Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Antibodies
2.2. Cytotoxicity Assay
2.3. Determination of Half Virus Inhibition Rate
2.4. Quantitative Real-Time PCR
2.5. Western Blotting
2.6. The Experiment of DNJ Inhibiting the Life Cycle of PEDV
2.7. Plaque Formation Assay
2.8. Measurement of Anti-Oxidative Stress Activity
2.9. Molecular Docking Simulation of the Binding of DNJ with the Protein Target of PEDV
2.10. Statistical Analysis
3. Results
3.1. Evaluation of the Cytotoxicity of DNJ
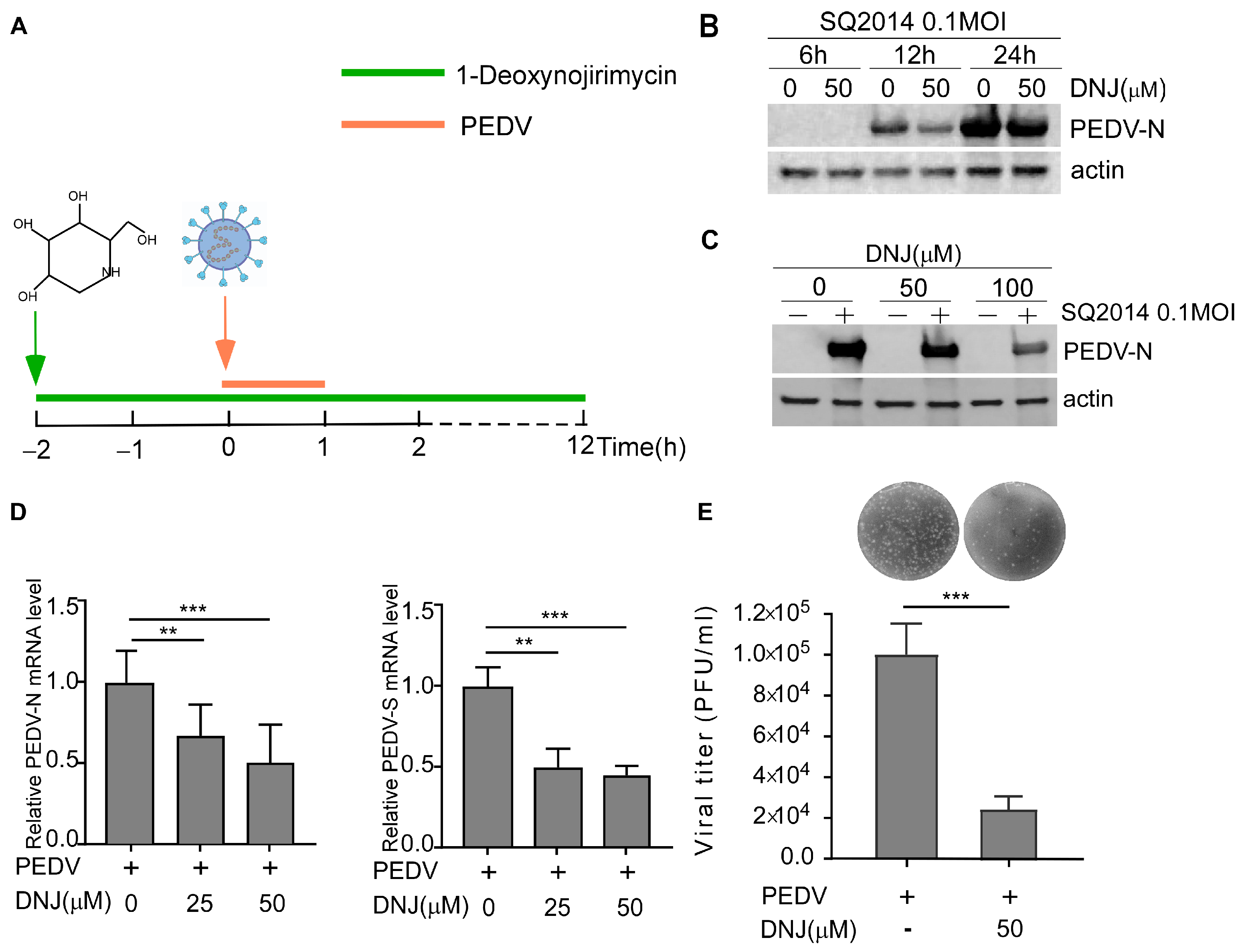
3.2. Antiviral Activity of DNJ in Vero-E6 Cells
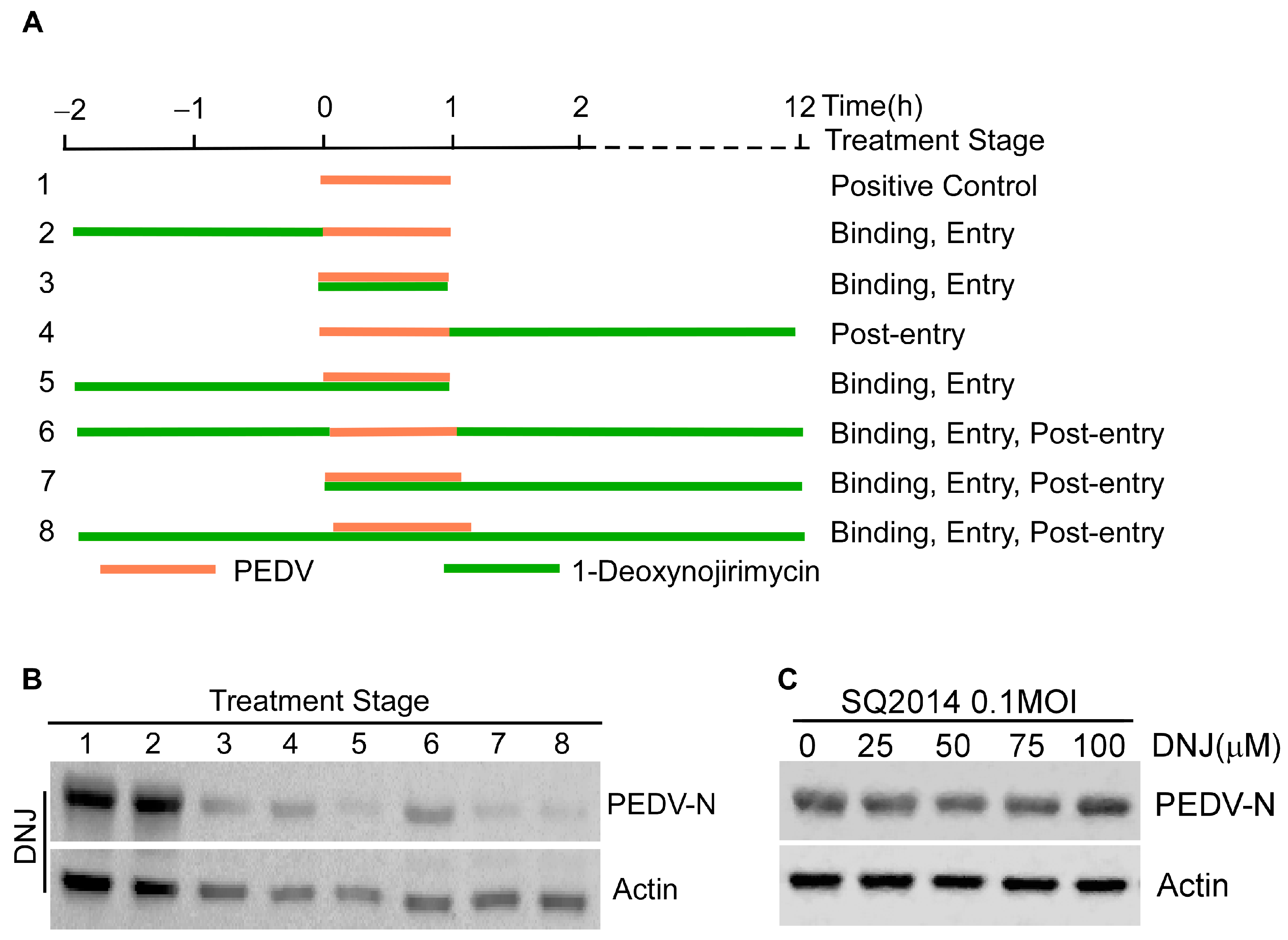
3.3. Effect of DNJ on Different Stages of PEDV Life Cycle
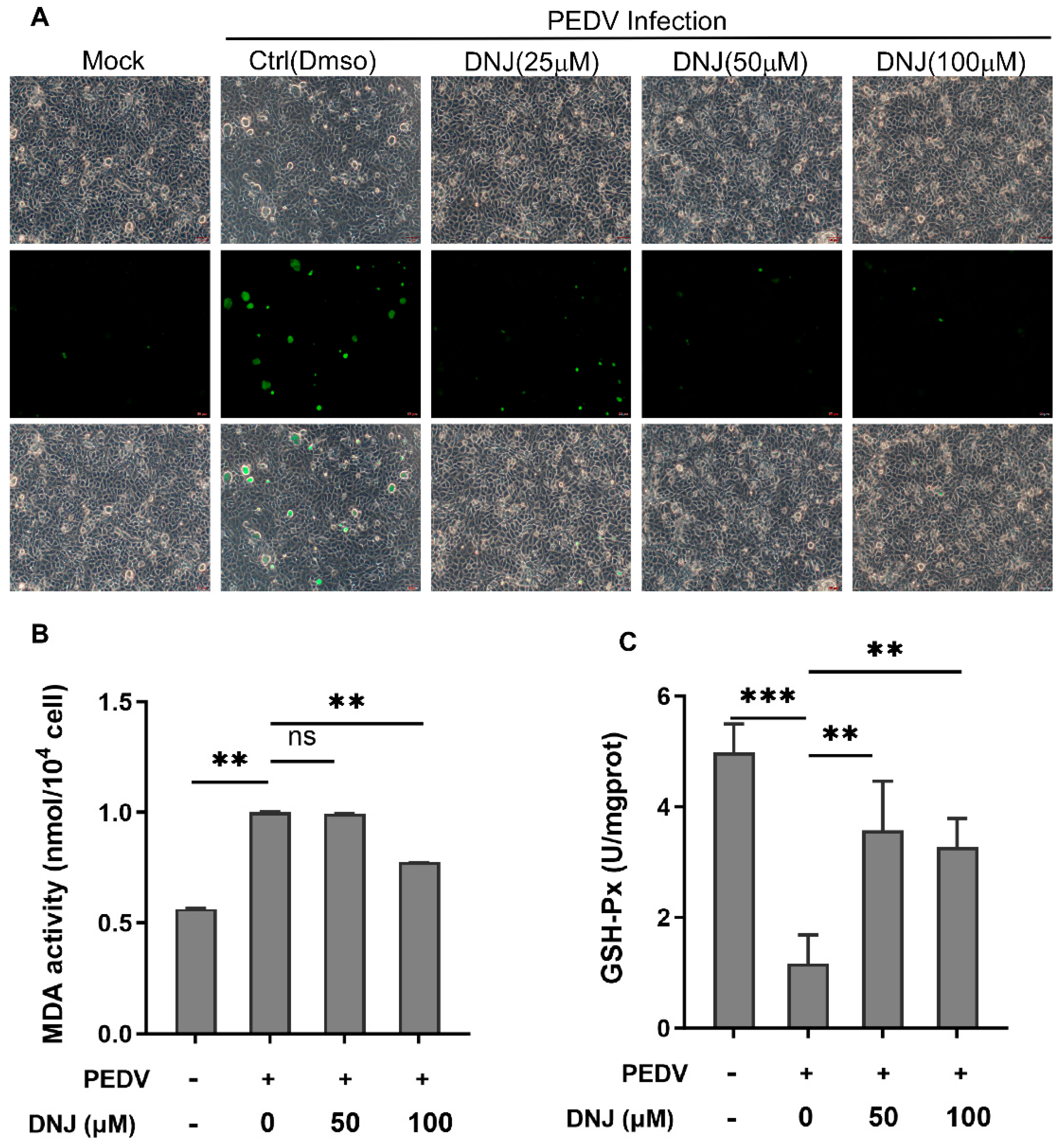
3.4. DNJ Anti-PEDV Activity Is Related to Inhibition of ROS Production
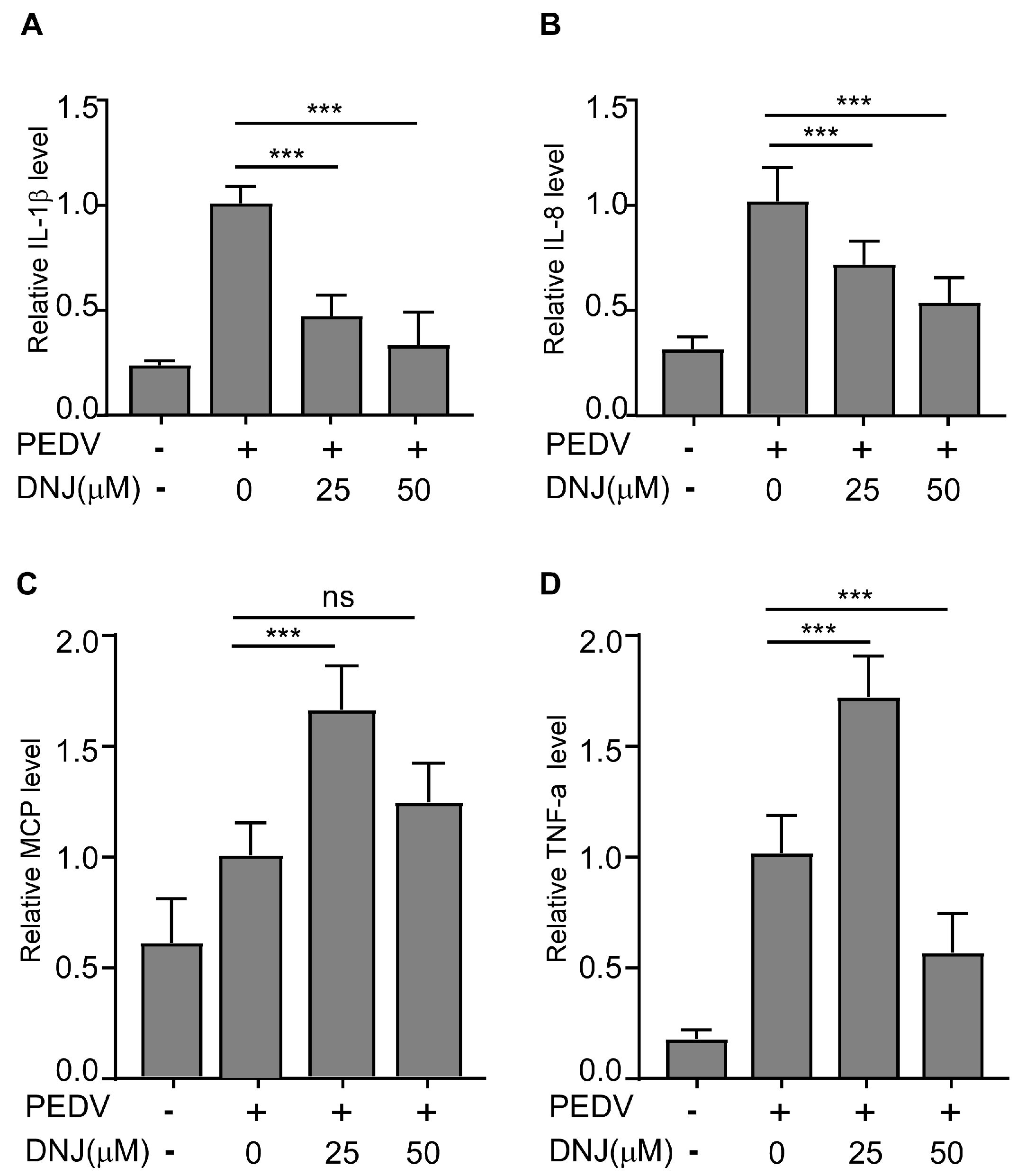
3.5. DNJ Alleviated the Inflammatory Cytokines Induced by PEDV Infection
3.6. Molecular Docking to Predict Protein Targets of DNJ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, C.; Peng, O.; Ashraf, U.; Xu, Q.; Gong, L.; Fan, B.; Zhang, Y.; Xu, Z.; Xue, C.; et al. Global Dynamics of Porcine Enteric Coronavirus PEDV Epidemiology, Evolution, and Transmission. Mol. Biol. Evol. 2023, 40, msad052. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, H.; Li, L.; Yang, Y.; Zou, X.; Chen, J.; Tang, X. PEDV: Insights and Advances into Types, Function, Structure, and Receptor Recognition. Viruses 2022, 14, 1744. [Google Scholar] [CrossRef]
- Liu, C.; Kong, N.; Liu, H.; Zhang, Y.; Qin, W.; Zhao, W.; Yang, X.; Wang, Y.; Cao, X.; Liu, T.; et al. FSTL1 and TLR4 interact with PEDV structural proteins to promote virus adsorption to host cells. J. Virol. 2025, 99, e0183724. [Google Scholar] [CrossRef]
- Wei, X.; She, G.; Wu, T.; Xue, C.; Cao, Y. PEDV enters cells through clathrin-, caveolae-, and lipid raft-mediated endocytosis and traffics via the endo-/lysosome pathway. Vet. Res. 2020, 51, 10. [Google Scholar] [CrossRef]
- Wang, H.; Shen, Y.; Zhao, L.; Ye, Y. 1-Deoxynojirimycin and its Derivatives: A Mini Review of the Literature. Curr. Med. Chem. 2021, 28, 628–643. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Nakagawa, K. A comprehensive review on the production, pharmacokinetics and health benefits of mulberry leaf iminosugars: Main focus on 1-deoxynojirimycin, d-fagomine, and 2-O-ɑ-d-galactopyranosyl-DNJ. Crit. Rev. Food Sci. Nutr. 2023, 63, 3468–3496. [Google Scholar] [CrossRef]
- Papandréou, M.J.; Barbouche, R.; Guieu, R.; Kieny, M.P.; Fenouillet, E. The alpha-glucosidase inhibitor 1-deoxynojirimycin blocks human immunodeficiency virus envelope glycoprotein-mediated membrane fusion at the CXCR4 binding step. Mol. Pharmacol. 2002, 61, 186–193. [Google Scholar] [CrossRef]
- Shimizu, H.; Tsuchie, H.; Yoshida, K.; Morikawa, S.; Tsuruoka, T.; Yamamoto, H.; Ushijima, H.; Kitamura, T. Inhibitory effect of novel 1-deoxynojirimycin derivatives on HIV-1 replication. Aids 1990, 4, 975–979. [Google Scholar] [CrossRef]
- Jacob, J.R.; Mansfield, K.; You, J.E.; Tennant, B.C.; Kim, Y.H. Natural iminosugar derivatives of 1-deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J. Microbiol. 2007, 45, 431–440. [Google Scholar]
- Block, T.M.; Lu, X.; Platt, F.M.; Foster, G.R.; Gerlich, W.H.; Blumberg, B.S.; Dwek, R.A. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc. Natl. Acad. Sci. USA 1994, 91, 2235–2239. [Google Scholar] [CrossRef]
- Perera, N.; Brun, J.; Alonzi, D.S.; Tyrrell, B.E.; Miller, J.L.; Zitzmann, N. Antiviral effects of deoxynojirimycin (DNJ)-based iminosugars in dengue virus-infected primary dendritic cells. Antivir. Res. 2022, 199, 105269. [Google Scholar] [CrossRef]
- Tyrrell, B.E.; Sayce, A.C.; Warfield, K.L.; Miller, J.L.; Zitzmann, N. Iminosugars: Promising therapeutics for influenza infection. Crit. Rev. Microbiol. 2017, 43, 521–545. [Google Scholar] [CrossRef]
- Karade, S.S.; Franco, E.J.; Rojas, A.C.; Hanrahan, K.C.; Kolesnikov, A.; Yu, W.; MacKerell, A.D., Jr.; Hill, D.C.; Weber, D.J.; Brown, A.N.; et al. Structure-Based Design of Potent Iminosugar Inhibitors of Endoplasmic Reticulum α-Glucosidase I with Anti-SARS-CoV-2 Activity. J. Med. Chem. 2023, 66, 2744–2760. [Google Scholar] [CrossRef]
- Ren, X.; Xing, Y.; He, L.; Xiu, Z.; Yang, L.; Han, A.; Jia, Q.; Dong, Y. Effect of 1-Deoxynojirimycin on insulin resistance in prediabetic mice based on next-generation sequencing and intestinal microbiota study. J. Ethnopharmacol. 2022, 289, 115029. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, M.; Li, T.; Li, D.; Feng, Y.; Wang, Y.; Qu, L.; Barcenas, A.R.; Serrano, B.R.; Shen, M.; et al. Effects of 1-Deoxynojirimycin Extracts of Mulberry Leaves on Oxidative Stress and the Function of the Intestinal Tract in Broilers Induced by H(2)O(2). Animals 2024, 14, 3319. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y.; Li, T.; Zhao, C.; Barcenas, A.R.; Serrano, B.R.; Qu, L.; Shen, M.; Zhao, W. The Effects of 1-Deoxynojirimycin from Mulberry on Oxidative Stress and Inflammation in Laying Hens and the Direct Effects on Intestine Epithelium Cells In Vitro. Animals 2023, 13, 2830. [Google Scholar] [CrossRef]
- Chun, F.; Zhang, B.B.; Lin, H.; Gao, C.F.; Min, W. d-Fagomine Attenuates High Glucose-Induced Endothelial Cell Oxidative Damage by Upregulating the Expression of PGC-1α. J. Agric. Food Chem. 2018, 66, 2758–2764. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, W.; Gu, Y.; Yu, S. 1-Deoxynojirimycin in Mulberry (Morus indica L.) Leaves Ameliorates Stable Angina Pectoris in Patients with Coronary Heart Disease by Improving Antioxidant and Anti-inflammatory Capacities. Front. Pharmacol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Piao, X.; Li, S.; Sui, X.; Guo, L.; Liu, X.; Li, H.; Gao, L.; Cai, S.; Li, Y.; Wang, T.; et al. 1-Deoxynojirimycin (DNJ) Ameliorates Indomethacin-Induced Gastric Ulcer in Mice by Affecting NF-kappaB Signaling Pathway. Front. Pharmacol. 2018, 9, 372. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Wang, F.; Li, H.; Peng, G. 1-Deoxynojirimycin Alleviates Liver Injury and Improves Hepatic Glucose Metabolism in db/db Mice. Molecules 2016, 21, 279. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, H.; Ming, X.; Bo, Z.; Shin, H.J.; Jung, Y.S.; Qian, Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021, 95, e02344-20. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.T.; Zhao, E.H.; Gao, H.L.; Xu, Y.H.; Zhao, Y.J.; Fu, G.; Cui, H.J. Recent research advances of 1-deoxynojirimycin and its derivatives. Zhongguo Zhong Yao Za Zhi 2018, 43, 1990–1997. [Google Scholar] [CrossRef]
- El Khoury, M.; Wanes, D.; Lynch-Miller, M.; Hoter, A.; Naim, H.Y. Glycosylation Modulation Dictates Trafficking and Interaction of SARS-CoV-2 S1 Subunit and ACE2 in Intestinal Epithelial Caco-2 Cells. Biomolecules 2024, 14, 537. [Google Scholar] [CrossRef]
- Clarke, E.C.; Nofchissey, R.A.; Ye, C.; Bradfute, S.B. The iminosugars celgosivir, castanospermine and UV-4 inhibit SARS-CoV-2 replication. Glycobiology 2021, 31, 378–384. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef]
- Sun, P.; Jin, J.; Wang, L.; Wang, J.; Zhou, H.; Zhang, Q.; Xu, X. Porcine epidemic diarrhea virus infections induce autophagy in Vero cells via ROS-dependent endoplasmic reticulum stress through PERK and IRE1 pathways. Vet. Microbiol. 2021, 253, 108959. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, L.; Yang, J.; Shi, H.; Zhu, H.; Zhai, M.; Lu, L.; Wang, X.; Li, X.Y.; Yu, S.; et al. 1-Deoxynojirimycin attenuates septic cardiomyopathy by regulating oxidative stress, apoptosis, and inflammation via the JAK2/STAT6 signaling pathway. Biomed. Pharmacother. 2022, 155, 113648. [Google Scholar] [CrossRef]
- Costa, T.; Fernandez-Villalba, E.; Izura, V.; Lucas-Ochoa, A.M.; Menezes-Filho, N.J.; Santana, R.C.; de Oliveira, M.D.; Araújo, F.M.; Estrada, C.; Silva, V.; et al. Combined 1-Deoxynojirimycin and Ibuprofen Treatment Decreases Microglial Activation, Phagocytosis and Dopaminergic Degeneration in MPTP-Treated Mice. J. Neuroimmune Pharmacol. 2021, 16, 390–402. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; He, W.; Wei, Y.; Han, S.; Xia, L.; Tan, B.; Yu, J.; Kang, H.; Ma, M.; et al. Effects of Small Peptide Supplementation on Growth Performance, Intestinal Barrier of Laying Hens During the Brooding and Growing Periods. Front. Immunol. 2022, 13, 925256. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.; Yan, C.; Wu, C.; Han, F.; Bo, Z.; Shen, M.; Sun, Y.; Wang, L.; Zheng, H.; et al. Phylogenetic and Genetic Variation Analysis of Porcine Epidemic Diarrhea Virus in East Central China during 2020–2023. Animals 2024, 14, 2185. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Xu, Y.; Zhang, W.; Ni, B.; Gao, S. Glycyrrhiza Polysaccharide Inhibits Pseudorabies Virus Infection by Interfering with Virus Attachment and Internalization. Viruses 2022, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Shen, S.; Zhang, M.; Liu, L.; Ghonaim, A.H.; Li, Y.; Zhang, S.; Li, W. Inhibition of porcine deltacoronavirus entry and replication by Cepharanthine. Virus Res. 2024, 340, 199303. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, W.; Chen, Y.; Wang, L.; An, W.; An, X.; Song, L.; Tong, Y.; Fan, H.; Lu, C. Transcriptome analysis of cepharanthine against a SARS-CoV-2-related coronavirus. Brief Bioinform. 2021, 22, 1378–1386. [Google Scholar] [CrossRef]
- Leng, L.; Xu, Z.; Hong, B.; Zhao, B.; Tian, Y.; Wang, C.; Yang, L.; Zou, Z.; Li, L.; Liu, K.; et al. Cepharanthine analogs mining and genomes of Stephania accelerate anti-coronavirus drug discovery. Nat. Commun. 2024, 15, 1537. [Google Scholar] [CrossRef]
- Chen, C.Z.; Xu, M.; Pradhan, M.; Gorshkov, K.; Petersen, J.D.; Straus, M.R.; Zhu, W.; Shinn, P.; Guo, H.; Shen, M.; et al. Identifying SARS-CoV-2 Entry Inhibitors through Drug Repurposing Screens of SARS-S and MERS-S Pseudotyped Particles. ACS Pharmacol. Transl. Sci. 2020, 3, 1165–1175. [Google Scholar] [CrossRef]
- Zou, H.; Niu, Z.; Tang, Z.; Cheng, P.; Yin, Y.; Luo, G.; Huang, S. The Mechanism of Action of the Active Ingredients of Coptidis rhizoma against Porcine Epidemic Diarrhea Was Investigated Using Network Pharmacology and Molecular Docking Technology. Viruses 2024, 16, 1229. [Google Scholar] [CrossRef]
- Xiang, H.; Qiao, J.; Lin, H.; Li, J.; Li, Y.; Sun, H.; Wang, X.; Bi, R.; Zhang, Z.; Bo, Z.; et al. Berbamine inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet. Microbiol. 2024, 298, 110244. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Wang, L.; Zhang, X.; Zhang, Y.; Gai, X.; Chen, L.; Liu, L.; Yang, L.; Wang, B. Corrigendum: Network pharmacology-based exploration identified the antiviral efficacy of Quercetin isolated from mulberry leaves against enterovirus 71 via the NF-κB signaling pathway. Front. Pharmacol. 2024, 15, 1421483. [Google Scholar] [CrossRef]
- Ouzounov, S.; Mehta, A.; Dwek, R.A.; Block, T.M.; Jordan, R. The combination of interferon alpha-2b and n-butyl deoxynojirimycin has a greater than additive antiviral effect upon production of infectious bovine viral diarrhea virus (BVDV) in vitro: Implications for hepatitis C virus (HCV) therapy. Antivir. Res. 2002, 55, 425–435. [Google Scholar] [CrossRef]
- Mehta, A.; Ouzounov, S.; Jordan, R.; Simsek, E.; Lu, X.; Moriarty, R.M.; Jacob, G.; Dwek, R.A.; Block, T.M. Imino sugars that are less toxic but more potent as antivirals, in vitro, compared with N-n-nonyl DNJ. Antivir. Chem. Chemother. 2002, 13, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bai, J.; Liu, X.; Wang, M.; Wang, X.; Jiang, P. Tomatidine inhibits porcine epidemic diarrhea virus replication by targeting 3CL protease. Vet. Res. 2020, 51, 136. [Google Scholar] [CrossRef] [PubMed]
- Diosa-Toro, M.; Troost, B.; van de Pol, D.; Heberle, A.M.; Urcuqui-Inchima, S.; Thedieck, K.; Smit, J.M. Tomatidine, a novel antiviral compound towards dengue virus. Antivir. Res. 2019, 161, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The steroidal alkaloids α-tomatine and tomatidine: Panorama of their mode of action and pharmacological properties. Steroids 2021, 176, 108933. [Google Scholar] [CrossRef]
- Gong, T.; Wu, D.; Feng, Y.; Liu, X.; Gao, Q.; Zheng, X.; Song, Z.; Wang, H.; Zhang, G.; Gong, L. Inhibitory effects of quercetin on porcine epidemic diarrhea virus in vitro and in vivo. Virology 2024, 589, 109923. [Google Scholar] [CrossRef]
- Li, Z.; Cao, H.; Cheng, Y.; Zhang, X.; Zeng, W.; Sun, Y.; Chen, S.; He, Q.; Han, H. Inhibition of Porcine Epidemic Diarrhea Virus Replication and Viral 3C-Like Protease by Quercetin. Int. J. Mol. Sci. 2020, 21, 8095. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Woolner, E.; Perry, J.K.; Feng, J.Y.; Porter, D.P.; Götte, M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, 295, 6785–6797. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Sanjuán, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef]



| Primer | Sequence (5′–3′) |
|---|---|
| PEDV-N-F | AAACCACGCAGCAGCAGAATG |
| PEDV-N-R | TGTATTTTTTCCGCTGTTGTC |
| PEDV-S-F | GAACTGCCATTCAGCGTATT |
| PEDV-S-R | ACCGAACTCAGGGTAACCAA |
| PEDV-M-F | TTGTATGGTGTCAAGATGGC |
| PEDV-M-R | AAGGATGCTGAAAGCGAAAA |
| PEDV-ORF3-F | TTTGCACTGTTTAAAGCGTCT |
| PEDV-ORF3-R | AGTAAAAGCAGACTAAACAAAGCCT |
| β-GAPDH-F | AACGGATTTGGTCGTATTGGG |
| β-GAPDH-R | CAGCTTTGGGGCAGCACTCA |
| IL-1β-F | GCGGCAACGAGGATGACTT |
| IL-1β-R | TGGCTACAACAACTGACACGG |
| IL-8-F | GGAACCATCTCGCTCTGTGTAA |
| IL-8-R | GGTCCACTCTCAATCACTCTCAG |
| MCP-F | ACTTGCTGCTGGTGATTCTTCT |
| MCP-R | CTTCTGTGCCTGCTGCTCATA |
| TNF-α-F | CACCACGCTCTTCTGTCT |
| TNF-α-R | AGATGATCTGACTGCCTGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, L.; Ma, K.; Shen, M.; Liu, J.; Zhang, Y.; Sun, L. Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus. Animals 2025, 15, 1207. https://doi.org/10.3390/ani15091207
Sun Y, Wang L, Ma K, Shen M, Liu J, Zhang Y, Sun L. Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus. Animals. 2025; 15(9):1207. https://doi.org/10.3390/ani15091207
Chicago/Turabian StyleSun, Yiwei, Liyan Wang, Keke Ma, Manman Shen, Jiying Liu, Yujuan Zhang, and Liumei Sun. 2025. "Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus" Animals 15, no. 9: 1207. https://doi.org/10.3390/ani15091207
APA StyleSun, Y., Wang, L., Ma, K., Shen, M., Liu, J., Zhang, Y., & Sun, L. (2025). Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus. Animals, 15(9), 1207. https://doi.org/10.3390/ani15091207






