Morphological, Histological and Morphometrical Aspects of Auditory Ossicles in Pig Fetuses (Sus scrofa domestica)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Image Processing Technique
2.2. Histological Technique
2.3. Morphometric Aspects and Measurements
3. Results
4. Discussion
4.1. Morphology and Morphometry of the Fetal Auditory Ossicle
4.2. Adult vs. Fetal Dimensional Features of the Auditory Ossicles
4.3. Middle Ear Lever Ratio
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simaei, N.; Soltanalinejad, F.; Najafi, G.; Shalizar Jalali, A. Anatomical and Morphometrical Study of Middle Ear Ossicles in 2 to 3-Month-Old Makouei Sheep Fetuses. Vet. Res. Forum 2017, 8, 237–241. [Google Scholar] [PubMed]
- Erdogan, S.; Kilinc, M. Gross Anatomy and Arterial Vascularization of the Tympanic Cavity and Osseous Labyrinth in Mid-Gestational Bovine Fetuses. Anat. Rec. 2010, 293, 2083–2093. [Google Scholar] [CrossRef]
- Parodi, A.-L.; Barone, R.; Simoes, P. Anatomie Comparée Des Mammifères Domestiques, Paris, Vigot Édit., 2010, 7, Neurologie 2, 836 p. Bull. Acad. Natl. Med. 2011, 195, 1148–1150. [Google Scholar] [CrossRef]
- Robert, M.P.; Carstens, A.; de Beer, F.C.; Hoffman, J.W.; Steenkamp, G. Micro-Anatomy of the Ear of the Southern White Rhinoceros (Ceratotherium simum simum). Anat. Histol. Embryol. 2021, 50, 316–323. [Google Scholar] [CrossRef]
- Barone, R.; Bortolami, R. Anatomie Comparee Des Mammiferes Domestiques. Tome Sixieme. Neurologie I. Systeme Nerveux Central; Vigot Frerres: Paris, France, 2004. [Google Scholar]
- Ugarteburu, M.; Withnell, R.H.; Cardoso, L.; Carriero, A.; Richter, C.-P. Mammalian Middle Ear Mechanics: A Review. Front. Bioeng. Biotechnol. 2022, 10, 983510. [Google Scholar] [CrossRef] [PubMed]
- Martonos, C.O.; Gudea, A.I.; Ratiu, I.A.; Stan, F.G.; Bolfă, P.; Little, W.B.; Dezdrobitu, C.C. Anatomical, Histological, and Morphometrical Investigations of the Auditory Ossicles in Chlorocebus aethiops sabaeus from Saint Kitts Island. Biology 2023, 12, 631. [Google Scholar] [CrossRef]
- Takechi, M.; Kuratani, S. History of Studies on Mammalian Middle Ear Evolution: A Comparative Morphological and Developmental Biology Perspective. J. Exp. Zool. Part B Mol. Dev. Evol. 2010, 314 B, 417–433. [Google Scholar] [CrossRef]
- Amin, S.; Tucker, A.S. Joint Formation in the Middle Ear: Lessons from the Mouse and Guinea Pig. Dev. Dyn. 2006, 235, 1326–1333. [Google Scholar] [CrossRef]
- Padmini, P.; Narasinga Rao, B. Morphological Variations in Human Fetal Ear Ossicles—A Study. Int. J. Anat. Res. 2013, 1, 40–42. [Google Scholar]
- Anson, B.J.; Bast, T.H. Development of the Incus of the Human Ear; Illustrated in Atlas Series. Q. Bull. Northwest Univ. Med. Sch. 1959, 33, 110–119. [Google Scholar]
- Anson, B.J.; Bast, T.H. Development of the Stapes of the Human Ear; Illustrated in Atlas Series. Q. Bull. Northwest Univ. Med. Sch. 1959, 33, 44–59. [Google Scholar]
- Whyte, J.; Cisneros, A.; Yus, C.; Obón, J.; Whyte, A.; Serrano, P.; Pérez-Castejón, C.; Vera, A. Development of the Dynamic Structure (Force Lines) of the Middle Ear Ossicles in Human Foetuses. Histol. Histopathol. 2008, 23, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.H.; Röösli, C.; Chatzimichalis, M.; Eiber, A.; Huber, A.M. Characterization of Stapes Anatomy: Investigation of Human and Guinea Pig. JARO J. Assoc. Res. Otolaryngol. 2013, 14, 159. [Google Scholar] [CrossRef]
- Mallo, M. Embryological and Genetic Aspects of Middle Ear Development. Int. J. Dev. Biol. 1998, 42, 11–22. [Google Scholar] [PubMed]
- Huy, P.T.B.; Teissier, N. Embryologie de l’oreille Moyenne. Oto-Rhino-Laryngologie 2011, 6, 1–9. [Google Scholar]
- Carlson, B.M. Human Embryology and Developmental Biology; Mosby: St. Louis, MI, USA, 1994; 447p, ISBN 0801664152/9780801664151. [Google Scholar]
- Martonos, C.; Gudea, A.; D’Amico, G.; Stan, F.; Stroe, T. Morphological and Morphometrical Anatomy of the Auditory Ossicles in Roe Deer (Capreolus capreolus). Eur. Zool. J. 2022, 89, 1094–1103. [Google Scholar] [CrossRef]
- Martonos, C.; Gudea, A.; Lațiu, C.; Blagojevic, M.; Stan, F. Morphological and Morphometrical Aspects of the Auditory Ossicles in the European Badger (Meles Meles). Vet. Sci. 2022, 9, 483. [Google Scholar] [CrossRef]
- Martonos, C.; Damian, A.; Gudea, A.I.; Bud, I.; Stan, G.F. Morphological and Morphometrical Study of the Auditory Ossicles in Chinchilla. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2019, 48, 340–345. [Google Scholar] [CrossRef]
- Wang, X.; Gan, R.Z. 3D Finite Element Model of the Chinchilla Ear for Characterizing Middle Ear Functions. Biomech. Model. Mechanobiol. 2016, 15, 1263–1277. [Google Scholar] [CrossRef]
- Guan, M.; Zhang, J.; Jia, Y.; Cao, X.; Lou, X.; Li, Y.; Gao, X. Middle Ear Structure and Transcanal Approach Appropriate for Middle Ear Surgery in Rabbits. Exp. Ther. Med. 2019, 17, 1248–1255. [Google Scholar] [CrossRef]
- Martins, L.L.; Almeida-Silva, I.; Rossato, M.; Murashima, A.A.B.; Hyppolito, M.A.; Machado, M.R.F. Macroscopic Description of the External and Middle Ear of Paca (Cuniculus Paca Linnaeus, 1766). Pesqui. Vet. Bras. 2015, 35, 583–589. [Google Scholar] [CrossRef]
- Mason, M.J.; Cornwall, H.L.; Smith, E.S.J. Ear Structures of the Naked Mole-Rat, Heterocephalus Glaber, and Its Relatives (Rodentia: Bathyergidae). PLoS ONE 2016, 11, e0167079. [Google Scholar] [CrossRef] [PubMed]
- Anthwal, N.; Joshi, L.; Tucker, A.S. Evolution of the Mammalian Middle Ear and Jaw: Adaptations and Novel Structures. J. Anat. 2013, 222, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Gurr, A.; Kevenhörster, K.; Stark, T.; Pearson, M.; Dazert, S. The Common Pig: A Possible Model for Teaching Ear Surgery. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 213–217. [Google Scholar] [CrossRef]
- Hoffstetter, M.; Lugauer, F.; Kundu, S.; Wacker, S.; Perea-Saveedra, H.; Lenarz, T.; Hoffstetter, P.; Schreyer, A.G.; Wintermantel, E. Middle Ear of Human and Pig: A Comparison of Structures and Mechanics. Biomed. Tech. 2011, 56, 159–165. [Google Scholar] [CrossRef]
- Pracy, J.P.; White, A.; Mustafa, Y.; Smith, D.; Perry, M.E. The Comparative Anatomy of the Pig Middle Ear Cavity: A Model for Middle Ear Inflammation in the Human? J. Anat. 1998, 192, 359–368. [Google Scholar] [CrossRef]
- Pinheiro, J.N.; dos Santos Ferraz, R.H.; Ribeiro, M.; Motheo, T.F. Fetal External Features and Morphology of the Umbilical Cord of Wild Boars. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2024, 53, e13041. [Google Scholar] [CrossRef]
- Karunasri, V.; Nagamalleswari, Y.; K, R.N. Morphogenesis and Morphometry of Middle Ear Ossicles in Nellore Sheep Fetus. Indian J. Vet. Anat. 2023, 35, 134–138. [Google Scholar]
- Marrable, A.W. The Embryonic Pig: A Chronological Account; Pitman: London, UK, 1971; ISBN 9780272672426. [Google Scholar]
- Casa, B.; Lorentz, S.; Hermoin, S. Evaluating external auditory exostosis severity using ImageJ: A clinical method applied to archaeological remains. Int. J. Osteoarchaeol. 2024, 34, e3342. [Google Scholar] [CrossRef]
- Fleming, S. Basic Histology. Histopathology 1990, 16, 511. [Google Scholar] [CrossRef]
- Stoessel, A.; Gunz, P.; David, R.; Spoor, F. Comparative Anatomy of the Middle Ear Ossicles of Extant Hominids—Introducing a Geometric Morphometric Protocol. J. Hum. Evol. 2016, 91, 1–25. [Google Scholar] [CrossRef]
- Quam, R.M.; Coleman, M.N.; Martínez, I. Evolution of the Auditory Ossicles in Extant Hominids: Metric Variation in African Apes and Humans. J. Anat. 2014, 225, 167–196. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, V.; Kachlik, D.; Bahşi, I.; Carlson, M.; Isaacson, B.; Broman, J.; Tubbs, R.S.; Baud, R.; ten Donkelaar, H.J. Relevant Temporal Bone Anatomy for Robotic Cochlear Implantation: An Updated Terminology Combined with Anatomical and Clinical Terms. Transl. Res. Anat. 2021, 25, 167–196. [Google Scholar] [CrossRef]
- Yi, H.; Guo, W.; Chen, L.; Wu, N.; Li, J.; Ren, L.; Yang, S. Microdissection of Miniature Pig Ear. J. Otol. 2013, 8, 91–96. [Google Scholar] [CrossRef]
- Unur, E.; Ülger, H.; Ekinci, N. Morphometrical and Morphological Variations of Middle Ear Ossicles in the Newborn. Erciyes Tip Derg. 2002, 24, 57–63. [Google Scholar]
- Wang, W.; Wang, X.; Chen, L.; Li, Z.; Zhang, S.; Su, B.; Zhao, F.F.; Weng, Z.; Guan, H.; Gao, M.; et al. Micro-Architecture Study of the Normal Infant Auditory Ossicles with Micro-Computed Tomography. Int. J. Morphol. 2019, 37, 1387–1390. [Google Scholar] [CrossRef]
- Nadeem, G. Can Fetal Ossicles Be Used as Prosthesis in Adults? A Morphometric Study. Anatomy 2013, 7, 52–57. [Google Scholar] [CrossRef]
- Bellmer, E.H. The Time of Embryonic Fusion of the Malleus and Incus of the Guinea Pig. Am. Midl. Nat. 1963, 69, 426. [Google Scholar] [CrossRef]
- Konig, M.; Libebich, H.G. Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas; Konig, M., Liebich, H.G., Eds.; Schattauer GmbH: Stuttgart, Germany; New York, NY, USA, 2014. [Google Scholar]
- Kamali, Y.; Gholami, S.; Ahrari-Khafi, M.S.; Rasouli, B.; Shayegh, H. The Architecture of the Middle Ear in the Small Indian Mongoose (Herpestes javanicus). Folia Morphol. 2015, 74, 340–345. [Google Scholar] [CrossRef]
- Demircioğlu, İ.; Aksünger Karaavci, F. Morphologic Examination of Ossicula Auditus in Different Ruminants. Vet. Med. Sci. 2024, 10, e70061. [Google Scholar] [CrossRef]
- Mohammadpour, A.A. Morphology and Morphometrical Study of Hamster Middle Ear Bones. Iran. J. Vet. Res. 2011, 12, 121–126. [Google Scholar] [CrossRef]
- Argyle, E.C.; Mason, M.J. Middle Ear Structures of Octodon degus (Rodentia: Octodontidae), in Comparison with Those of Subterranean Caviomorphs. J. Mammal. 2008, 89, 1447–1455. [Google Scholar] [CrossRef]
- Kurtul, I.; Cevik, A.; Bozkurt, E.U.; Dursun, N. A Detailed Subgross Morphometric Study on the Auditory Ossicles of the New Zealand Rabbit. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2003, 32, 249–252. [Google Scholar] [CrossRef]
- Carter, Y. Monkey Hear: A Morphometric Analysis of the Primate Auditory Ossicles; University of Manitoba: Winnipeg, MB, Canada, 2009. [Google Scholar]
- Nourinezhad, J.; Abedini, M.; Shamsi, M.M.; Dabbaghi, A.; Janeczek, M. Evaluation of the Middle Ear in Water Buffaloes (Bubalus bubalis) by Gross Anatomy and Cone-Beam Computed Tomography. Folia Morphol. 2021, 80, 177–185. [Google Scholar] [CrossRef]
- Martonos, C.O.; Gudea, A.I.; Little, W.B.; Stan, F.G.; Lațiu, C.; Bolfa, P.; Dezdrobitu, C.C. The Gross Anatomical and Histological Features of the Humerus in African Green Monkeys (Chlorocebus sabaeus) from Saint Kitts and Nevis, West Indies. Life 2024, 14, 1295. [Google Scholar] [CrossRef]
- Proops, D.; Hawke, M.; Berger, G.; MacKay, A. The Anterior Process of the Malleus. J. Otolaryngol. 1984, 1, 39–43. [Google Scholar]
- Hadžiomerović, N.; Gundemir, O.; Tandir, F.; Avdić, R.; Katica, M. Geometric and Morphometric Analysis of the Auditory Ossicles in the Red Fox (Vulpes vulpes). Animals 2023, 13, 1230. [Google Scholar] [CrossRef]
- Gürbüz, İ.; Demiraslan, Y.; Orhun Dayan, M.; Aslan, K. Morphometric and Macroanatomic Examination of Auditory Ossicles in Male Wolves (Canis lupus). Folia Morphol. 2019, 78, 600–605. [Google Scholar] [CrossRef]
- Whyte; Cisneros; Yus; Blasco; Torres. Sarrat Contribution to the Development of the Stapedius Muscle Structure in Human Fetuses. Anat. Histol. Embryol. 2001, 30, 175–178. [Google Scholar] [CrossRef]
- Whyte, J.; Cisneros, A.; Yus, C.; Fraile, J.; Obón, J.; Vera, A. Tympanic Ossicles and Pharyngeal Arches. Anat. Histol. Embryol. 2009, 38, 31–33. [Google Scholar] [CrossRef]
- Sarrat, R.; Garcia Guzman, A.; Torres, A. Morphology Variations of Human Ossicula Tympani. Acta Anat. 1988, 131, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Padmini, M.P.; Rao, B.N. Morphometry of Human Fetal Ear Ossicles: A Human Cadaveric Study. J. Adv. Med. Med. Res. 2014, 4, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Kaul, J.M.; Agarwal, A.K. Morphometric Study of Stapes and Its Clinical Implications. J. Anat. Soc. India 2005, 54, 1–9. [Google Scholar]
- Martonos, C.; Gudea, A.; Damian, A.; Lăcătuș, R.; Purdoiu, R.; Cocan, D.; Stan, F.G. Morphological and Morphometrical Aspects of the Auditory Ossicles in Goat (Capra hircus). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2020, 50, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Nazih, A.M. Anatomical Study on the Middle Ear of Donkey (Equus acinus). Int. J. Adv. Res. Biol. Sci. 2017, 4, 110–121. [Google Scholar] [CrossRef]
- Nummela, S.; Aguirre-Fernández, G.; Smith, K.K.; Sánchez-Villagra, M.R. Growth Pattern of the Middle Ear in the Gray Short-Tailed Opossum, Monodelphis Domestica. Vertebr. Zool. 2022, 72, 487–494. [Google Scholar] [CrossRef]
- Nummela, S. Scaling of the Mammalian Middle Ear. Hear. Res. 1995, 85, 18–30. [Google Scholar] [CrossRef]
- Rosowski, J.J. Comparative Middle Ear Structure and Function in Vertebrates. In The Middle Ear: Science, Otosurgery, and Technology; Springer: New York, NY, USA, 2013; pp. 31–65. [Google Scholar] [CrossRef]
- Koyabu, D.; Hosojima, M.; Endo, H. Into the Dark: Patterns of Middle Ear Adaptations in Subterranean Eulipotyphlan Mammals. R. Soc. Open Sci. 2017, 4, 170608. [Google Scholar] [CrossRef]
- Chelli, D.; Chanoufi, B. Audition Fœtale. Mythe Ou Réalité? J. Gynecol. Obs. Biol. Reprod. 2008, 37, 554–558. [Google Scholar] [CrossRef]
- Richard, C.; Courbon, G.; Laroche, N.; Prades, J.M.; Vico, L.; Malaval, L. Inner Ear Ossification and Mineralization Kinetics in Human Embryonic Development—Microtomographic and Histomorphological Study. Sci. Rep. 2017, 7, 4825. [Google Scholar] [CrossRef]
- Sohmer, H.; Perez, R.; Sichel, J.Y.; Priner, R.; Freeman, S. The Pathway Enabling External Sounds to Reach and Excite the Fetal Inner Ear. Audiol. Neurootol. 2001, 6, 109–116. [Google Scholar] [CrossRef] [PubMed]
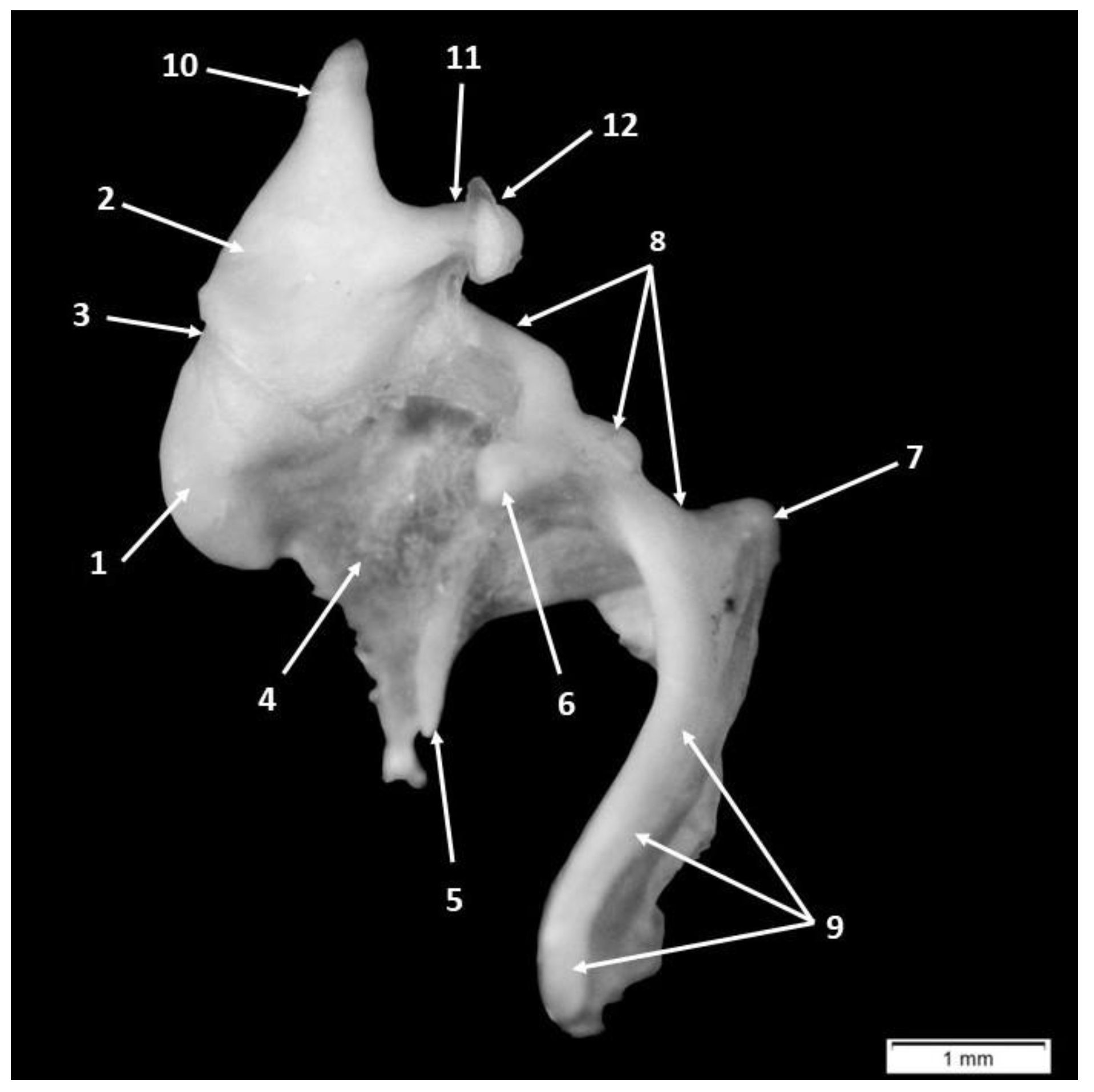
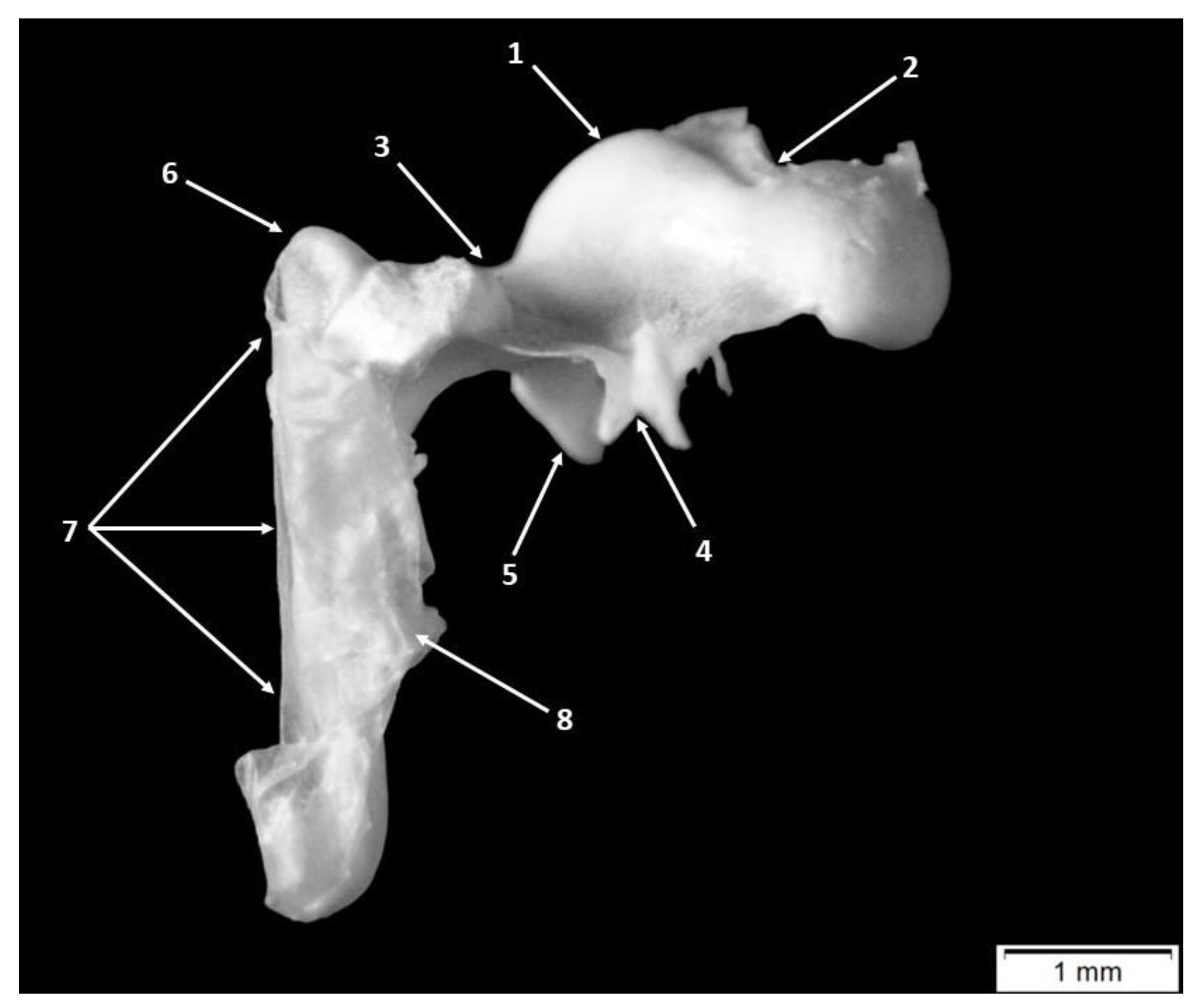
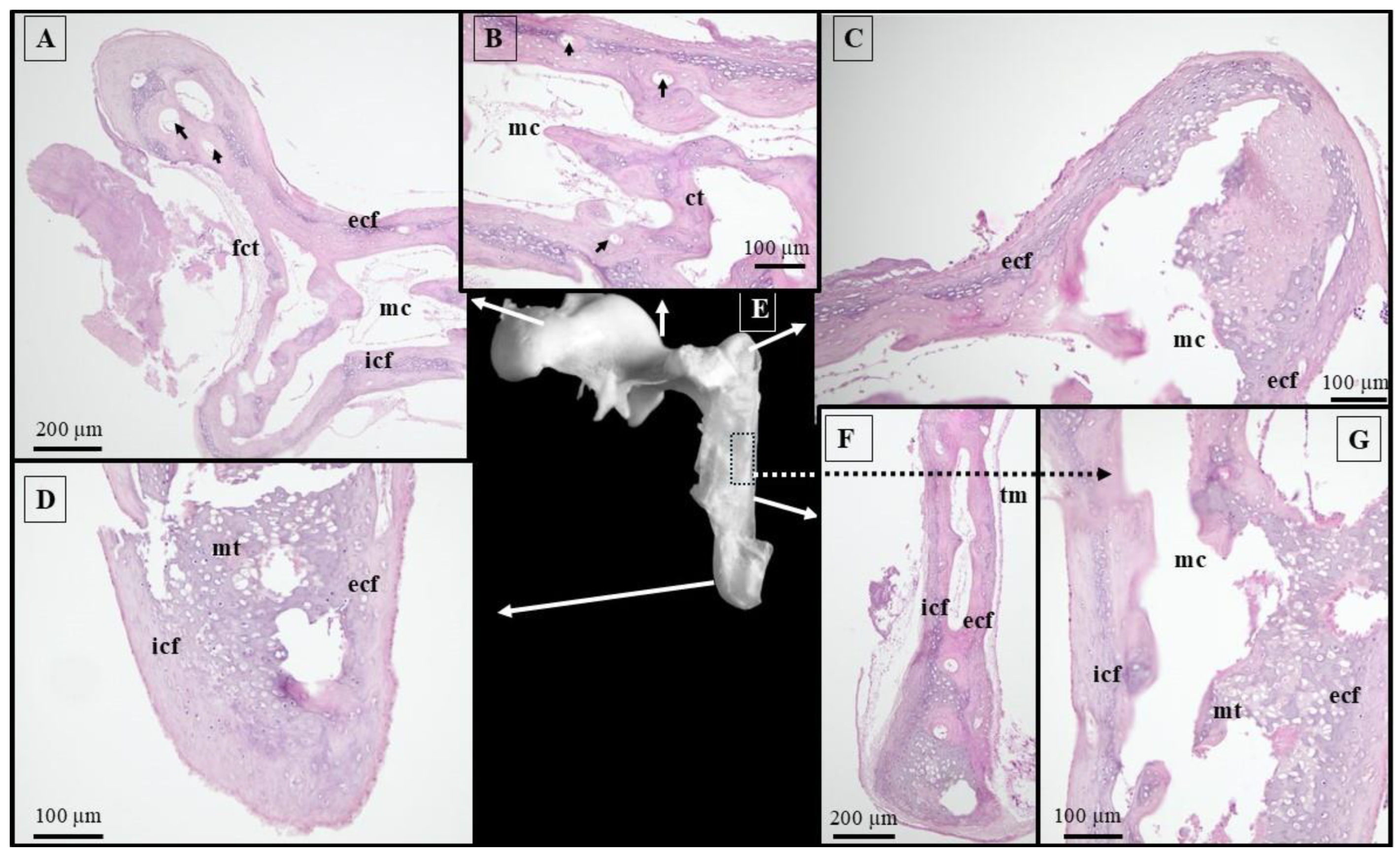
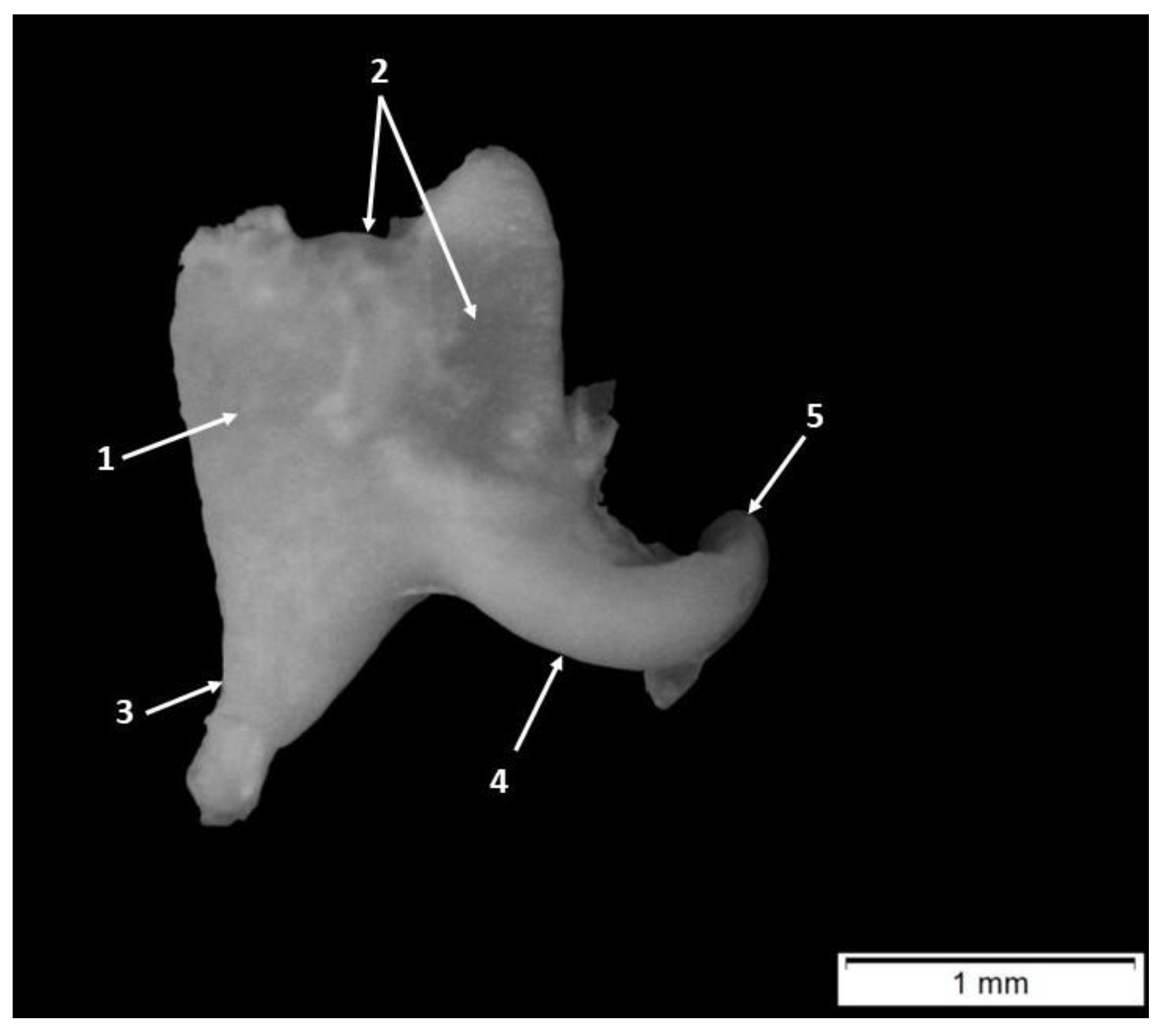
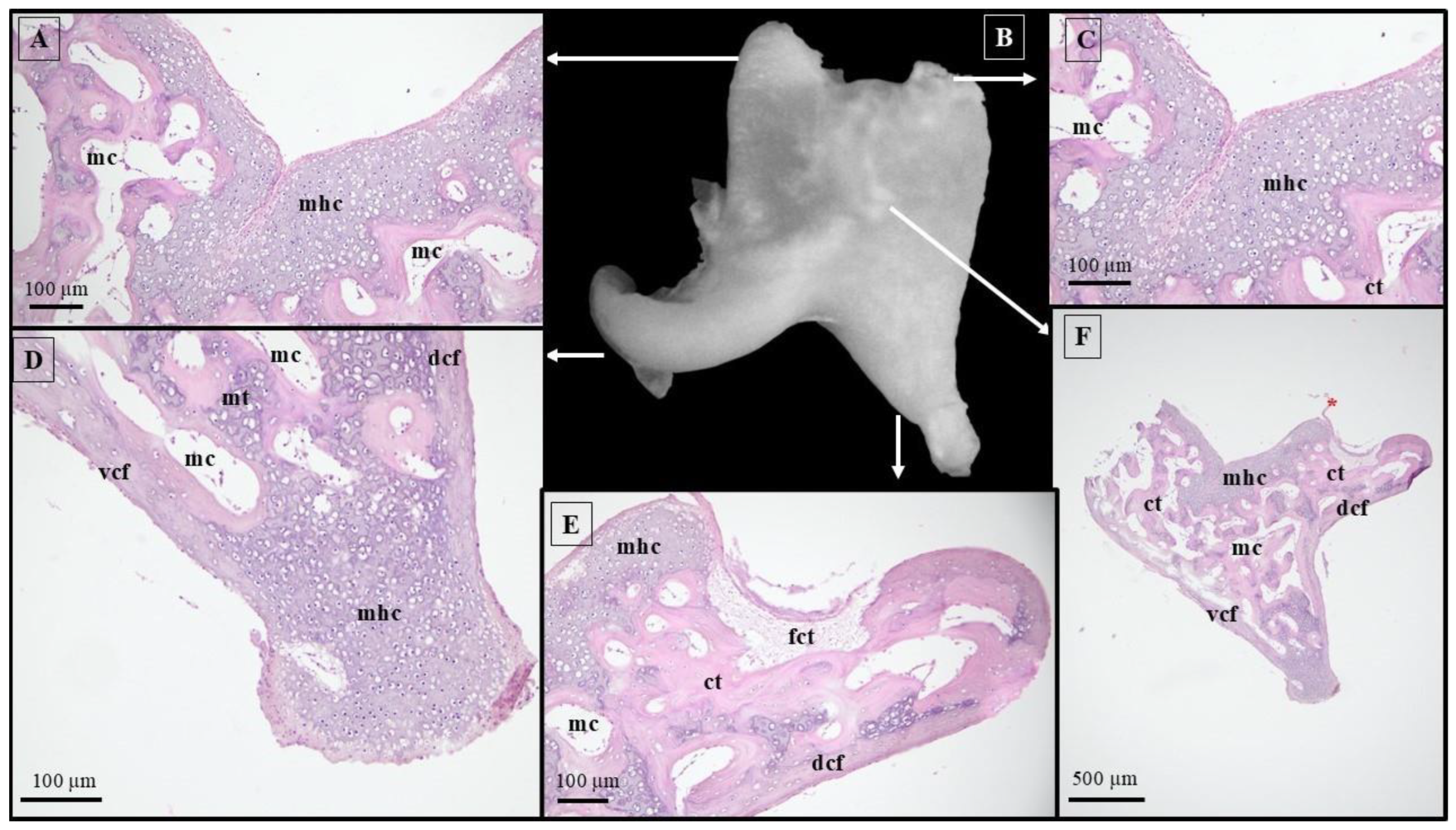
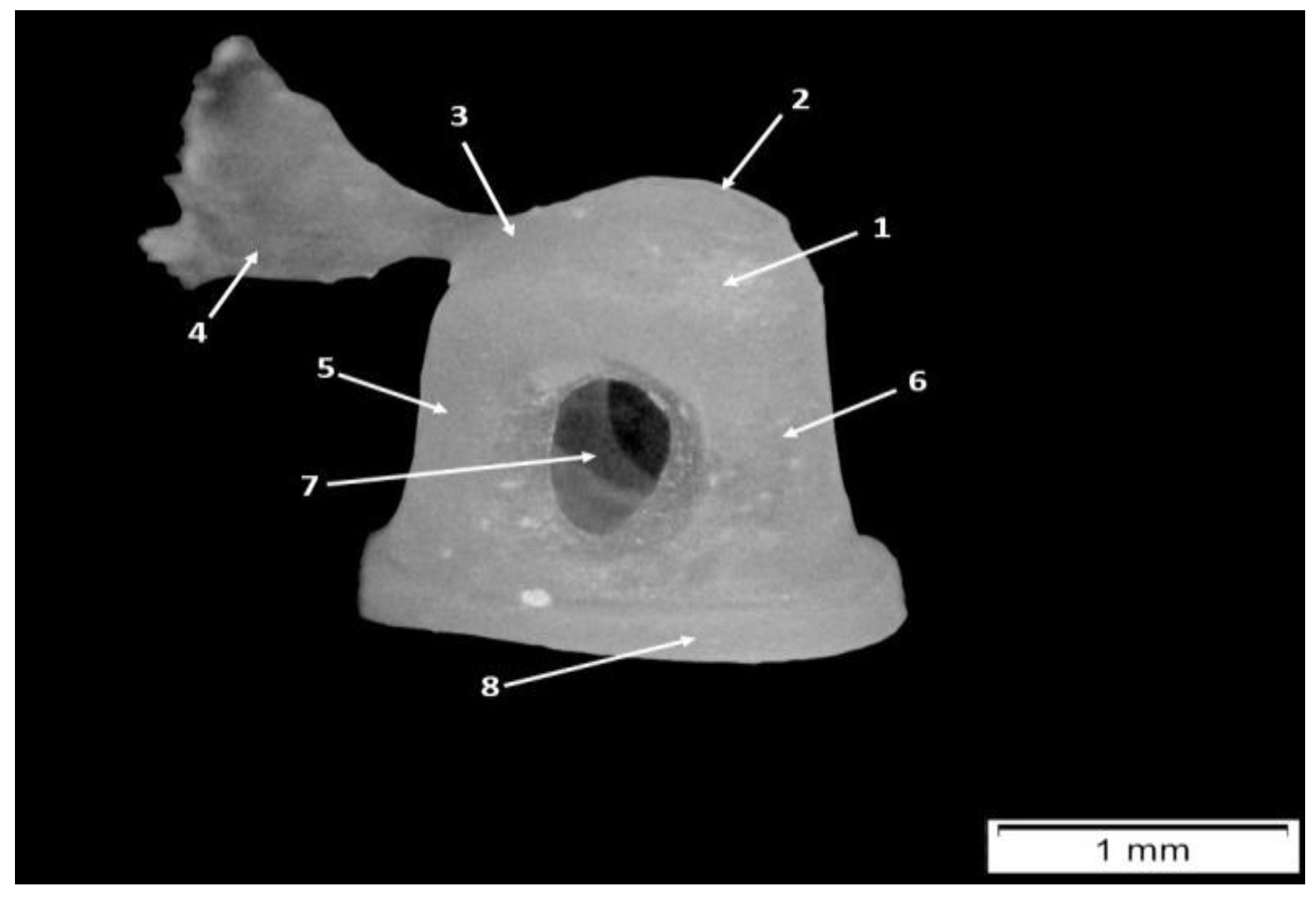

| Embryo Number | Estimated Gestational Age | Maternal Origin |
|---|---|---|
| 1 | 48 (days) | B |
| 2 | 66 (days) | D |
| 5 | 72 (days) | C |
| 6 | 66 (days) | D |
| 8 | 66 (days) | A |
| 9 | 66 (days) | A |
| 10 | 66 (days) | A |
| 13 | 66 (days) | D |
| X axis | Midpoint of the minimum neck width—the most noticeable point along the top of the head |
| Y axis | Most inferior point of the short process and the manubrium: |
| 1 Total length | tip of the manubrium to the top of the head |
| 2 Manubrium length | Tip of the short process to the tip of the manubrium following X axis |
| 3 Manubrium M-L thickness | M-L thickness of the manubrium at mid-manubrium length, perpendicular to X axis |
| 4 Manubrium arc depth | Maximum depth of the curvature of the arc of the manubrium, following X axis |
| 5 Corpus length | Tip of the head to the lower border of the manubrium following X axis |
| 6 Neck width | Anterior and posterior borders of the neck |
| 7 S-L head width | Maximum distance between 2 parallel lines marking the widest points of the margin of the head, taken following the X axis |
| 8 Angle between axes | X-Y angle |
| Manubrium/length index | (manubrium length/total length) × 100 |
| Manubrium robusticity index | (manubrium ML thickness/corpus length) × 100 |
| Manubrium/corpus index | (manubrium length/corpus length) × 100 |
| Corpus/length index | (corpus length/total length) × 100 |
| X axis | Line that joins the most salient point along the anterior portion of the superior border of the body |
| Y axis | Line that joins the tip of the long process to the most salient point along the superior border of the body |
| Z axis | Line joining the tip of the long process to the most external point along the margin of the anterior facet |
| 9 Short process length | Maximum distance from the tip of the short process to the most salient point along the anterior portion of the superior border of the body, following X axis |
| 10 Long process length | Maximum distance from the tip of the long process to the most salient point along the superior border of the body |
| 11 Functional length of the long process | Perpendicular distance from the Z axis (rotational axis) to the tip of the long process |
| 12 Arc depth of the long process | Maximum depth of the arc along the long process measured from the plane defined by the lateral outmost point along the tip of the long process |
| 13 Articular facet height | Max height of the articular facet with the bone oriented along the rotational axis |
| 14 Angle between the axes | Angle formed by the X and Y axes |
| 15 Interprocess length | Maximum distance between the most salient points along the superior margin of the short process and the tip of the long process |
| 16 Interprocess arc depth | Maximum depth of the curvature between the short and long process tips |
| Incudal index | 9/10 × 100 |
| Long process index | 11/10 × 100 |
| Relative articular facet height | 13/10 × 100 |
| X axis | Line joining the antero-superior corner of the footplate and the tip of the head |
| Y axis | Line joining the posterior-superior corner of the footplate and the tip of the head |
| Z axis | Line joining the most inferior points along the footplate margin anteriorly and posteriorly |
| 19 Total height of the stapes | Maximum height from the lower margin of the footplate to the tip of the head perpendicular to the Z axis |
| 20 Head height | Minimum distance between the superior margin of the obturator foramen and the top of the head, taken perpendicular to the Z axis |
| 21 Obturator foramen height | Maximum height of the obturator foramen taken perpendicular to the Z axis |
| 22 Obturator foramen width | Maximum width of the obturator foramen taken parallel to the Z axis |
| 23 Maximum width of the crura | Maximum width across the anterior and posterior crurae, taken on the external aspect and parallel to the Z axis |
| 24 Posterior crus length | Maximum distance from the posterior-superior corner of the footplate to the tip of the head, following Y axis |
| 25 Posterior crus arc depth | Maximum depth of the curvature of the posterior crus taken parallel to the Y axis |
| 26 Anterior crus length | Maximum distance from the anterio-superior corner of the footplate to the tip of the head following X axis |
| 27 Anterior crus arc depth | Maximum depth of the curvature of the anterior crus taken parallel to the X axis |
| 28 Angle A | Angle between the anterior and posterior crurae or between the X and Y axes |
| 29 Angle B | Angle between the anterior crus and the footplate or between the X and Z axes |
| 30 Angle C | Angle between the posterior crus and the footplate between Y and Z axes |
| 31 Footplate length | Maximum length of the footplate |
| 32 Footplate width | Maximum width of the footplate |
| 33 Footplate area | Measured area of the footplate |
| Stapedial index | 31/19 × 100 |
| Relative head height | 20/19 × 100 |
| Obturator foramen index | 21/22 × 100 |
| Footplate index | 31/32 × 100 |
| Crural index | 36/24 × 100 |
| Specimen | Measurement Number | Mean Values | |
|---|---|---|---|
| 1 | 1 | 5.34 mm (n = 4) | |
| 2 | 4.51 mm (n = 5) | ||
| 3 | 0.58 mm (n = 4) | ||
| 5 | 3.17 mm (n = 4) | ||
| 6 | 0.86 mm (n = 4) | ||
| 7 | 1.14 mm (n = 3) | ||
| 8 | 75.52° (n = 3) | ||
| 2 | 1 | 5.84 mm (n = 3) | |
| 2 | 3.85 mm (n = 6) | ||
| 3 | 0.44 mm (n = 5) | ||
| 5 | 4.21 mm (n = 4) | ||
| 6 | 1.07 mm (n = 2) | ||
| 7 | 1,12 mm (n = 5) | ||
| 8 | 77.01° (n = 3) | ||
| 5 | 1 | 5.62 mm (n = 13) | |
| 2 | 3.97 mm (n = 14) | ||
| 3 | 0.45 mm (n = 14) | ||
| 5 | 4.16 mm (n = 13) | ||
| 6 | 0.71 mm (n = 13) | ||
| 7 | 1.24 mm (n = 15) | ||
| 8 | 85.2° (n = 13) | ||
| 6 | 1 | 6.39 mm (n = 2) | |
| 2 | 4.65 mm (n = 2) | ||
| 3 | 0.48 mm (n = 2) | ||
| 5 | 4.53 mm (n = 2) | ||
| 6 | 0.85 mm (n = 2) | ||
| 7 | 1.47 mm (n = 2) | ||
| 8 | 80.25° (n = 2) | ||
| 8 | 1 | 5.85 mm (n = 2) | |
| 2 | 4.11 mm (n = 2) | ||
| 3 | 0.40 mm (n = 2) | ||
| 5 | 4.30 mm (n = 2) | ||
| 6 | 0.99 mm (n = 2) | ||
| 7 | 1.63 mm (n = 1) | ||
| 8 | 90.4° (n = 1) | ||
| 9 | 1 | - | |
| 2 | - | ||
| 3 | 3.94 (n = 1) | ||
| 5 | - | ||
| 6 | - | ||
| 7 | - | ||
| 8 | 85.3 (n = 1) | ||
| 10 | 1 | 5.25 mm (n = 2) | |
| 2 | 3.55 mm (n = 2) | ||
| 3 | 0.35 mm (n = 2) | ||
| 5 | 3.98 mm (n = 2) | ||
| 6 | 0.68 mm (n = 1) | ||
| 7 | 1.50 mm (n = 2) | ||
| 8 | 85.21° (n = 2) |
| Measurement Number | Mean Values | Standard Deviation | Shapiro–Wilk Test for Normality |
|---|---|---|---|
| 1 | 56,448 | 347 | The Shapiro–Wilk test did not show a significant departure from normality, W(27) = 0.96, p = 0.328 |
| 2 | 4.0503 | 0.383 | The Shapiro–Wilk test did not show a significant departure from normality, W(33) = 0.95, p = 0.115 |
| 3 | 0.4633 | 0.103 | The Shapiro–Wilk test did not show a significant departure from normality, W(30) = 0.96, p = 0.255 |
| 5 | 4.04 | 0.404 | The Shapiro–Wilk test showed a significant departure from normality, W(27) = 0.8, p < 0.001 |
| 6 | 0.8004 | 0.16 | The Shapiro–Wilk test showed a significant departure from normality, W(25) = 0.89, p = 0.013 |
| 7 | 1.2713 | 0.196 | The Shapiro–Wilk test showed a significant departure from normality, W(29) = 0.9, p = 0.008 |
| 8 | 82.9857 | 5.532 | The Shapiro–Wilk test did not show a significant departure from normality, W(26) = 0.95, p = 0.192 |
| Specimen | Measurement Number | Mean Values |
|---|---|---|
| 2 | 9 | 1.87 mm (n = 2) |
| 10 | 2.305 mm (n = 2) | |
| 11 | 1.635 mm | |
| 12 | 0.145 mm | |
| 13 | 1.3 mm (n = 1) | |
| 14 | 52.4° (n = 2) | |
| 15 | 1.87 mm | |
| 16 | 0.45 mm | |
| 5 | 9 | 2.065 mm (n = 4) |
| 10 | 2.29 mm (n = 4) | |
| 11 | 1.351 mm (n = 4) | |
| 12 | 0.292 mm (n = 4) | |
| 13 | 1.12 mm (n = 4) | |
| 14 | 50.19° (n = 4) | |
| 15 | 1.74 mm (n = 4) | |
| 16 | 0.452 mm (n = 4) | |
| 8 | 9 | 1.98 mm (n = 2) |
| 10 | 2.37 mm (n = 2) | |
| 11 | 1.62 mm (n = 2) | |
| 12 | 0.1 mm (n = 1) | |
| 13 | 1.04 mm (n = 2) | |
| 14 | 52.6° (n = 1) | |
| 15 | 1.96 mm (n = 2) | |
| 16 | 0.385 mm (n = 2) | |
| 9 | 9 | 1.88 mm (n = 1) |
| 10 | 2.39 mm (n = 1) | |
| 11 | 1.6 mm (n = 1) | |
| 12 | - | |
| 13 | 1.23 mm (n = 1) | |
| 14 | 53° (n = 1) | |
| 15 | 2.21 mm (n = 1) | |
| 16 | 0.4 mm (n = 1) | |
| 10 | 9 | 1,96 mm (n = 2) |
| 10 | 2.215 mm (n = 2) | |
| 11 | 1.55 mm (n = 2) | |
| 12 | 0.26 mm (n = 2) | |
| 13 | 1.09 mm (n = 2) | |
| 14 | 49.15° (n = 2) | |
| 15 | 2 mm (n = 2) | |
| 16 | 0.435 mm (n = 2) | |
| 13 | 9 | 1.85 mm (n = 3) |
| 10 | 2.49 mm (n = 3) | |
| 11 | 1.62 mm (n = 3) | |
| 12 | 0.2 mm (n = 3) | |
| 13 | 1.11 mm (n = 3) | |
| 14 | 51.72° (n = 3) | |
| 15 | 2.17 mm (n = 3) | |
| 16 | 0.453 mm (n = 3) |
| Measurement Number | Mean Values (mm) | Standard Deviation | Shapiro–Wilk Test for Normality |
|---|---|---|---|
| 9 | 1.95 | 0.111 | The Shapiro–Wilk test did not show a significant departure from normality, W(14) = 0.89, p = 0.088 |
| 10 | 2.343 | 0.151 | The Shapiro–Wilk test did not show a significant departure from normality, W(14) = 0.96, p = 0.654 |
| 11 | 1.535 | 0.142 | The Shapiro–Wilk test did not show a significant departure from normality, W(14) = 0.91, p = 0.166 |
| 12 | 0.223 | 0.079 | The Shapiro–Wilk test did not show a significant departure from normality, W(12) = 0.97, p = 0.951 |
| 13 | 1.123 | 0.108 | The Shapiro–Wilk test did not show a significant departure from normality, W(13) = 0.91, p = 0.188 |
| 14 | 51.124 | 3.15 | The Shapiro–Wilk test did not show a significant departure from normality, W(13) = 0.95, p = 0.574 |
| 15 | 1.953 | 0.199 | The Shapiro–Wilk test did not show a significant departure from normality, W(14) = 0.92, p = 0.233 |
| 16 | 0.436 | 0.038 | The Shapiro–Wilk test did not show a significant departure from normality, W(14) = 0.96, p = 0.672 |
| Specimen | Measurement Number | Mean Values |
|---|---|---|
| 6 | 19 | 2.18 mm (n = 1) |
| 20 | 0.78 mm (n = 1) | |
| 21 | 0.83 mm (n = 1) | |
| 22 | 0.86 mm (n = 1) | |
| 23 | 1.65 mm (n = 1) | |
| 24 | 2.09 mm (n = 1) | |
| 25 | 0.36 mm (n = 1) | |
| 26 | 1.98 mm (n = 1) | |
| 27 | 0.36 mm (n = 1) | |
| 28 | 50.7° (n = 1) | |
| 29 | 69° (n = 1) | |
| 30 | 61° (n = 1) | |
| 31 | 2.02 mm (n = 1) | |
| 32 | - | |
| 8 | 19 | 1.92 mm (n = 1) |
| 20 | 0.75 mm (n = 1) | |
| 21 | 0.87 mm (n = 1) | |
| 22 | 0.73 mm (n = 1) | |
| 23 | 1.65 mm (n = 1) | |
| 24 | 2.05 mm (n = 1) | |
| 25 | 0.4 mm (n = 1) | |
| 26 | 1.82 mm (n = 1) | |
| 27 | 0.31 mm (n = 1) | |
| 28 | 60° (n = 1) | |
| 29 | 64° (n = 1) | |
| 30 | 52° (n = 1) | |
| 31 | 1.78 mm (n = 1) | |
| 32 | - | |
| 9 | 19 | 2.08 mm (n = 3) |
| 20 | 0.91 mm (n = 3) | |
| 21 | 0.89 mm (n = 3) | |
| 22 | 0.753 mm (n = 3) | |
| 23 | 1.57 mm (n = 3) | |
| 24 | 2.05 mm (n = 3) | |
| 25 | 0.42 mm (n = 3) | |
| 26 | 1.94 mm (n = 3) | |
| 27 | 0.396 mm (n = 3) | |
| 28 | 53.26° (n = 3) | |
| 29 | 67° (n = 3) | |
| 30 | 58.33° (n = 3) | |
| 31 | 1.83 mm (n = 3) | |
| 32 | - | |
| 10 | 19 | 1.89 mm (n = 2) |
| 20 | 0.71 mm (n = 2) | |
| 21 | 0.745 mm (n = 2) | |
| 22 | 0.655 mm (n = 2) | |
| 23 | 1.58 mm (n = 2) | |
| 24 | 1.915 mm (n = 2) | |
| 25 | 0.465 mm (n = 2) | |
| 26 | 1.69 mm (n = 2) | |
| 27 | 0.48 mm (n = 2) | |
| 28 | 58° (n = 2) | |
| 29 | 68° (n = 2) | |
| 30 | 55° (n = 2) | |
| 31 | 1.685 mm (n = 2) | |
| 32 | - | |
| 13 | 19 | 2.07 mm (n = 2) |
| 20 | 0.82 mm (n = 2) | |
| 21 | 0.88 mm (n = 2) | |
| 22 | 0.715 mm (n = 2) | |
| 23 | 1.59 mm (n = 2) | |
| 24 | 1.99 mm (n = 2) | |
| 25 | 0.415 mm (n = 2) | |
| 26 | 1.865 mm (n = 2) | |
| 27 | 0.47 mm (n = 2) | |
| 28 | 53.5° (n = 2) | |
| 29 | 66.5° (n = 2) | |
| 30 | 57.5° (n = 2) | |
| 31 | 1.846 mm (n = 3) | |
| 32 | 1.08 mm (n = 1) |
| Measurement Number | Mean Values (mm) | Standard Deviation | Shapiro–Wilk Test for Normality |
|---|---|---|---|
| 19 | 2.028 | 0.117 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.9, p = 0.239 |
| 20 | 0.815 | 0.089 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.94, p = 0.694 |
| 21 | 0.846 | 0.073 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.95, p = 0.806 |
| 22 | 0.732 | 0.065 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.95, p = 0.725 |
| 23 | 1.594 | 0.043 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.95, p = 0.762 |
| 24 | 2.012 | 0.105 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.87, p = 0.131 |
| 25 | 0.42 | 0.051 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.96, p = 0.910 |
| 26 | 1.858 | 0.129 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.97, p = 0.941 |
| 27 | 0.417 | 0.074 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.86, p = 0.104 |
| 28 | 54.836 | 3.198 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.94, p = 0.579 |
| 29 | 67 | 2.692 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.95, p = 0.720 |
| 30 | 57 | 2.958 | The Shapiro–Wilk test did not show a significant departure from normality, W(9) = 0.94, p = 0.666 |
| 31 | 1.82 | 0.102 | The Shapiro–Wilk test did not show a significant departure from normality, W(10) = 0.95, p = 0.735 |
| 32 | 1.08 | - | Not applicable (n = 1) |
| Index Malleus | Value (Average/Variation) |
|---|---|
| Manubrium/length index | 71.46 ± 6.342 |
| Manubrium robusticity index | 11.21 ± 4.242 |
| Manubrium/corpus index | 101.02 ± 18.551 |
| Length index | 69.35 ± 15.159 |
| Index | Value (Average/Variation) |
|---|---|
| Incudal index | 83.621 ± 7.862 |
| Long process index | 65.727 ± 7.223 |
| Relative articular facet height | 44.312 ± 13.696 |
| Index | Value (Average/Variation) |
|---|---|
| Stapedial index | 89.522 ± 4.324 |
| Relative head height | 40.178 ± 3.540 |
| Obturator foramen index | 116.150 ± 11.409 |
| Footplate index | 173.148 |
| Crural index | 92.374 ± 4.077 |
| Pigs (Adult) | Miniature Pigs (Adult) | Fetal Pig | |
|---|---|---|---|
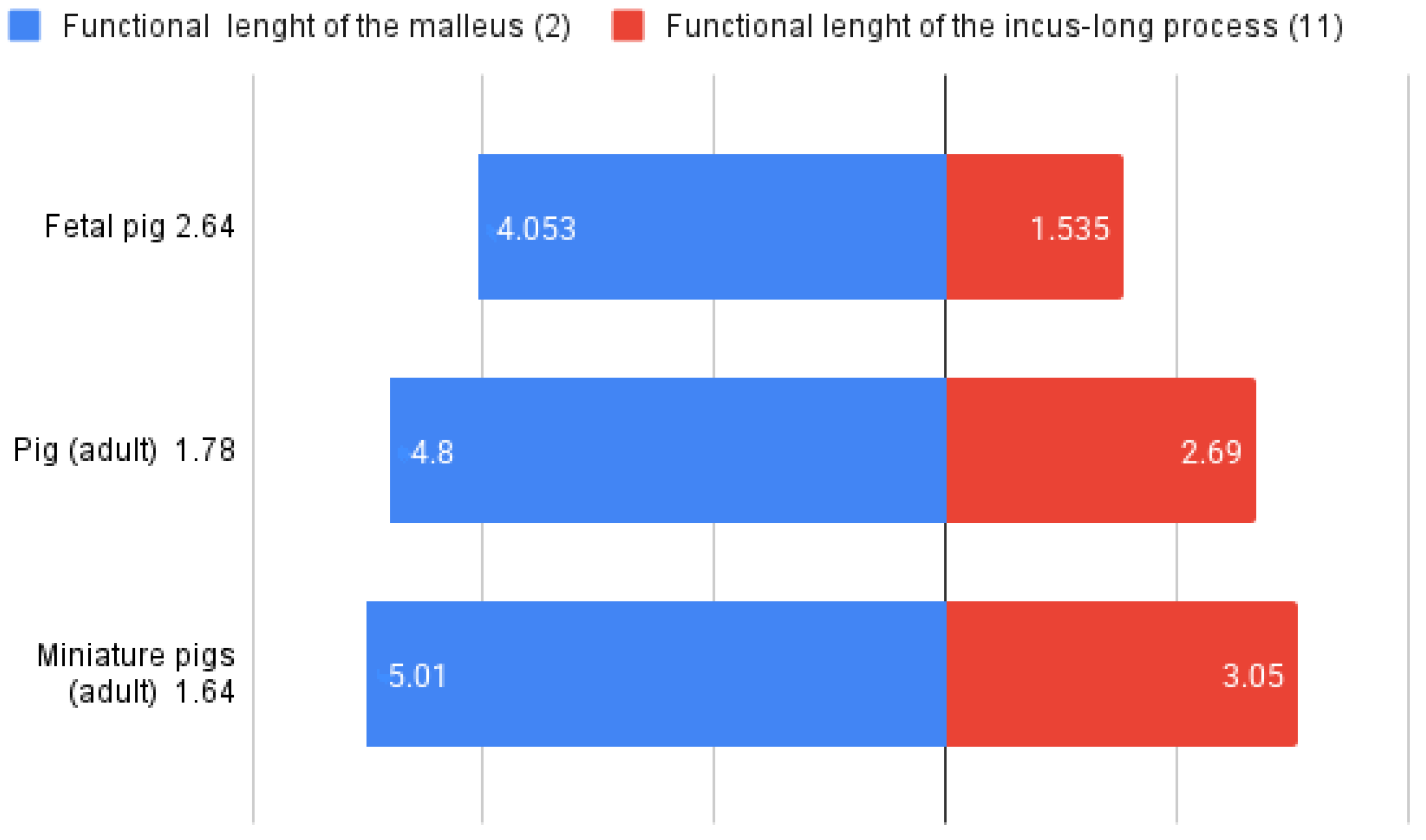
| Malleus: length of the manubrium (mm) | 4.8 ± 0.4 | 5.01 ± 0.5 | 4.05 ± 0.38 |
| Incus: length of the short process (9), incus width | 3.12 ± 0.15 | 2.59 ± 0.16 | 1.95 ± 0.111 |
| Incus: functional length of the long process (11) or the effective lever | 2.69 ± 0.15 | 3.05 ± 0.14 | 1.53 ± 0.14 |
| Stapes: total height (19) | 2.16 ± 0.14 | 2 | 2.02 ± 0.11 |
| Stapes: footplate width (31), total width | 2.05 ± 0.14 | N/A | 1.82 ± 0.102 |
| Specimen Number | Functional Length of Malleus (mm) | Functional Length of Long Process (mm) | Lever Ratio |
|---|---|---|---|
| 1 | 4.51 | No available data | - |
| 2 | 3.85 | 1.635 | 2.35 |
| 5 | 3.97 | 1.351 | 2.93 |
| 6 | 4.65 | No available data | - |
| 8 | 4.11 | 1.62 | 2.53 |
| 9 | No available data | 1.6 | - |
| 10 | 3.55 | 1.55 | - |
| 13 | No available data | 1.62 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martonos, C.O.; Bolfa, P.; Nagy, A.-L.; Hilchie, D.; Little, W.B.; Dezdrobitu, C.C.; Gudea, A.I. Morphological, Histological and Morphometrical Aspects of Auditory Ossicles in Pig Fetuses (Sus scrofa domestica). Animals 2025, 15, 1129. https://doi.org/10.3390/ani15081129
Martonos CO, Bolfa P, Nagy A-L, Hilchie D, Little WB, Dezdrobitu CC, Gudea AI. Morphological, Histological and Morphometrical Aspects of Auditory Ossicles in Pig Fetuses (Sus scrofa domestica). Animals. 2025; 15(8):1129. https://doi.org/10.3390/ani15081129
Chicago/Turabian StyleMartonos, Cristian Olimpiu, Pompei Bolfa, Andras-Laszlo Nagy, David Hilchie, William Brady Little, Cristian Constantin Dezdrobitu, and Alexandru Ion Gudea. 2025. "Morphological, Histological and Morphometrical Aspects of Auditory Ossicles in Pig Fetuses (Sus scrofa domestica)" Animals 15, no. 8: 1129. https://doi.org/10.3390/ani15081129
APA StyleMartonos, C. O., Bolfa, P., Nagy, A.-L., Hilchie, D., Little, W. B., Dezdrobitu, C. C., & Gudea, A. I. (2025). Morphological, Histological and Morphometrical Aspects of Auditory Ossicles in Pig Fetuses (Sus scrofa domestica). Animals, 15(8), 1129. https://doi.org/10.3390/ani15081129







