar] [CrossRef]
- Tsutsui, T.; Stabenfeldt, G.H. Biology of ovarian cycles, pregnancy and pseudopregnancy in the domestic cat. J. Reprod. Fertil. Suppl. 1993, 47, 29–35. [Google Scholar]
- Braun, B.C.; Vargas, A.; Jewgenow, K. The molecular detection of relaxin and its receptor RXFP1 in reproductive tissue of Felis catus and Lynx pardinus during pregnancy. Reproduction 2012, 143, 399–410. [Google Scholar] [CrossRef]
- Braun, B.C.; Zschockelt, L.; Dehnhard, M.; Jewgenow, K. Progesterone and estradiol in cat placenta--biosynthesis and tissue concentration. J. Steroid Biochem. Mol. Biol. 2012, 132, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Siemieniuch, M.J.; Jursza, E.; Szóstek, A.Z.; Zschockelt, L.; Boos, A.; Kowalewski, M.P. Placental origin of prostaglandin F2α in the domestic cat. Mediators Inflamm. 2014, 2014, 364787. [Google Scholar] [CrossRef] [PubMed]
- Wildt, D.E.; Chan, S.Y.; Seager, S.W.; Chakraborty, P.K. Ovarian activity, circulating hormones, and sexual behavior in the cat. I. Relationships during the coitus-induced luteal phase and the estrous period without mating. Biol. Reprod. 1981, 25, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Jost, A. Problems of fetal endocrinology: The gonadal and hypophyseal hormones. Recent Prog. Horm. Res. 1953, 8, 379–418. [Google Scholar]
- Durlinger, A.L.L.; Gruijters, M.J.G.; Kramer, P.; Karels, B.; Ingraham, H.A.; Nachtigal, M.W.; Uilenbroek, J.T.J.; Grootegoed, J.A.; Themmen, A.P.N. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 2002, 143, 1076–1084. [Google Scholar] [CrossRef]
- Durlinger, A.L.L.; Visser, J.A.; Themmen, A.P.N. Regulation of ovarian function: The role of anti-Müllerian hormone. Reproduction 2002, 124, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Ojha, K.; Whitehead, S.; Mason, H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Müllerian hormone type II receptor in single, isolated, human preantral follicles: Relevance to polycystic ovaries. J. Clin. Endocrinol. Metab. 2007, 92, 1034–1040. [Google Scholar] [CrossRef]
- Baarends, W.M.; Uilenbroek, J.T.; Kramer, P.; Hoogerbrugge, J.W.; van Leeuwen, E.C.; Themmen, A.P.; Grootegoed, J.A. Anti-müllerian hormone and anti-müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 1995, 136, 4951–4962. [Google Scholar] [CrossRef]
- Snoeck, F.; Sarrazin, S.; Wydooghe, E.; van Soom, A. Age and anti-Müllerian hormone levels predict the success of in vitro maturation of cat oocytes. Reprod. Domest. Anim. 2017, 52 (Suppl. S2), 98–102. [Google Scholar] [CrossRef]
- Stocker, W.A.; Olenick, L.; Maskey, S.; Skrombolas, D.; Luan, H.; Harrison, S.G.; Wilson, M.; Traas, A.; Heffernan, M.; Busfield, S.; et al. Gene therapy with feline anti-Müllerian hormone analogs disrupts folliculogenesis and induces pregnancy loss in female domestic cats. Nat. Commun. 2025, 16, 1668. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.-L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundström-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef]
- Lawler, D.F.; Johnston, S.D.; Hegstad, R.L.; Keltner, D.G.; Owens, S.F. Ovulation without cervical stimulation in domestic cats. J. Reprod. Fertil. Suppl. 1993, 47, 57–61. [Google Scholar]
- Paape, S.R.; Shille, V.M.; Seto, H.; Stabenfeldt, G.H. Luteal activity in the pseudopregnant cat. Biol. Reprod. 1975, 13, 470–474. [Google Scholar] [CrossRef]
- Verstegen, J.P.; Onclin, K.; Silva, L.D.; Donnay, I. Abortion induction in the cat using prostaglandin F2 alpha and a new anti-prolactinic agent, cabergoline. J. Reprod. Fertil. Suppl. 1993, 47, 411–417. [Google Scholar] [PubMed]
- Zschockelt, L.; Amelkina, O.; Siemieniuch, M.J.; Koster, S.; Jewgenow, K.; Braun, B.C. Corpora lutea of pregnant and pseudopregnant domestic cats reveal similar steroidogenic capacities during the luteal life span. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 373–381. [Google Scholar] [CrossRef]
- Zschockelt, L.; Amelkina, O.; Siemieniuch, M.J.; Kowalewski, M.P.; Dehnhard, M.; Jewgenow, K.; Braun, B.C. Synthesis and reception of prostaglandins in corpora lutea of domestic cat and lynx. Reproduction 2016, 152, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Agaoglu, O.K.; Agaoglu, A.R.; Guzeloglu, A.; Kurar, E.; Kayis, S.A.; Ozmen, O.; Schäfer-Somi, S.; Aslan, S. Expression of hypoxia-inducible factors and vascular endothelial growth factor during pregnancy in the feline uterus. Theriogenology 2015, 84, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Agaoglu, O.K.; Agaoglu, A.R.; Guzeloglu, A.; Aslan, S.; Kurar, E.; Kayis, S.A.; Schäfer-Somi, S. Gene expression profiles of some cytokines, growth factors, receptors, and enzymes (GM-CSF, IFNγ, MMP-2, IGF-II, EGF, TGF-β, IGF-IIR) during pregnancy in the cat uterus. Theriogenology 2016, 85, 638–644. [Google Scholar] [CrossRef]
- Boudreaux, C.E.; Chumbley, L.B.; Scott, V.L.; Wise, D.A.; Coats, K.S. Imbalance of placental regulatory T cell and Th17 cell population dynamics in the FIV-infected pregnant cat. Virol. J. 2012, 9, 88. [Google Scholar] [CrossRef]
- Paulson, E.E.; Comizzoli, P. Endometrial receptivity and embryo implantation in carnivores-commonalities and differences with other mammalian species. Biol. Reprod. 2021, 104, 771–783. [Google Scholar] [CrossRef]
- Tsutsui, T.; Suzuki, Y.; Toyonaga, M.; Oba, H.; Mizutani, T.; Hori, T. The role of the ovary for the maintenance of pregnancy in cats. Reprod. Domest. Anim. 2009, 44 (Suppl. S2), 120–124. [Google Scholar] [CrossRef]
- García Mitacek, M.C.; Stornelli, M.C.; Praderio, R.; Stornelli, M.A.; de La Sota, R.L. Efficacy of cloprostenol or aglepristone at 21–22 and 35–38 days of gestation for pregnancy termination in queens. Reprod. Domest. Anim. 2012, 47 (Suppl. S6), 200–203. [Google Scholar] [CrossRef]
- García Mitacek, M.C.; Bonaura, M.C.; Praderio, R.G.; Nuñez Favre, R.; de La Sota, R.L.; Stornelli, M.A. Progesterone and ultrasonographic changes during aglepristone or cloprosternol treatment in queens at 21 to 22 or 35 to 38 days of pregnancy. Theriogenology 2017, 88, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Nachreiner, R.F.; Marple, D.N. Termination of pregnancy in cats with prostaglandin F2 alpha. Prostaglandins 1974, 7, 303–308. [Google Scholar] [CrossRef]
- Georgiev, P.; Bostedt, H.; Goericke-Pesch, S.; Dimitrov, M.; Petkov, P.; Stojanthev, K.; Tsoneva, V.; Wehrend, A. Induction of abortion with aglepristone in cats on day 45 and 46 after mating. Reprod. Domest. Anim. 2010, 45, e161–e167. [Google Scholar] [CrossRef]
- Fieni, F.; Martal, J.; Marnet, P.G.; Siliart, B.; Guittot, F. Clinical, biological and hormonal study of mid-pregnancy termination in cats with aglepristone. Theriogenology 2006, 66, 1721–1728. [Google Scholar] [CrossRef]
- Erünal-Maral, N.; Aslan, S.; Findik, M.; Yüksel, N.; Handler, J.; Arbeiter, K. Induction of abortion in queens by administration of cabergoline (Galastop) solely or in combination with the PGF2alpha analogue Alfaprostol (Gabbrostim). Theriogenology 2004, 61, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Jöchle, M.; Jöchle, W. Interruption of pregnancies in the queen with a prolactin inhibitor, cabergoline. Riv. Zootec. Vet. 1988, 16, 85–88. [Google Scholar]
- Onclin, K.; Verstegen, J. Termination of pregnancy in cats using a combination of cabergoline, a new dopamine agonist, and a synthetic PGF2 alpha, cloprostenol. J. Reprod. Fertil. Suppl. 1997, 51, 259–263. [Google Scholar]
- Harris, E.A.; Stephens, K.K.; Winuthayanon, W. Extracellular Vesicles and the Oviduct Function. Int. J. Mol. Sci. 2020, 21, 8280. [Google Scholar] [CrossRef]
- Ferraz, M.d.A.M.M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef]
- O’Neil, E.V.; Burns, G.W.; Spencer, T.E. Extracellular vesicles: Novel regulators of conceptus-uterine interactions? Theriogenology 2020, 150, 106–112. [Google Scholar] [CrossRef]
- Leiser, R.; Koob, B. Development and characteristics of placentation in a carnivore, the domestic cat. J. Exp. Zool. 1993, 266, 642–656. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Mavrogianis, P.A.; Verhage, H.G. Changes in endometrial and placental protein synthesis and morphology during pregnancy and pseudopregnancy in the cat. Biol. Reprod. 1991, 44, 345–356. [Google Scholar] [CrossRef]
- Miglino, M.A.; Ambrósio, C.E.; dos Santos Martins, D.; Wenceslau, C.V.; Pfarrer, C.; Leiser, R. The carnivore pregnancy: The development of the embryo and fetal membranes. Theriogenology 2006, 66, 1699–1702. [Google Scholar] [CrossRef]
- Wilsterman, K.; Bao, X.; Estrada, A.D.; Comizzoli, P.; Bentley, G.E. Sex steroids influence organizational but not functional decidualization of feline endometrial cells in a 3D culture system†. Biol. Reprod. 2019, 101, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Kautz, E.; de Carvalho Papa, P.; Reichler, I.M.; Gram, A.; Boos, A.; Kowalewski, M.P. In vitro decidualisation of canine uterine stromal cells. Reprod. Biol. Endocrinol. 2015, 13, 85. [Google Scholar] [CrossRef]
- Schäfer-Somi, S.; Sabitzer, S.; Klein, D.; Reinbacher, E.; Kanca, H.; Beceriklisoy, H.B.; Aksoy, O.A.; Kucukaslan, I.; Macun, H.C.; Aslan, S. Vascular endothelial (VEGF) and epithelial growth factor (EGF) as well as platelet-activating factor (PAF) and receptors are expressed in the early pregnant canine uterus. Reprod. Domest. Anim. 2013, 48, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Agaoglu, A.R.; Korkmaz Agaoglu, Ö.; Kurar, E.; Güzeloglu, A.; Kayis, S.A.; Atli, M.O.; Schäfer-Somi, S.; Aslan, A. Expression of platelet activating factors and its receptor in cat uterus during early pregnancy. Ankara Üniv Vet. Fak. Derg. 2013, 60, 47–52. [Google Scholar] [CrossRef]
- Korkmaz Ağaoğlu, Ö.; Ağaoğlu, A.R.; Özmen, Ö.; Saatci, M.; Schäfer-Somi, S.; Aslan, S. Expression of the insulin-like growth factor (IGF) gene family in feline uterus during pregnancy. Biotech. Histochem. 2021, 96, 439–449. [Google Scholar] [CrossRef]
- Gudenschwager-Basso, E.K.; Frydman, G.; Weerakoon, S.; Andargachew, H.; Piltaver, C.M.; Huckle, W.R. Morphological evaluation of the feline placenta correlates with gene expression of vascular growth factors and receptors†. Biol. Reprod. 2024, 110, 569–582. [Google Scholar] [CrossRef]
- Zambelli, D.; Castagnetti, C.; Belluzzi, S.; Bassi, S. Correlation between the age of the conceptus and various ultrasonographic measurements during the first 30 days of pregnancy in domestic cats (Felis catus). Theriogenology 2002, 57, 1981–1987. [Google Scholar] [CrossRef]
- Michel, E.; Spörri, M.; Ohlerth, S.; Reichler, I. Prediction of parturition date in the bitch and queen. Reprod. Domest. Anim. 2011, 46, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.P.; Nyland, T.G.; Tsutsui, T. Pregnancy diagnosis with ultrasound in the domestic cat. Vet. Radiol. 1986, 27, 109–114. [Google Scholar] [CrossRef]
- Zambelli, D.; Prati, F. Ultrasonography for pregnancy diagnosis and evaluation in queens. Theriogenology 2006, 66, 135–144. [Google Scholar] [CrossRef] [PubMed]
- García Mitacek, M.C.; Stornelli, M.C.; Praderio, R.G.; de La Sota, R.L.; Stornelli, M.A. Ultrasonographic and progesterone changes during Days 21 to 63 of pregnancy in queens. Theriogenology 2015, 84, 1131–1141. [Google Scholar] [CrossRef]
- Lopate, C. Gestational Aging and Determination of Parturition Date in the Bitch and Queen Using Ultrasonography and Radiography. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 617–638. [Google Scholar] [CrossRef]
- Górka, A.; Ochota, M.; Niżański, W. Ultrasound biometry for estimating delivery dates in small and medium breed cats. Theriogenology 2025, 232, 56–69. [Google Scholar] [CrossRef]
- Gatel, L.; Rault, D.N.; Chalvet-Monfray, K.; de Rooster, H.; Levy, X.; Chiers, K.; Saunders, J.H. Ultrasonography of the normal reproductive tract of the female domestic cat. Theriogenology 2020, 142, 328–337. [Google Scholar] [CrossRef]
- Zambelli, D.; Caneppele, B.; Bassi, S.; Paladini, C. Ultrasound aspects of fetal and extrafetal structures in pregnant cats. J. Feline Med. Surg. 2002, 4, 95–106. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Lim, B.; Lee, S.-Y.; Won, S.-H.; Jang, G. Establishment and characterization of endometrial organoids from different placental types. BMB Rep. 2025, 58, 95–103. [Google Scholar] [CrossRef]
| Measure | Dosages | Day of Gestation | Nr of Cats That Aborted/Total Nr of Treated Cats | Authors |
|---|---|---|---|---|
| OE | 35 | 5/5 | Tsutsui et al., 2009 [41] | |
| OE | 40 | 4/5 | Tsutsui et al., 2009 [41] | |
| OE | 45 | 2/5 | Tsutsui et al., 2009 [41] | |
| OE | 50 | 3/5 | Tsutsui et al., 2009 [41] | |
| Cloprostenol | 5 µg/kg s.c. on three days | 21–22 | 0/6 | Garcia-Mitacek et al., 2012 [42] |
| Cloprostenol | 5 µg/kg s.c. on three days | 21–22 | 0/10 | Garcia-Mitacek et al., 2017 [43] |
| Cloprostenol | 5 µg/kg s.c. on three days | 35–38 | 1/7 | Garcia-Mitacek et al., 2012 [42] |
| Cloprostenol | 5 µg/kg s.c. on three days | 35–38 | 1/10 | Garcia-Mitacek et al., 2017 [43] |
| Natural PGF2α | 2 mg per cat s.c. | 33 | 4/4 | Verstegen et al., 1993 [34] |
| Aglepristone | 10 mg/kg s.c. on two days | 21 + 25 or 35 + 36 | 10/10 10/10 | Garcia-Mitacek et al., 2017 [43] |
| Aglepristone | 10 mg/kg s.c. on two days | 45 | 4/6 | Georgiev et al., 2010 [45] |
| Aglepristone | 15 mg/kg s.c. on two days | 33.3 ± 4.2 days | 88.5% (54/61) | Fieni et al., 2006 [46] |
| Caberboline Injections | 1.65 µg/kg/day s.c. for 5 d | Daily from day 30 on | 80% (4/5) | Verstegen et al., 1993 [34] |
| Caberboline | 15 µg/kg orally | Daily between d 34 and 42 (until abortion) Daily starting between d 45 and 47 | 100% (8/8) Failure in two cats | Erünal-Maral et al., 2004 [47] |
| Caberboline Oral appl. | 5–15 µg/kg orally | Daily between d 36 ± 6.17 and 40.8 ± 6.96 Daily starting at on average d 48.5 | 100% (41/41) Premature parturition | Jöchle and Jöchle 1988 [48] |
| Cabergoline + prostagl. | 5 µg/kg orally 5 µg/kg s.c. every other day | Daily from day 30 on (11 d until abortion), PG every other day | 100% | Onclin and Verstegen 1997 [49] |
| Cabergoline + prostagl. | 5 µg/kg orally 5 µg/kg s.c. every other day | Daily from day 25–47 on (11 d until abortion), PG every other day | 100% | Onclin and Verstegen 1997 [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer-Somi, S. Establishment and Maintenance of Feline Pregnancy—A Comprehensive Review. Animals 2025, 15, 1249. https://doi.org/10.3390/ani15091249
Schäfer-Somi S. Establishment and Maintenance of Feline Pregnancy—A Comprehensive Review. Animals. 2025; 15(9):1249. https://doi.org/10.3390/ani15091249
Chicago/Turabian StyleSchäfer-Somi, Sabine. 2025. "Establishment and Maintenance of Feline Pregnancy—A Comprehensive Review" Animals 15, no. 9: 1249. https://doi.org/10.3390/ani15091249
APA StyleSchäfer-Somi, S. (2025). Establishment and Maintenance of Feline Pregnancy—A Comprehensive Review. Animals, 15(9), 1249. https://doi.org/10.3390/ani15091249






