Evaluation of Body and Udder Temperatures and Mammary Gland Health Status Throughout Lactation in Manchega Dairy Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Facilities and Management
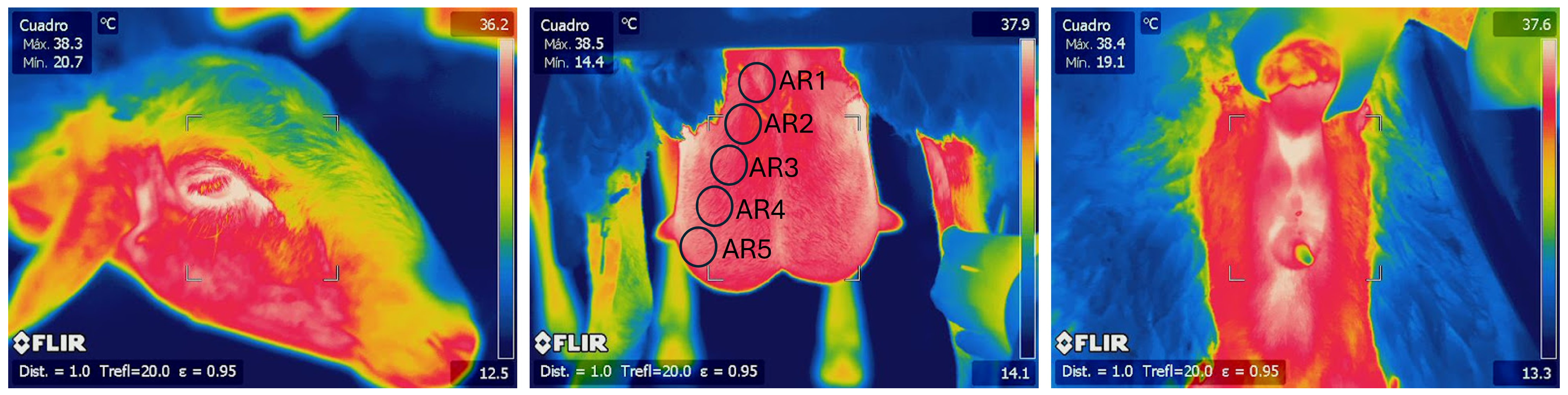
2.2. Temperature Measurement and Thermographic Image Description
2.3. Mammary Glands’ Health Status
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teixeira, D.L.; Boyle, L.A.; Enríquez-Hidalgo, D. Skin Temperature of Slaughter Pigs with Tail Lesions. Front. Vet. Sci. 2020, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.N.; Silva, S.R. Use of Infrared Thermography to Assess Body Temperature as a Physiological Stress Indicator in Horses during Ridden and Lunging Sessions. Animals 2022, 12, 3255. [Google Scholar] [CrossRef] [PubMed]
- Cugmas, B.; Šušterič, P.; Ružić Gorenjec, N.; Plavec, T. Comparison between Rectal and Body Surface Temperature in Dogs by the Calibrated Infrared Thermometer. Vet. Anim. Sci. 2020, 10, 100120. [Google Scholar] [CrossRef]
- Easterwood, L.; Cohen, N.D. Agreement of Temperatures Measured Using a Non-Contact Infrared Thermometer with a Rectal Digital Thermometer in Horses. J. Equine Vet. Sci. 2023, 123, 104243. [Google Scholar] [CrossRef]
- Ibáñez, C.; Moreno-Manrique, M.; Villagrá, A.; Bueso-Ródenas, J.; Mínguez, C. Evaluation of Non-Contact Device to Measure Body Temperature in Sheep. Animals 2024, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Gelasakis, A.I.; Kalogianni, A.I.; Moschovas, M.; Tsimpouri, E.; Pnevmatikos, T.; Bossis, I.; Arsenos, G.; Simitzis, P. Evaluation of Infrared Thermography for the Detection of Footrot and White Line Disease Lesions in Dairy Sheep. Vet. Sci. 2021, 8, 219. [Google Scholar] [CrossRef]
- Coe, A.; Blackie, N. Comparison of Low- and High-Cost Infrared Thermal Imaging Devices for the Detection of Lameness in Dairy Cattle. Vet. Sci. 2022, 9, 414. [Google Scholar] [CrossRef]
- Castells, E.; Lacasta, D.; Climent, M.; Pérez, M.; Sanromán, F.; Jiménez, C.; Ferrer, L.M. Diagnostic Imaging Techniques of the Respiratory Tract of Sheep. Small Rumin. Res. 2019, 180, 112–126. [Google Scholar] [CrossRef]
- Scott, S.L.; Schaefer, A.L.; Tong, A.K.W.; Lacasse, P. Use of Infrared Thermography for Early Detection of Mastitis in Dairy Cows. Can. J. Anim. Sci. 2000, 80, 764. [Google Scholar]
- Colak, A.; Polat, B.; Okumus, Z.; Kaya, M.; Yanmaz, L.E.; Hayirli, A. Early Detection of Mastitis Using Infrared Thermography in Dairy Cows. J. Dairy Sci. 2008, 91, 4244–4248. [Google Scholar] [CrossRef]
- Hovinen, M.; Siivonen, J.; Taponen, S.; Hänninen, L.; Pastell, M.; Aisla, A.M.; Pyörälä, S. Detection of Clinical Mastitis with the Help of a Thermal Camera. J. Dairy Sci. 2008, 91, 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Klee, W. Infrared Thermography of the Udder Surface of Dairy Cattle: Characteristics, Methods, and Correlation with Rectal Temperature. Vet. J. 2014, 199, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Pampariene, I.; Veikutis, V.; Oberauskas, V.; Zymantiene, J.; Zelvyte, R.; Stankevicius, A.; Marciulionyte, D.; Palevicius, P. Thermography-Based Inflammation Monitoring of Udder State in Dairy Cows: Sensitivity and Diagnostic Priorities Comparing with Routine California Mastitis Test. J. Vibroeng. 2016, 18, 511–521. [Google Scholar]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef]
- Bortolami, A.; Fiore, E.; Gianesella, M.; Corrò, M.; Catania, S.; Morgante, M. Evaluation of the Udder Health Status in Sub-Clinical Mastitis-Affected Dairy Cows through Bacteriological Culture, Somatic Cell Count and Thermographic Imaging. Pol. J. Vet. Sci. 2015, 18, 799–805. [Google Scholar] [CrossRef]
- Porcionato, M.A.F.; Canata, T.F.; De Oliveira, C.E.L.; Dos Santos, M.V. Udder Thermography of Gyr Cows for Subclinical Mastitis Detection. Rev. Bras. Eng. Biossistemas 2009, 3, 251–257. [Google Scholar] [CrossRef]
- Byrne, D.T.; Berry, D.P.; Esmonde, H.; McHugh, N. Investigation of the Relationship between Udder Quarter Somatic Cell Count and Udder Skin Surface Temperature of Dairy Cows Measured by Infrared Thermography. J. Anim. Sci. 2018, 96, 4458–4470. [Google Scholar] [CrossRef]
- De la Cruz, M.; Serrano, E.; Montoro, V.; Marco, J.; Romeo, M.; Baselga, R.; Albizu, I.; Amorena, B. Etiology and Prevalence of Subclinical Mastitis in the Manchega Sheep at Mid-Late Lactation. Small Rumin. Res. 1994, 14, 175–180. [Google Scholar] [CrossRef]
- Ariznabarreta, A.; Gonzalo, C.; San Primitivo, F. Microbiological Quality and Somatic Cell Count of Ewe Milk with Special Reference to Staphylococci. J. Dairy Sci. 2002, 85, 1370–1375. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in Sheep–The Last 10 Years and the Future of Research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef]
- Romero, G.; Roca, A.; Alejandro, M.; Muelas, R.; Díaz, J.R. Relationship of Mammary Gland Health Status and Other Non-Infectious Factors with Electrical Conductivity of Milk in Manchega Ewes. J. Dairy Sci. 2017, 100, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Menzies, P. Udder Health for Dairy Goats. Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Martí-De Olives, A.; Le Roux, Y.; Rubert-Alemán, J.; Peris, C.; Molina, M.P. Effect of Subclinical Mastitis on Proteolysis in Ovine Milk. J. Dairy Sci. 2011, 94, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.; Sánchez, A.; Contreras, A.; Luengo, C.; Corrales, J.C.; Morales, C.T.; de la Fe, C.; Guirao, I.; Gonzalo, C. Detection Limits of Four Antimicrobial Residue Screening Tests for Beta-Lactams in Goat’s Milk. J. Dairy Sci. 2009, 92, 3585–3591. [Google Scholar] [CrossRef]
- Simões, J.; Abecia, J.A.; Cannas, A.; Delgadillo, J.A.; Lacasta, D.; Voigt, K.; Chemineau, P. Managing Sheep and Goats for Sustainable High-Yield Production. Animal 2021, 15, 100293. [Google Scholar] [CrossRef]
- Martins, R.F.; do Prado Paim, T.; de Abreu Cardoso, C.; Stéfano Lima Dallago, B.; de Melo, C.B.; Louvandini, H.; McManus, C. Mastitis Detection in Sheep by Infrared Thermography. Res. Vet. Sci. 2013, 94, 722–724. [Google Scholar] [CrossRef]
- Castro-Costa, A.; Caja, G.; Salama, A.A.K.; Rovai, M.; Flores, C.; Aguiló, J. Thermographic Variation of the Udder of Dairy Ewes in Early Lactation and Following an Escherichia coli Endotoxin Intramammary Challenge in Late Lactation. J. Dairy Sci. 2014, 97, 1377–1387. [Google Scholar] [CrossRef]
- Marnet, P.G.; Velasquez, A.B.; Dzidic, A. Infrared Thermography of Teat in French Dairy Alpine Goats: A Promising Tool to Study Animal–Machine Interaction during Milking but Not to Detect Mastitis. Animals 2024, 14, 882. [Google Scholar] [CrossRef]
- Bueso-Ródenas, J.; Alejandro, M.; Romero, G.; Roca, A.; Díaz, J.R. Effects of Automatic Prestimulation in the Milking of Manchega Sheep. Livest. Sci. 2022, 255, 104813. [Google Scholar] [CrossRef]
- Mazdeyasna, S.; Ghassemi, P.; Wang, Q. Best Practices for Body Temperature Measurement with Infrared Thermography: External Factors Affecting Accuracy. Sensors 2023, 23, 8011. [Google Scholar] [CrossRef]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary Applications of Infrared Thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Leite, R.F.; Rodrigues, L.A.; de Freitas Neto, M.A.; Nogueira, F.R.B.; da Silva Lima, J.D.; de Souza, B.B.; de Miranda Neto, E.G. Principles and Cautions in the Infrared Thermography Application in Sheep. Multidiscip. Rev. 2022, 5, 2022001. [Google Scholar] [CrossRef]
- McManus, C.; Bianchini, E.; Paim, T.D.P.; De Lima, F.G.; Neto, J.B.; Castanheira, M.; Esteves, G.I.F.; Cardoso, C.C.; Dalcin, V.C. Infrared Thermography to Evaluate Heat Tolerance in Different Genetic Groups of Lambs. Sensors 2015, 15, 17258–17273. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.; Sullivan, M.; Gaughan, J.B.; Phillips, C.J.C. The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures. Animals 2024, 14, 2898. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef]
- Blond, B.; Majkić, M.; Spasojević, J.; Hristov, S.; Radinović, M.; Nikolić, S.; Anđušić, L.; Čukić, A.; Došenović Marinković, M.; Vujanović, B.D.; et al. Influence of Heat Stress on Body Surface Temperature and Blood Metabolic, Endocrine, and Inflammatory Parameters and Their Correlation in Cows. Metabolites 2024, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Lees, J.; Sejian, V.; Wallage, A.; Gaughan, J. Using Infrared Thermography as an In Situ Measure of Core Body Temperature in Lot-Fed Angus Steers. Int. J. Biometeorol. 2018, 62, 3–8. [Google Scholar] [CrossRef]
- Karthik, D.; Suresh, J.; Reddy, Y.R.; Sharma, G.R.K.; Ramana, J.V.; Gangaraju, G.; Reddy, P.R.K. Farming Systems in Sheep Rearing: Impact on Growth and Reproductive Performance, Nutrient Digestibility, Disease Incidence and Heat Stress Indices. PLoS ONE 2021, 16, e0244922. [Google Scholar] [CrossRef]
- Carnovale, F.; Phillips, C.J.C. The Effects of Heat Stress on Sheep Welfare during Live Export Voyages from Australia to the Middle East. Animals 2020, 10, 694. [Google Scholar] [CrossRef]
- Redaelli, V.; Zaninelli, M.; Martino, P.; Luzi, F.; Costa, L.N. A Precision Livestock Farming Technique from Breeding to Slaughter: Infrared Thermography in Pig Farming. Appl. Sci. 2024, 14, 5780. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-Invasive Measurement of Stress in Dairy Cows Using Infrared Thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Pyörälä, S. Indicators of Inflammation in the Diagnosis of Mastitis. Vet. Res. 2003, 34, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Zaias, J.; Altman, N.H. Acute Phase Response in Animals: A Review. Comp. Med. 2009, 59, 517–526. [Google Scholar]
- Spiegel, S.; Spiegel, F.; Luepke, M.; Wendt, M.; von Altrock, A. Ultrasonography and Infrared Thermography as a Comparative Diagnostic Tool to Clinical Examination to Determine Udder Health in Sows. Animals 2022, 12, 2713. [Google Scholar] [CrossRef] [PubMed]
- Paterna, A.; Gómez-Martín, Á.; Van Der Ham, M.P.; Tatay-Dualde, J.; Amores, J.; Corrales, J.C.; Sánchez, A.; Contreras, A.; De La Fe, C. Mycoplasma Excretion in Reproductive Male and Female Goats. Jpn. J. Vet. Res. 2017, 65, 83–88. [Google Scholar]
- Sutra, L.; Poutrel, B. Virulence Factors Involved in the Pathogenesis of Bovine Intramammary Infections Due to Staphylococcus aureus. J. Med. Microbiol. 1994, 40, 79–89. [Google Scholar] [CrossRef]
- Haveri, M.; Taponen, S.; Vuopio-Varkila, J.; Salmenlinna, S.; Pyörälä, S. Bacterial Genotype Affects the Manifestation and Persistence of Bovine Staphylococcus aureus Intramammary Infection. J. Clin. Microbiol. 2005, 43, 959–961. [Google Scholar] [CrossRef]
- Pedersen, L.H.; Aalbaek, B.; Røntved, C.M.; Ingvartsen, K.L.; Sørensen, N.S.; Heegaard, P.M.; Jensen, H.E. Early Pathogenesis and Inflammatory Response in Experimental Bovine Mastitis Due to Streptococcus uberis. J. Comp. Pathol. 2003, 128, 156–164. [Google Scholar] [CrossRef]
- Leitner, G.; Merin, U.; Silanikove, N. Changes in Milk Composition as Affected by Subclinical Mastitis in Sheep. J. Dairy Sci. 2004, 87, 46–52. [Google Scholar] [CrossRef]
| Variable | Average | SD | Minimum | Maximum |
|---|---|---|---|---|
| AT (°C) | 18.70 | 6.65 | 9.3 | 30.0 |
| AR01Max (°C) | 35.16 | 2.52 | 20.9 | 39.4 |
| AR02Max (°C) | 35.24 | 2.44 | 21.8 | 39.2 |
| AR03Max (°C) | 35.23 | 2.32 | 21.6 | 39.4 |
| AR04Max (°C) | 34.94 | 2.26 | 22.5 | 38.8 |
| AR05Max (°C) | 34.45 | 2.30 | 23.2 | 39.0 |
| AR01Avg (°C) | 34.12 | 2.65 | 20.2 | 38.6 |
| AR02Avg (°C) | 34.07 | 2.58 | 21.1 | 38.5 |
| AR03Avg (°C) | 33.83 | 2.61 | 20.8 | 38.5 |
| AR04Avg (°C) | 33.39 | 2.68 | 22.5 | 38.0 |
| AR05Avg (°C) | 32.81 | 2.81 | 25.1 | 37.8 |
| PT (°C) | 38.69 | 1.13 | 34.2 | 40.9 |
| RT (°C) | 38.86 | 0.361 | 37.2 | 39.9 |
| LT (°C) | 37.93 | 0.89 | 35.1 | 40.1 |
| SCC (°C) | 338 | 1964 | 4 | 27,007 |
| AT | AR01 Max | AR02 Max | AR03 Max | AR04 Max | AR05 Max | AR01 Avg | AR02 Avg | AR03 Avg | AR04 Avg | AR05 Avg | PT | RT | LT | SCC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AT | 1.00 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | 0.06 | ** | 0.12 |
| AR01Max | 0.72 | 1.00 | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | * | ** | 0.09 |
| AR02Max | 0.76 | 0.97 | 1.00 | ** | ** | ** | ** | ** | ** | ** | ** | ** | 0.09 | ** | 0.10 |
| AR03Max | 0.80 | 0.92 | 0.95 | 1.00 | ** | ** | ** | ** | ** | ** | ** | ** | 0.44 | ** | 0.07 |
| AR04Max | 0.79 | 0.83 | 0.87 | 0.94 | 1.00 | ** | ** | ** | ** | ** | ** | ** | 0.43 | ** | * |
| AR05Max | 0.77 | 0.76 | 0.80 | 0.85 | 0.91 | 1.00 | ** | ** | ** | ** | ** | ** | 0.23 | ** | * |
| AR01Avg | 0.77 | 0.98 | 0.96 | 0.92 | 0.84 | 0.79 | 1.00 | ** | ** | ** | ** | ** | * | ** | * |
| AR02Avg | 0.80 | 0.95 | 0.98 | 0.95 | 0.88 | 0.81 | 0.97 | 1.00 | ** | ** | ** | ** | 0.25 | ** | * |
| AR03Avg | 0.83 | 0.90 | 0.94 | 0.97 | 0.93 | 0.85 | 0.93 | 0.97 | 1.00 | ** | ** | ** | 0.61 | ** | * |
| AR04Avg | 0.82 | 0.82 | 0.86 | 0.91 | 0.96 | 0.92 | 0.86 | 0.89 | 0.95 | 1.00 | ** | ** | 0.75 | ** | * |
| AR05Avg | 0.81 | 0.76 | 0.79 | 0.83 | 0.89 | 0.96 | 0.80 | 0.83 | 0.87 | 0.93 | 1.00 | ** | 0.43 | ** | * |
| PT | 0.59 | 0.57 | 0.57 | 0.57 | 0.55 | 0.52 | 0.59 | 0.59 | 0.59 | 0.57 | 0.56 | 1.00 | * | ** | 0.05 |
| RT | −0.06 | 0.08 | 0.05 | 0.02 | 0.04 | 0.07 | 0.04 | 0.01 | 0.02 | 0.01 | 0.03 | 0.19 | 1.00 | 0.45 | 0.06 |
| LT | 0.77 | 0.63 | 0.64 | 0.67 | 0.66 | 0.65 | 0.64 | 0.65 | 0.68 | 0.68 | 0.66 | 0.58 | 0.04 | 1.00 | 0.79 |
| SCC | −0.05 | −0.06 | −0.06 | −0.06 | −0.08 | −0.07 | −0.07 | −0.07 | −0.08 | −0.08 | −0.08 | −0.06 | −0.06 | 0.01 | 1.00 |
| Variable | MG SCC Status | Mean | SR | p-Value | Variable | MG MC Status | Mean | SR | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| AR01Max (°C) | Low SCC | 35.22 | 0.12 | <0.01 | AR01Max (°C) | − | 35.19 | 0.12 | 0.39 |
| High SCC | 34.08 | 0.36 | + | 34.97 | 0.24 | ||||
| AR02Max (°C) | Low SCC | 35.30 | 0.10 | <0.01 | AR02Max (°C) | − | 35.26 | 0.11 | 0.56 |
| High SCC | 34.16 | 0.34 | + | 35.12 | 0.23 | ||||
| AR03Max (°C) | Low SCC | 35.30 | 0.08 | <0.01 | AR03Max (°C) | − | 35.26 | 0.09 | 0.46 |
| High SCC | 34.14 | 0.31 | + | 35.10 | 0.21 | ||||
| AR04Max (°C) | Low SCC | 35.01 | 0.08 | <0.01 | AR04Max (°C) | − | 34.99 | 0.09 | 0.17 |
| High SCC | 33.87 | 0.31 | + | 34.68 | 0.21 | ||||
| AR05Max (°C) | Low SCC | 34.52 | 0.09 | <0.01 | AR05Max (°C) | − | 34.51 | 0.09 | 0.11 |
| High SCC | 33.51 | 0.31 | + | 34.14 | 0.21 | ||||
| AR01Avg (°C) | Low SCC | 34.18 | 0.12 | <0.01 | AR01Avg (°C) | − | 34.16 | 0.12 | 0.27 |
| High SCC | 32.98 | 0.37 | + | 33.87 | 0.25 | ||||
| AR02Avg (°C) | Low SCC | 34.14 | 0.10 | <0.01 | AR02Avg (°C) | − | 34.12 | 0.11 | 0.20 |
| High SCC | 32.95 | 0.36 | + | 33.80 | 0.24 | ||||
| AR03Avg (°C) | Low SCC | 33.89 | 0.09 | <0.01 | AR03Avg (°C) | − | 33.89 | 0.10 | 0.11 |
| High SCC | 32.66 | 0.35 | + | 33.48 | 0.23 | ||||
| AR04Avg (°C) | Low SCC | 33.45 | 0.10 | <0.01 | AR04Avg (°C) | − | 33.45 | 0.10 | 0.08 |
| High SCC | 32.34 | 0.36 | + | 32.96 | 0.24 | ||||
| AR05Avg (°C) | Low SCC | 32.86 | 0.10 | 0.02 | AR05Avg (°C) | − | 32.86 | 0.11 | 0.04 |
| High SCC | 31.96 | 0.38 | + | 31.96 | 0.38 | ||||
| PT (°C) | Low SCC | 38.69 | 0.05 | 0.52 | PT (°C) | − | 38.74 | 0.05 | <0.01 |
| High SCC | 38.59 | 0.16 | + | 38.40 | 0.11 | ||||
| RT (°C) | Low SCC | 38.87 | 0.03 | 0.12 | RT (°C) | − | 38.87 | 0.03 | 0.80 |
| High SCC | 38.79 | 0.05 | + | 38.86 | 0.04 | ||||
| LT (°C) | Low SCC | 38.01 | 0.04 | 0.62 | LT (°C) | − | 37.94 | 0.05 | 0.49 |
| High SCC | 37.92 | 0.17 | + | 37.86 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueso-Ródenas, J.; Moreno-Manrique, M.; Gascó, P.; Arias, R.; Romero, G.; Díaz, J.R. Evaluation of Body and Udder Temperatures and Mammary Gland Health Status Throughout Lactation in Manchega Dairy Sheep. Animals 2025, 15, 773. https://doi.org/10.3390/ani15060773
Bueso-Ródenas J, Moreno-Manrique M, Gascó P, Arias R, Romero G, Díaz JR. Evaluation of Body and Udder Temperatures and Mammary Gland Health Status Throughout Lactation in Manchega Dairy Sheep. Animals. 2025; 15(6):773. https://doi.org/10.3390/ani15060773
Chicago/Turabian StyleBueso-Ródenas, Joel, María Moreno-Manrique, Pilar Gascó, Ramón Arias, Gema Romero, and José Ramón Díaz. 2025. "Evaluation of Body and Udder Temperatures and Mammary Gland Health Status Throughout Lactation in Manchega Dairy Sheep" Animals 15, no. 6: 773. https://doi.org/10.3390/ani15060773
APA StyleBueso-Ródenas, J., Moreno-Manrique, M., Gascó, P., Arias, R., Romero, G., & Díaz, J. R. (2025). Evaluation of Body and Udder Temperatures and Mammary Gland Health Status Throughout Lactation in Manchega Dairy Sheep. Animals, 15(6), 773. https://doi.org/10.3390/ani15060773






