Perioperative Pain Management for Mastectomy in Dogs: A Narrative Review
Simple Summary
Abstract
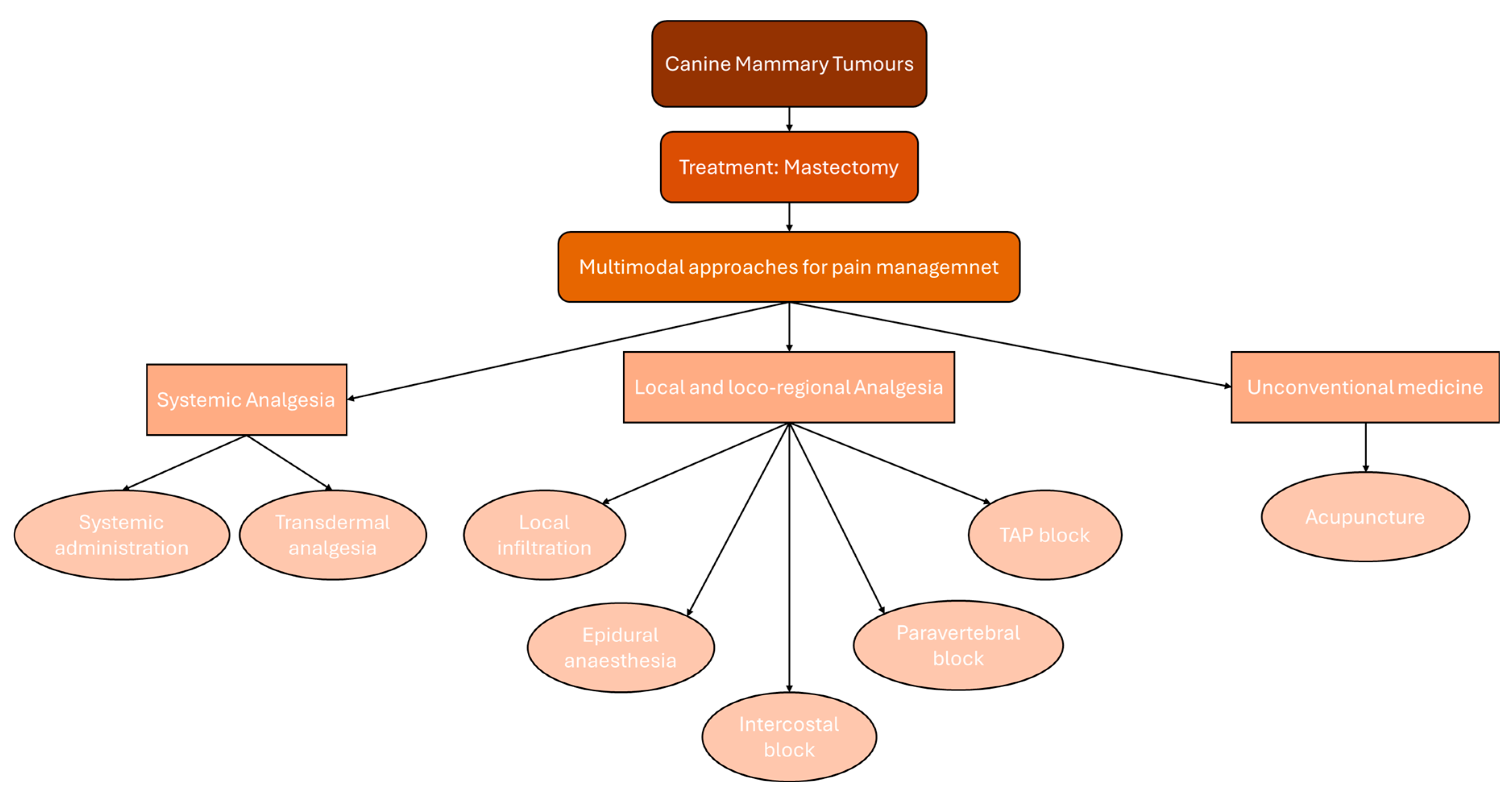
1. Introduction
2. Materials and Methods
3. Systemic Analgesia
3.1. Systemic Administration
3.2. Transdermal Analgesia
| Type | Paper | Drugs and Study Design | Pain Scale |
|---|---|---|---|
| Systemic Administration | Nakagawa et al. [21] | Meloxicam + Butorphanol Vs. Butorphanol | Serum cortisol levels |
| Crociolli et al. [22] | Gabapentin + Meloxicam Vs. Placebo + Meloxicam | GCMPS 1 + VAS 2 | |
| Friton et al. [25] | Robenacoxib Vs. Placebo | GCMPS-SF 3 | |
| Texeira et al. [26] | Tramadol Vs. Tramadol + Meloxicam Vs. Tramadol + Dipyrone | GCMPS 1 + VAS 2 | |
| Uscategui et al. [31] | Methadone Vs. Tramadol | UMPS 4 | |
| Uscategui et al. [33] | Methadone | UMPS 4 | |
| Sarrau et al. [35] | Morphine + low-dose Ketamine Vs. Morphine + high-dose Ketamine Vs. Morphine | VAS 2 + 4A VET pain scale 5 | |
| Soares et al. [38] | Ketamine + Lidocaine + Maropitant Vs. Ketamine + Lidocaine | DIVAS 6 + NCS 7 + GCMPS-SF 3 | |
| deMoura et al. [43] | Ketamine + Fentanyl Vs. Ketamine Vs. Fentanyl | GCMPS-SF 3 + MNT 8 | |
| Marques et al. [44] | Fentanyl Vs. Fentanyl + Lidocaine + Ketamine | GCMPS-SF 3 | |
| Cardozo et al. [47] | Fentanyl + Lidocaine + Ketamine Vs. Dexmedetomidine + Lidocaine + Ketamine | GCMPS-SF 3 | |
| Beier et al. [48] | Low-dose Remifentanil Vs. High-dose Remifentanil Vs. Control | - | |
| Interlandi et al. [49] | Cisatracurium Vs. Control | - | |
| Transdermal Analgesia | Cicirelli et al. [51] | Fentanyl patch Vs. Tramadol SC | NAS 9 + GCMPS-SF 3 |
| Galosi et al. [52] | Buprenorphine patch Vs. Buprenorphine IV | UMPS 4 + GCMPS-SF 3 |
4. Local and Loco-Regional Analgesia
4.1. Local Infiltration
4.2. Epidural Anaesthesia, Intercostal and Paravertebral Block
4.3. TAP Block
| Type | Paper | Drugs and Study Design | Pain Scale |
|---|---|---|---|
| Local infiltration | Costa et al. [58] | Lidocaine (through Comfort-in® device) | CPS 1 + CSU-CAPS 2 |
| Suarez-Redondo et al. [60] | Bupivacaine (through WSC) | GCMPS-SF 3 | |
| Vilhegas et al. [61] | BoNT-A | VAS 4 + GCMPS 5 | |
| Credie et al. [70] | TA (Lidocaine + Adrenaline) Vs. Fentanyl IV | DIVAS 6 + GCMPS 5 + UMPS 7 + VAS 4 + von Frey Filament test | |
| Abimussi et al. [72] | TA (0.1% Ropivacaine + Epinephrine) Vs. TA (0.05% Ropivacaine + Epinephrine) | UMPS 7 + CMPS 8 + von Frey Filament test | |
| Del Lama Rocha et al. [73] | TA (Lidocaine + Adrenaline) Vs. TA (Ropivacaine + Adrenaline) | GCMPS-SF 3 + von Frey Filament test | |
| Vullo et al. [74] | TA (Lidocaine + Adrenaline) Vs. Lidocaine CRI Vs. TA (Lidocaine + Adrenaline) + Lidocaine CRI | ICMP-SF 9 | |
| Del Lama Rocha et al. [77] | Heated TA | - | |
| Epidural infiltration + Intercostal block | Sanches et al. [82] | TA (Lidocaine + Epinephrine) Vs. Epidural Lidocaine + Morphine + Intercostal block with Lidocaine | GCMPS 5 |
| Epidural infiltration | Caramalac et al. [83] | Epidural Levobupivacaine Vs. Epidural Levobupivacaine + Methadone Vs. Epidural Levobupivacaine + Dexmedetomidine | UMPS 7 + GCMPS 5 |
| Herrera-Becerra et al. [84] | Epidural Ropivacaine + Morphine Vs. Epidural Ropivacaine + Xylazine Vs. Epidural Ropivacaine + Morphine + Xylazine | GCMPS-SF 3 | |
| Tayari et al. [85] | Epidural Ropivacaine Vs. Epidural Ropivacaine + 0.5% Morphine Vs. Epidural Ropivacaine + 0.35% Morphine Vs. Epidural Ropivacaine + 0.25% Morphine Vs. Control | GCMPS-SF 3 | |
| Thoracic paravertebral block | Santoro et al. [87] | TPVB with Ropivacaine Vs. Control | GCMPS-SF 3 |
| TAP block | Portela et al. [90] | TAP with Bupivacaine + Intercostal block with Bupivacaine | GCMPS 5 |
| Teixeira et al. [91] | TAP with Bupivacaine + SP block with Bupivacaine | CSU-CAPS 2 |
5. Unconventional Medicine: Acupuncture
6. Limitations of the Review
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4AVET | French Veterinary Association for Anaesthesiology and Analgesia pain scoring scale |
| CMPS | Composite Measure Pain Scale |
| CPS | Composite Pain Scale |
| CRI | Continuous Rate Infusion |
| CSU-CAPS | Canine Acute Pain Scale from Colorado State University |
| DIVAS | Visual Interactive and Dynamic Analogue |
| GCMPS | Glasgow Composite Measure Pain Scale |
| GCMPS-SF | Short-Form Glasgow Composite Measure Pain Scale |
| ICMP-SF | Italian version of GCMP-SF |
| IM | Intramuscular |
| IV | Intravenous |
| IVAS | Interactive VAS |
| MNT | Mechanical Nociceptive Threshold |
| NAS | Numerical Analogue Scale |
| NCS | Numerical Classification Scale |
| SC | Subcutis |
| SP | Serratus Plane |
| TA | Tumescent Anaesthesia |
| TAP | Transverse Abdominis Plane |
| TPVB | Thoracic ParaVertebral Block |
| UMPS | University of Melbourne Pain Scale |
| VAS | Visual Analogue Scale |
| WSC | Wound Soaker Catheters |
| YNSA | Yamamoto New Scalp Acupuncture |
References
- Sleeckx, N.; de Rooster, H.; Veldhuis Kroeze, E.J.; Van Ginneken, C.; Van Brantegem, L. Canine mammary tumours, an overview. Reprod. Domest. Anim. 2011, 46, 1112–1131. [Google Scholar] [CrossRef] [PubMed]
- Novosad, C.A. Principles of treatment for mammary gland tumors. Clin. Tech. Small Anim. Pract. 2003, 18, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Moe, L. Population-based incidence of mammary tumours in some dog breeds. J. Reprod. Fertil. Suppl. 2001, 57, 439–443. [Google Scholar]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002-2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Gilbertson, S.R.; Kurzman, I.D.; Zachrau, R.E.; Hurvitz, A.I.; Black, M.M. Canine mammary epithelial neoplasms: Biologic implications of morphologic characteristics assessed in 232 dogs. Vet. Pathol. 1983, 20, 127–142. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Worley, D.R.; Goldschmidt, M.H. Tumors of the mammary gland. In Withrow & MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Eds.; Elsevier: St Louis, MO, USA, 2013; pp. 538–556. [Google Scholar]
- Horta, R.S.; Figueiredo, M.S.; Lavalle, G.E.; Costa, M.P.; Cunha, R.M.; Araújo, R.B. Surgical stress and postoperative complications related to regional and radical mastectomy in dogs. Acta Vet. Scand. 2015, 57, 34. [Google Scholar] [CrossRef]
- Polton, G. Mammary tumours in dogs. Irish Vet. J. 2009, 62, 50–56. [Google Scholar]
- Pinheiro, A.V.; Petrucci, G.N.; Dourado, A.; Pires, I. Anaesthesia in Veterinary Oncology: The Effects of Surgery, Volatile and Intravenous Anaesthetics on the Immune System and Tumour Spread. Animals 2023, 13, 3392. [Google Scholar] [CrossRef]
- Allen, C. Animal pain. Nous 2004, 38, 617–643. [Google Scholar] [CrossRef]
- Rutherford, K.M.D. Assessing Pain in Animals. Anim. Welf. 2002, 11, 31–53. [Google Scholar] [CrossRef]
- 2022 AAHA Pain Management Guidelines for Dogs and Cats. Available online: https://www.aaha.org/resources/2022-aaha-pain-management-guidelines-for-dogs-and-cats/ (accessed on 15 February 2025).
- Lemke, K.A.; Creighton, C.M. Analgesia for anesthetized patients. Top. Companion Anim. Med. 2010, 25, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Oderda, G. Challenges in the management of acute postsurgical pain. Pharmacotherapy 2012, 32 (Suppl. 9), 6S–11S. [Google Scholar] [CrossRef]
- Gupta, K.; Kshirsagar, S.; Chang, L.; Schwartz, R.; Law, P.Y.; Yee, D.; Hebbel, R.P. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002, 62, 4491–4498. [Google Scholar]
- Shavit, Y.; Ben-Eliyahu, S.; Zeidel, A.; Beilin, B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation 2004, 11, 255–260. [Google Scholar] [CrossRef]
- Santamaria, L.B.; Schifilliti, D.; La Torre, D.; Fodale, V. Drugs of anaesthesia and cancer. Surg. Oncol. 2010, 19, 63–81. [Google Scholar] [CrossRef]
- Gach, K.; Wyrębska, A.; Fichna, J.; Janecka, A. The role of morphine in regulation of cancer cell growth. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 384, 221–230. [Google Scholar] [CrossRef]
- Berry, S.H. Analgesia in the Perioperative Period. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 1013–1027. [Google Scholar] [CrossRef]
- Nakagawa, K.; Miyagawa, Y.; Takemura, N.; Hirose, H. Influence of preemptive analgesia with meloxicam before resection of the unilateral mammary gland on postoperative cardiovascular parameters in dogs. J. Vet. Med. Sci. 2007, 69, 939–944. [Google Scholar] [CrossRef]
- Crociolli, G.C.; Cassu, R.N.; Barbero, R.C.; Rocha, T.L.; Gomes, D.R.; Nicácio, G.M. Gabapentin as an adjuvant for postoperative pain management in dogs undergoing mastectomy. J. Vet. Med. Sci. 2015, 77, 1011–1015. [Google Scholar] [CrossRef]
- Ochroch, E.A.; Mardini, I.A.; Gottschalk, A. What is the role of NSAIDs in pre-emptive analgesia? Drugs 2003, 63, 2709–2723. [Google Scholar] [CrossRef] [PubMed]
- Kong, V.K.; Irwin, M.G. Gabapentin: A multimodal perioperative drug? Br. J. Anaesth. 2007, 99, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Friton, G.; Thompson, C.; Karadzovska, D.; King, S.; King, J.N. Efficacy and Safety of Injectable Robenacoxib for the Treatment of Pain Associated With Soft Tissue Surgery in Dogs. J. Vet. Intern. Med. 2017, 31, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.C.; Monteiro, E.R.; Campagnol, D.; Coelho, K.; Bressan, T.F.; Monteiro, B.S. Effects of tramadol alone, in combination with meloxicam or dipyrone, on postoperative pain and the analgesic requirement in dogs undergoing unilateral mastectomy with or without ovariohysterectomy. Vet. Anaesth. Analg. 2013, 40, 641–649. [Google Scholar] [CrossRef]
- Shipton, E.A. Tramadol—Present and future. Anaesth. Intensive Care 2000, 28, 363–374. [Google Scholar] [CrossRef]
- Martins, T.L.; Kahvegian, M.A.; Noel-Morgan, J.; Leon-Román, M.A.; Otsuki, D.A.; Fantoni, D.T. Comparison of the effects of tramadol, codeine, and ketoprofen alone or in combination on postoperative pain and on concentrations of blood glucose, serum cortisol, and serum interleukin-6 in dogs undergoing maxillectomy or mandibulectomy. Am. J. Vet. Res. 2010, 71, 1019–1026. [Google Scholar] [CrossRef]
- Vettorato, E.; Zonca, A.; Isola, M.; Villa, R.; Gallo, M.; Ravasio, G.; Beccaglia, M.; Montesissa, C.; Cagnardi, P. Pharmacokinetics and efficacy of intravenous and extradural tramadol in dogs. Vet. J. 2010, 183, 310–315. [Google Scholar] [CrossRef]
- Camu, F.; Vanlersberghe, C. Pharmacology of systemic analgesics. Best. Pract. Res. Clin. Anaesthesiol. 2002, 16, 475–488. [Google Scholar] [CrossRef]
- Uscategui, R.A.R.; Tiosso, C.; Moro, J.V.; Mostachio, G.Q.; Padilha-Nakaghi, L.C.; Feliciano, M.A.R.; Vicente, W.R.R. Pre-emptive methadone or tramadol analgesia for mastectomy and ovariohysterectomy in bitches. Rev. Colomb. Cienc. Pecu. 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Firth, A.M.; Haldane, S.L. Development of a scale to evaluate postoperative pain in dogs. J. Am. Vet. Med. Assoc. 1999, 214, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Uscategui, R.A.R.; Feliciano, M.A.R.; Tiosso, C.F.; Moro, J.V.; Coutinho, L.N.; Brito, M.B.S.; Vicente, W.R.R. Comparative Evaluation of Methadone Administration at Different Periods in Bitches Undergoing Mastectomy. Pak. Vet. J. 2017, 37, 345–349. [Google Scholar]
- Donati, P.A.; Tarragona, L.; Franco, J.V.A.; Kreil, V.; Fravega, R.; Diaz, A.; Verdier, N.; Otero, P.E. Efficacy of tramadol for postoperative pain management in dogs: Systematic review and meta-analysis. Vet. Anaesth. Analg. 2021, 48, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Sarrau, S.; Jourdan, J.; Dupuis-Soyris, F.; Verwaerde, P. Effects of postoperative ketamine infusion on pain control and feeding behaviour in bitches undergoing mastectomy. J. Small Anim. Pract. 2007, 48, 670–676. [Google Scholar] [CrossRef]
- Verwaerde, P.; Estrade, C. (Eds.) Les e’tapes de l’anesthe’sie ge’ne’rale. In Vade-Mecum d’Anesthe’sie des Carnivores Domestiques; Editions med’com: Paris, France, 2005; pp. 122–125. [Google Scholar]
- Young, L.E.; Brearley, J.C.; Richards, D.L.S. Medetomidine as a premedicant in dogs and its reversal by atipamezole. J. Small Anim. Pract. 1990, 31, 554–559. [Google Scholar] [CrossRef]
- Soares, P.C.L.R.; Corrêa, J.M.X.; Niella, R.V.; de Oliveira, J.N.S.; Costa, B.A.; Silva Junior, A.C.; Sena, A.S.; Pinto, T.M.; Munhoz, A.D.; Martins, L.A.F.; et al. Continuous Infusion of Ketamine and Lidocaine Either with or without Maropitant as an Adjuvant Agent for Analgesia in Female Dogs Undergoing Mastectomy. Vet. Med. Int. 2021, 2021, 4747301. [Google Scholar] [CrossRef]
- Marquez, M.; Boscan, P.; Weir, H.; Vogel, P.; Twedt, D.C. Comparison of NK-1 Receptor Antagonist (Maropitant) to Morphine as a Pre-Anaesthetic Agent for Canine Ovariohysterectomy. PLoS ONE 2015, 10, e0140734. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Hill, R. NK1 (substance P) receptor antagonists—Why are they not analgesic in humans? Trends Pharmacol. Sci. 2000, 21, 244–246. [Google Scholar] [CrossRef]
- Kinobe, R.T.; Miyake, Y. Evaluating the anti-inflammatory and analgesic properties of maropitant: A systematic review and meta-analysis. Vet. J. 2020, 259–260, 105471. [Google Scholar] [CrossRef]
- de Moura, R.S.; Bittar, I.P.; Gomes, J.H.; de Oliveira, Y.V.R.; de Sousa Filho, G.D.; de Faria Soares, G.C.F.; Lima, E.M.; Franco, L.G. Plasma concentration, cardiorespiratory and analgesic effects of ketamine-fentanyl infusion in dogs submitted to mastectomy. BMC Vet. Res. 2022, 18, 225. [Google Scholar] [CrossRef]
- Marques, É.J.; Monteiro, E.R.; Herrera-Becerra, J.R.; Tomazeli, D.; Rovaris, I.B.; de Oliveira, T.F.; Valle, S.F.; Alievi, M.M. Influence of Constant Rate Infusions of Fentanyl Alone or in Combination With Lidocaine and Ketamine on the Response to Surgery and Postoperative Pain in Isoflurane Anesthetized Dogs Undergoing Unilateral Mastectomy: A Randomized Clinical Trial. Top. Companion Anim. Med. 2023, 52, 100759. [Google Scholar] [CrossRef] [PubMed]
- Slingsby, L.S.; Waterman-Pearson, A.E. The post-operative analgesic effects of ketamine after canine ovariohysterectomy—A comparison between pre- or post-operative administration. Res. Vet. Sci. 2000, 69, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Shih, A.; Miletic, G.; Miletic, V. Continual systemic infusion of lidocaine provides analgesia in an animal model of neuropathic pain. Pain 2002, 97, 267–273. [Google Scholar] [CrossRef]
- Cardozo, H.G.; Monteiro, E.R.; Correia, B.S.; Ferronatto, V.B.J.; Almeida-Filho, F.T.; Alievi, M.M.; Valle, S.F. Influence of intravenous fentanyl or dexmedetomidine infusions, combined with lidocaine and ketamine, on cardiovascular response, sevoflurane requirement and postoperative pain in dogs anesthetized for unilateral mastectomy. Vet. Anaesth. Analg. 2024, 51, 381–390. [Google Scholar] [CrossRef]
- Beier, S.L.; Rosa, A.C.; da Mattoso, C.R.S.; Moraes, A.N.; de Oleskovicz, N.; Klein, A.V.; Dallabrida, A.L. Evaluation of the isoflurane-sparing effects of a constant rate infusion of remifentanil undergoing mastectomy in dogs. Semin. Ciências Agrárias 2015, 36, 3139–3148. [Google Scholar] [CrossRef]
- Interlandi, C.; Di Pietro, S.; Costa, G.L.; Spadola, F.; Iannelli, N.M.; Macrì, D.; Ferrantelli, V.; Macrì, F. Effects of Cisatracurium in Sevoflurane and Propofol Requirements in Dog-Undergoing-Mastectomy Surgery. Animals 2022, 12, 3134. [Google Scholar] [CrossRef]
- Pascoe, P.J. Opioid analgesics. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 757–772. [Google Scholar] [CrossRef]
- Cicirelli, V.; Aiudi, G.G.; Mrenoshki, D.; Lacalandra, G.M. Fentanyl patch versus tramadol for the control of postoperative pain in canine ovariectomy and mastectomy. Vet. Med. Sci. 2022, 8, 469–475. [Google Scholar] [CrossRef]
- Galosi, M.; Troisi, A.; Toniolo, P.; Pennasilico, L.; Cicirelli, V.; Palumbo Piccionello, A.; Di Bella, C. Comparison of the Transdermal and Intravenous Administration of Buprenorphine in the Management of Intra- and Postoperative Pain in Dogs Undergoing a Unilateral Mastectomy. Animals 2022, 12, 3468. [Google Scholar] [CrossRef]
- Steagall, P.V.; Ruel, H.L.M.; Yasuda, T.; Monteiro, B.P.; Watanabe, R.; Evangelista, M.C.; Beaudry, F. Pharmacokinetics and analgesic effects of intravenous, intramuscular or subcutaneous buprenorphine in dogs undergoing ovariohysterectomy: A randomised, prospective, masked, clinical trial. BMC Vet. Res. 2020, 16, 154. [Google Scholar] [CrossRef]
- Enomoto, H.; Love, L.; Madsen, M.; Wallace, A.; Messenger, K.M. Pharmacokinetics of intravenous, oral transmucosal and intranasal buprenorphine in healthy male dogs. J. Vet. Pharmacol. Ther. 2022, 45, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Møiniche, S.; Mikkelsen, S.; Wetterslev, J.; Dahl, J.B. A qualitative systematic review of incisional local anaesthesia for postoperative pain relief after abdominal operations. Br. J. Anaesth. 1998, 81, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.E.; Wilson, D.V.; Evans, A.T. Evaluation of intraperitoneal and incisional lidocaine or bupivacaine for analgesia following ovariohysterectomy in the dog. Vet. Anaesth. Analg. 2004, 31, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Engwerda, E.E.; Abbink, E.J.; Tack, C.J.; de Galan, B.E. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care 2011, 34, 1804–1808. [Google Scholar] [CrossRef]
- Costa, G.L.; Bruno, F.; Leonardi, F.; Licata, P.; Macrì, F.; Fernández Parra, R.; Bruschetta, G.; Nava, V.; Pugliese, M.; Spadola, F. Surgical Site Infiltration with Comfort-in Device and Traditional Syringe in Dogs Undergoing Regional Mastectomy: Evaluation of Intra- and Postoperative Pain and Oxidative Stress. Animals 2024, 14, 1902. [Google Scholar] [CrossRef]
- Abelson, A.L.; McCobb, E.C.; Shaw, S.; Armitage-Chan, E.; Wetmore, L.A.; Karas, A.Z.; Blaze, C. Use of wound soaker catheters for the administration of local anesthetic for post-operative analgesia: 56 cases. Vet. Anaesth. Analg. 2009, 36, 597–602. [Google Scholar] [CrossRef]
- Suárez-Redondo, M.; Fuertes-Recuero, M.; Guzmán-Soltero, A.; Aguado, D.; Del Carmen Martín-Espada, M.; Espinel-Rupérez, J.; Ortiz-Diez, G. Description of postoperative complications and bacterial contamination of wound soaker catheters used to administer postoperative local analgesia after mastectomy in 11 dogs: Case series. Vet. Res. Commun. 2024, 48, 2707–2712. [Google Scholar] [CrossRef]
- Vilhegas, S.; Cassu, R.N.; Barbero, R.C.; Crociolli, G.C.; Rocha, T.L.; Gomes, D.R. Botulinum toxin type A as an adjunct in postoperative pain management in dogs undergoing radical mastectomy. Vet. Rec. 2015, 177, 391. [Google Scholar] [CrossRef]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef]
- Bach-Rojecky, L.; Lacković, Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009, 94, 234–238. [Google Scholar] [CrossRef]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A. Tumescent technique for local anesthesia improves safety in large-volume liposuction. Plast. Reconstr. Surg. 1993, 92, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Ostad, A.; Kageyama, N.; Moy, R.L. Tumescent anesthesia with a lidocaine dose of 55 mg/kg is safe for liposuction. Dermatol. Surg. 1996, 22, 921–927. [Google Scholar] [CrossRef]
- Klein, J.A. Anesthetic formulation of tumescent solutions. Dermatol. Clin. 1999, 17, 751–759. [Google Scholar] [CrossRef]
- Thomas, J. Adjunctive tumescent technique in massive resections. Aesthetic Plast. Surg. 2001, 25, 343–346. [Google Scholar] [CrossRef]
- Lehnhardt, M.; Homann, H.H.; Daigeler, A.; Hauser, J.; Palka, P.; Steinau, H.U. Major and lethal complications of liposuction: A review of 72 cases in Germany between 1998 and 2002. Plast. Reconstr. Surg. 2008, 121, 396–403. [Google Scholar] [CrossRef]
- Credie, L.d.F.; Luna, S.P.; Futema, F.; da Silva, L.C.; Gomes, G.B.; Garcia, J.N.; de Carvalho, L.R. Perioperative evaluation of tumescent anaesthesia technique in bitches submitted to unilateral mastectomy. BMC Vet. Res. 2013, 9, 178. [Google Scholar] [CrossRef]
- Sztark, F.; Malgat, M.; Dabadie, P.; Mazat, J.P. Comparison of the effects of bupivacaine and ropivacaine on heart cell mitochondrial bioenergetics. Anesthesiology 1998, 88, 1340–1349. [Google Scholar] [CrossRef]
- Abimussi, C.J.; Menegheti, T.M.; Wagatsuma, J.T.; Floriano, B.P.; Arruda, A.M.; dos Santos, P.S.; Oliva, V.N. Tumescent local anesthesia with ropivacaine in different concentrations in bitches undergoing mastectomy: Plasma concentration and post-operative analgesia. Vet. Anaesth. Analg. 2014, 41, 516–525. [Google Scholar] [CrossRef]
- Del Lama Rocha, F.; Nunes, N.; Chiconi Dacunto dos Santos, P.; Kazuo Ido, C.; do Espírito Santo Silva, P.; Simões Azenha Aidar, E.; Amaral da Silva, H.R.; Carmagnani Prada, T. Postoperative Analgesia Time in Dogs Submitted to Mastectomy and Anesthetized with Tumescent Solutions of Lidocaine or Ropivacaine. Acta Sci. Vet. 2020, 48, 1747. [Google Scholar] [CrossRef]
- Vullo, C.; Tambella, A.M.; Falcone, A.; Marino, G.; Catone, G. Constant Rate Infusion of Lidocaine, Tumescent Anesthesia and Their Combination in Dogs Undergoing Unilateral Mastectomy. Animals 2021, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, G.; Colpo, R.; Reid, J.; Di Salvo, A.; Scott, M. Creation And Validation Of The Italian Version Of The Glasgow Composite Measure Pain Scale-Short Form (ICMPS-SF). Vet. Ital. 2018, 54, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Govendir, M.; Zaki, S.; Miyake, Y.; Packiarajah, P.; Malik, R. Evaluation of four warming procedures to minimise heat loss induced by anaesthesia and surgery in dogs. Aust. Vet. J. 2004, 82, 65–68. [Google Scholar] [CrossRef]
- Del Lama Rocha, F.; Nunes, N.; Kazuo Ido, C.; Belchior Vela, D.; Rene Vargas Estrada, C.; Silva, P.; Fernanda Firmo, B. Effects of Heated Tumescence Solution in Bitches after Unilateral Mastectomy. Acta Sci. Vet. 2022, 50, 1855. [Google Scholar] [CrossRef]
- Boeer, B.; Helms, G.; Pasternak, J.; Roehm, C.; Kofler, L.; Haefner, H.M.; Moehrle, M.; Heim, E.; Fischer, H.; Brucker, S.Y.; et al. Back to the future: Breast surgery with tumescent local anesthesia (TLA)? Arch. Gynecol. Obstet. 2023, 308, 935–940. [Google Scholar] [CrossRef]
- Steagall, P.V.M.; Simon, B.T.; Teixeira Neto, F.J.; Luna, S.P.L. An Update on Drugs Used for Lumbosacral Epidural Anesthesia and Analgesia in Dogs. Front. Vet. Sci. 2017, 4, 68. [Google Scholar] [CrossRef]
- Fernandez-Parra, R.; Zilberstein, L.; Fontaine, C.; Adami, C. Comparison of intratesticular lidocaine, sacrococcygeal epidural lidocaine and intravenous methadone in cats undergoing castration: A prospective, randomized, investigator-blind clinical trial. Vet. Anaesth. Analg. 2017, 44, 356–363. [Google Scholar] [CrossRef]
- Groeben, H. Epidural anesthesia and pulmonary function. J. Anesth. 2006, 20, 290–299. [Google Scholar] [CrossRef]
- Sanches, M.C.; Naspolini, B.M.; Salame, J.P.; Maroneze, B.P.; Guim, T.N.; Gehrcke, M.I. Tumescent anesthesia or epidural anesthesia combined with intercostal block in bitches submitted to mastectomy. Ciência Anim. Bras. 2020, 21, e-53552. [Google Scholar] [CrossRef]
- Caramalac, S.M.; Albuquerque, V.B.; Oliveira, A.R.; Caramalac, S.M.; Jardim, P.H.A.; Barbosa, C.F.; Frazílio, F.O. Analgesic, cardiorespiratory effects and motor block characteristics of epidural levobupivacaine alone or in combination with methadone or dexmedetomidine in bitches undergoing unilateral total mastectomy. An. Acad. Bras. Cienc. 2022, 94 (Suppl. 3), e20210082. [Google Scholar] [CrossRef]
- Herrera Becerra, J.R.; Monteiro, E.R.; Martins, L.G.; Baier, M.E.; Santos, E.A.; Bianchi, S.P. Epidural administration of combinations of ropivacaine, morphine and xylazine in bitches undergoing total unilateral mastectomy: A randomized clinical trial. Vet. Anaesth. Analg. 2022, 49, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Tayari, H.; Otero, P.E.; D’Agostino, M.; Bartolini, F.; Briganti, A. Epidural Volume of Injectate Using a Dose Regimen Based on Occipito-Coccygeal Spinal Length (OCL): Randomized Clinical Study Comparing Different Ropivacaine Concentrations, with or without Morphine, in Bitches Undergoing Total Unilateral Mastectomy. Animals 2022, 12, 587. [Google Scholar] [CrossRef]
- Karmakar, M.K. Thoracic paravertebral block. Anesthesiology 2001, 95, 771–780. [Google Scholar] [CrossRef]
- Santoro, F.; Debidda, P.; Franci, P. Single-injection caudal thoracic paravertebral block improves pain control and recovery quality in female dogs undergoing unilateral radical mastectomy: A randomized controlled trial. J. Am. Vet. Med. Assoc. 2021, 260 (Suppl. 1), S53–S58. [Google Scholar] [CrossRef]
- Abdallah, F.W.; Chan, V.W.; Brull, R. Transversus abdominis plane block: A systematic review. Reg. Anesth. Pain Med. 2012, 37, 193–209. [Google Scholar] [CrossRef]
- Taylor, R.J.; Pergolizzi, J.V.; Sinclair, A.; Raffa, R.B.; Aldington, D.; Plavin, S.; Apfel, C.C. Transversus abdominis block: Clinical uses, side effects, and future perspectives. Pain Pract. 2013, 13, 332–344. [Google Scholar] [CrossRef]
- Portela, D.A.; Romano, M.; Briganti, A. Retrospective clinical evaluation of ultrasound guided transverse abdominis plane block in dogs undergoing mastectomy. Vet. Anaesth. Analg. 2014, 41, 319–324. [Google Scholar] [CrossRef]
- Teixeira, L.G.; Pujol, M.D.; Pazzim, F.A.; Souza, R.P.; Fadel, L. Combination of Transversus abdominis plane block and Serratus plane block anesthesia in dogs submitted to mastectomy. Pesqui. Veterinária Bras. 2018, 38, 315–319. [Google Scholar] [CrossRef]
- de la Torre, P.A.; García, P.D.; Alvarez, S.L.; Miguel, F.J.; Pérez, M.F. A novel ultrasound-guided block: A promising alternative for breast analgesia. Aesthet. Surg. J. 2014, 34, 198–200. [Google Scholar] [CrossRef]
- Gakiya, H.H.; Silva, D.A.; Gomes, J.; Stevanin, H.; Cassu, R.N. Electroacupuncture versus morphine for the postoperative control pain in dogs. Acta Cir. Bras. 2011, 26, 346–351. [Google Scholar] [CrossRef]
- Chrisman, C.; Xie, H. Canine Transpositional Acupoints. In Xie’s Veterinary Acupuncture; Wiley: Hoboken, NJ, USA, 2007; pp. 129–215. [Google Scholar] [CrossRef]
- Bacarin, C.C.; Nicácio, G.M.; Cerazo, L.M.L.; Peruchi, L.G.; Cassu, R.N. Perioperative Analgesic Efficacy of Yamamoto New Scalp Acupuncture for Canine Mastectomy Combined with Ovariohysterectomy: A Randomized, Controlled Clinical Trial. J. Acupunct. Meridian Stud. 2022, 15, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Shimizu, N. YNSA and Tail Acupuncture: New Acupuncture System for Canines and Felines. Dtsch. Z. Akupunkt. 2007, 50, 6–9. [Google Scholar] [CrossRef]
- Bergh, A.; Lund, I.; Boström, A.; Hyytiäinen, H.; Asplund, K. A Systematic Review of Complementary and Alternative Veterinary Medicine: “Miscellaneous Therapies”. Animals 2021, 11, 3356. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Xie, H. The scientific basis of acupuncture for veterinary pain management: A review based on relevant literature from the last two decades. Open Vet. J. 2021, 11, 203–209. [Google Scholar] [CrossRef]
- Monteiro, B.P.; Lascelles, B.D.X.; Murrell, J.; Robertson, S.; Steagall, P.V.M.; Wright, B. 2022 WSAVA guidelines for the recognition, assessment and treatment of pain. J. Small Anim. Pract. 2023, 64, 177–254. [Google Scholar] [CrossRef]
| Paper | Drugs and Study Design | Pain Scale |
|---|---|---|
| Gakiya et al. [93] | Morphine IM Vs. Electroacupuncture Vs. Sham procedure | Numbering rating scale + serum cortisol level |
| Bacarin et al. [95] | YNSA Vs. Control group | IVAS 1 + GCMPS-SF 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giambrone, G.; Catone, G.; Marino, G.; Sfacteria, A.; Miloro, R.; Vullo, C. Perioperative Pain Management for Mastectomy in Dogs: A Narrative Review. Animals 2025, 15, 1214. https://doi.org/10.3390/ani15091214
Giambrone G, Catone G, Marino G, Sfacteria A, Miloro R, Vullo C. Perioperative Pain Management for Mastectomy in Dogs: A Narrative Review. Animals. 2025; 15(9):1214. https://doi.org/10.3390/ani15091214
Chicago/Turabian StyleGiambrone, Giada, Giuseppe Catone, Gabriele Marino, Alessandra Sfacteria, Renato Miloro, and Cecilia Vullo. 2025. "Perioperative Pain Management for Mastectomy in Dogs: A Narrative Review" Animals 15, no. 9: 1214. https://doi.org/10.3390/ani15091214
APA StyleGiambrone, G., Catone, G., Marino, G., Sfacteria, A., Miloro, R., & Vullo, C. (2025). Perioperative Pain Management for Mastectomy in Dogs: A Narrative Review. Animals, 15(9), 1214. https://doi.org/10.3390/ani15091214








