Ontogenesis from Embryo to Juvenile in Threadsail Filefish, Stephanolepis cirrhifer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Fertilized Eggs
2.2. Egg Incubation
2.3. Culture of Larvae and Juveniles
2.4. Observation of Embryonic Development and Larval/Juvenile Morphology
2.5. Image Processing and Statistical Analysis
3. Results
3.1. Embryonic Development
3.2. Morphological Characteristics of Larvae and Juveniles
3.3. Growth Pattern of Larvae and Juvenile
4. Discussion
4.1. Characteristics of Fertilized Eggs
4.2. Embryonic Development
4.3. Growth of Larvae and Juveniles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Alien, G.R.; Amaoka, K.; Anderson, W.D., Jr.; Bellwood, D.R.; Bohlke, E.B.; Bradbury, M.G.; Carpenter, K.E.; Caruso, J.H.; Cohen, A.C.; Cohen, D.M.; et al. A checklist of the fishes of the South China Sea. Raffles Bull. Zool. 2000, 8, 569–667. Available online: https://www.science.nus.edu.sg/wp-content/uploads/sites/11/2024/05/s08rbz569-667-1.pdf (accessed on 31 August 2022).
- Nakabo, T. (Ed.) Fishes of Japan: With Pictorial Keys to the Species; Tokai University Press: Tokyo, Japan, 2022; Volume 2, Available online: https://www.nhbs.com/fishes-of-japan-with-pictorial-keys-to-the-species-3-volume-set-japanese-book (accessed on 26 July 2024).
- Miyajima-Taga, Y.; Masuda, R.; Yamashita, Y. Larvae of the threadsail filefish Stephanolepis cirrhifer feed on eggs and planulae of the jellyfish Aurelia sp. under laboratory conditions. Plankton Benthos Res. 2016, 11, 96–99. [Google Scholar] [CrossRef][Green Version]
- Youn, C.H. Fishes of Korea with Pictorial Key and Systematic List; Academic Seojeok: Seoul, Republic of Korea, 2002; Volume 448. [Google Scholar]
- Kim, P.D. Miniature Guide for Whole Korean Fish Seoul; Poong Deung Press: Seoul, Republic of Korea, 2007. [Google Scholar]
- Minami, T.; Kanemaru, M.; Iwata, K.; Kuwahara, M.; Amano, K.; Mizuta, A.; Maeda, N.; Nishiki, I.; Tue, Y.; Yoshida, T. Pathogenicity of Streptococcus iniae and Lactococcus garvieae in farmed thread-sail filefish and efficacy of the formalin-killed vaccines against these bacteria. Fish Pathol. 2013, 48, 81–87. [Google Scholar] [CrossRef]
- Mizuno, K.; Miura, C.; Miura, T. Relationships between Temperature and Growth of Thread-sail Filefish Stephanolepis cirrhifer and Black Scraper Thamnaconus modestus. Aquacult. Sci. 2014, 62, 23–30. [Google Scholar] [CrossRef]
- Yamada, T.; Sugihara, Y.; Takami, I.; Suga, K.; Kanai, K. Protective efficacy of a commercial β-hemolytic Streptococcus vaccine for Japanese flounder against Streptococcus iniae infection of threadsail filefish. Fish Pathol. 2013, 48, 29–31. [Google Scholar]
- Park, S.Y.; Lee, N.Y.; Kim, S.H.; Cho, J.I.; Lee, H.J.; Ha, S.D. Effect of ultraviolet radiation on the reduction of major food spoilage molds and sensory quality of the surface of dried filefish (Stephanolepis cirrhifer) fillets. Food Res. Int. 2014, 62, 1108–1112. [Google Scholar] [CrossRef]
- Miyajima, Y.; Masuda, R.; Kurihara, A.; Kamata, R.; Yamashita, Y.; Takeuchi, T. Juveniles of threadsail filefish, Stephanolepis cirrhifer, can survive and grow by feeding on moon jellyfish Aurelia aurita. Fish. Sci. 2011, 77, 41–48. [Google Scholar] [CrossRef]
- Garibaldi, L.; Caddy, J.F. Depleted Marine Resources: An Approach to Quantification Based on the FAO Capture Database; Fisheries and Aquaculture Organization, FAO Fisheries Circular: Rome, Italy, 2004; Volume 1011, pp. 18–19. [Google Scholar]
- An, C.M.; An, H.S.; Lee, J.W.; Hong, S.W. New polymorphic microsatellite loci of threadsail filefish, Stephanolepis cirrhifer (Teleostei, Monacanthidae), from Korean waters. Genet. Mol. Res. 2013, 12, 1679–1690. [Google Scholar] [CrossRef]
- Matsuura, K.; Motomura, H.; Khan, M. Stephanolepis cirrhifer . IUCN Red List. Threat. Species 2019, e.T79803245A79803253. [Google Scholar] [CrossRef]
- Markevich, A.I.; Balanov, A.A. Finding of threadsail filefish Stephanolepis cirrhifer (Temminck et Schlegel, 1850) rare for Peter the Great Bay, Sea of Japan. J. Ichthyol. 2011, 51, 678–682. [Google Scholar] [CrossRef]
- Allen, J.J.; Akkaynak, D.; Sugden, A.U.; Hanlon, R.T. Adaptive body patterning, three-dimensional skin morphology, and camouflage measures of the slender filefish Monacanthus tuckeri on a Caribbean coral reef. Biol. J. Linn. Soc. 2015, 116, 377–396. [Google Scholar] [CrossRef]
- An, H.S.; Hong, S.W.; Kim, E.M.; Myeong, J.I. Comparative genetic diversity of wild and hachery populations of Korean threadsail filefish Stephanolepis cirrhifer using cross-species microsatellite markers. Genes Genom. 2011, 33, 605–611. [Google Scholar] [CrossRef]
- Yoon, M.; Park, W.; Nam, Y.K.; Kim, D.S. Shallow population genetic structures of Thread-sail Filefish (Stephanolepis cirrhifer) populations from Korean coastal waters. Asian Australas. J. Anim. Sci. 2012, 25, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, P.M.; Chen, G.B.; Fang, L.C.; Tang, Y. Acoustic target strength measurement of banded grouper (Epinephelus awoara (Temming and Schlegel, 1842)) and threadsial filefish (Stephanolepis cirrhifer (Temming & Schlegel, 1850)) in the South China Sea. J. Appl. Ichthyol. 2014, 29, 1453–1455. [Google Scholar] [CrossRef]
- Hossain, M.A.; Furuichi, M. Necessity of dietary calcium supplement in file fish (Monacanthus cirrhifer). Bangladesh J. Fish. Res. 2008, 12, 157–162. [Google Scholar]
- Khosravi, S.; Lee, S.M. Optimum dietary protein and lipid levels in juvenile filefish, Stephanolepis cirrhifer, feed. J. World Aquacult. Soc. 2017, 48, 867–876. [Google Scholar] [CrossRef]
- Narita, A.; Kashiwagura, M.; Saito, H.; Okada, Y.; Akiyama, N. Effect of different rearing conditions on larval feeding activity, food consumption, survival and growth of filefish Stephanolepis cirrhifer larvae. Aquacult. Sci. 2011, 59, 551–561. [Google Scholar] [CrossRef]
- An, H.S.; Lee, J.W.; Hong, S.W.; Myeong, J.I.; An, C.M. Population genetic structure of the Korean threadsail filefish (Stephanolepis cirrhifer) based on microsatellite marker analysis. Biochem. Syst. Ecol. 2013, 50, 397–405. [Google Scholar] [CrossRef]
- Xu, W.; Zeng, J.; Mei, W.; Jiang, L.; Manabe, S.; Wu, Y.; Liu, L. Changes in Growth and Feeding Characteristics during Early Ontogenesis in Threadsail Filefish, Stephanolepis cirrhifer. Animals 2023, 13, 3420. [Google Scholar] [CrossRef]
- Boehlert, G.W.; Yamada, J. (Eds.) Rockfishes of the genus Sebastes: Their reproduction and early life history. In Developments in Environmental Biology of Fishes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; Volume 11, 288p. [Google Scholar]
- Du, R.; Wang, Y.; Jiang, H.; Liu, L.; Wang, M.; Li, T.; Zhang, S. Embryonic and larval development in barfin flounder Verasper moseri (Jordan and Gilbert). Chin. J. Oceanol. Limnol. 2010, 28, 18–25. [Google Scholar] [CrossRef]
- Løkkeborg, S.; Siikavuopio, S.I.; Humborstad, O.B.; Utne-Palm, A.C.; Ferter, K. Towards more efficient longline fisheries: Fish feeding behaviour, bait characteristics and development of alternative baits. Rev. Fish Biol. Fisher. 2014, 24, 985–1003. [Google Scholar] [CrossRef]
- Song, Y.Q.; Cheng, F.; Zhao, S.S.; Xie, S.G. Ontogenetic development and otolith microstructure in the larval and juvenile stages of mandarin fish Siniperca chuatsi. Ichthyol. Res. 2019, 66, 57–66. [Google Scholar] [CrossRef]
- Van Snik, G.M.J.; Van den Boogaart, J.G.M.; Osse, W.M. Larval growth patterns in Cyprinus carpio and Clarias gariepinus with attention to the finfold. J. Fish Biol. 1997, 50, 1339–1352. [Google Scholar] [CrossRef]
- Liu, L.M.; Zeng, J.; Wang, J.L.; Liu, Y.; Mei, W.P.; Wu, Y.Q.; Wang, C.W.; Xu, W.G. Early ontogenesis from embryo to juvenile in Senegalese sole Solea senegalensis under laboratory conditions. J. Fish Biol. 2024, 104, 1800–1812. [Google Scholar] [CrossRef]
- Gallego, V.; Yoshida, M.; Kurokawa, D.; Asturiano, J.F.; Fraser, G.J. Embryonic development of the grass pufferfish (Takifugu niphobles): From egg to larvae. Theriogenology 2017, 90, 191–196. [Google Scholar] [CrossRef]
- Guan, J.; Ma, Z.; Zheng, Y.; Guan, S.; Li, C.; Liu, H. Breeding and larval rearing of bluefin leatherjacket, Thamnaconus modestus (Gunther, 1877) under commercial scales. Int. J. Aquac. 2013, 3, 55–62. [Google Scholar] [CrossRef]
- Hu, F.W.; Pan, L.; Gao, F.X.; Jian, Y.X.; Zhang, S.C.; Wang, X.; Guo, W. Embryonic development of Hexagrammos otakii and its relationship with incubation temperature. Prog. Fish. Sci. 2012, 33, 28–33. [Google Scholar] [CrossRef]
- Huang, X.L.; Yang, Y.K.; Li, T.; Huang, Z.; Yu, W.; Lin, H.Z. Morphology and growth of larval, juvenile and young Siganus oramin. South China Fish. Sci. 2018, 14, 88–94. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, H.J.; Yu, D.D.; Li, Y.Q.; Guan, S.G.; Liu, Y. Observation of embryonic development of marine medaka Oryzias melastigma. Mar. Sci. 2017, 41, 18–25. [Google Scholar]
- Akagawa, I.; Tsukamoto, Y.; Okiyama, M. Sexual dimorphism and pair spawning into a sponge by the filefish, Brachaluteres ulvarum, with a description of the eggs and larvae. Jap. J. Ichthyol. 1995, 41, 397–407. [Google Scholar] [CrossRef]
- Kawase, H.; Nakazono, A. Embryonic and pre-larval development and otolith increments in two filefishes, Rudarius ercodes and Paramonacanthus japonicus (Monacanthidae). Jap. J. Ichthyol. 1994, 41, 57–63. [Google Scholar] [CrossRef]
- Gao, X.Q.; Liu, Z.F.; Huang, B.; Wang, Y.H.; Xue, G.P.; Qin, W.L.; Guan, C.T.; Hong, L. Morphological and histological observation of the embryo of American Shad (Alosa sapidissima). Prog. Fish. Sci. 2017, 38, 9–18. [Google Scholar] [CrossRef]
- He, T.; Xiao, Z.Z.; Liu, Q.H.; Ma, D.Y.; Xu, S.H.; Xiao, Y.S.; Li, J. Histological observation of eye ontogeny in rock bream larvae (Oplegnathus fasciatus). Mar. Sci. 2012, 36, 49–53. Available online: https://cstj.cqvip.com/Qikan/Article/Detail?id=42065461&from=Qikan_Article_Detail (accessed on 24 May 2022).
- Mo, G.Y.; Hu, G.D.; Zhou, Y.F. Observation on the embryonic development of Takifugu obscurus. Freshw. Fish. 2009, 39, 22–27. Available online: https://dsyy.cbpt.cnki.net/WKC/WebPublication/paperDigest.aspx?paperID=3a123e0f-0292-4d9f-8a85-a486077f5055 (accessed on 18 July 2022).
- Brummett, A.R.; Dumont, J.N. Kupffer’s vesicle in Fundulus heteroclitus: A scanning and transmission electron microscope study. Tissue Cell 1978, 10, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, H.; Fond, M. Biological observations during rearing experiments with the garfish Belone belone. Mar. Biol. 1973, 21, 203–218. [Google Scholar] [CrossRef]
- Essner, J.J.; Amack, J.D.; Nyholm, M.K.; Harris, E.B.; Yost, H.J. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 2005, 132, 1247–1260. [Google Scholar] [CrossRef]
- Okabe, N.; Burdine, R.D. Fluid dynamics in zebrafish Kupffer’s vesicle. Dev. Dynam. 2008, 237, 3602–3612. [Google Scholar] [CrossRef]
- Humphrey, C.; Weber, M.; Lott, C.; Cooper, T.; Fabricius, K.J.C.R. Effects of suspended sediments, dissolved inorganic nutrients and salinity on fertilisation and embryo development in the coral Acropora millepora (Ehrenberg, 1834). Coral Reefs 2008, 27, 837–850. [Google Scholar] [CrossRef]
- Duray, M.; Kohno, H.; Pascual, F. The effect of lipid-enriched broodstock diets on spawning and on egg and larval quality of hatchery-bred rabbitfish Siganus guttatus. Philipp. Sci. 1994, 31, 42–57. Available online: http://hdl.handle.net/10862/1452 (accessed on 19 November 2024).
- Furuita, H.; Tanaka, H.; Yamamoto, T.; Shiraishi, M.; Takeuchi, T. Effects of n-3 HUFA levels in broodstock diet on the reproductive performance and egg and larval quality of the Japanese flounder, Parolichthys olivaceus. Aquaculture 2000, 187, 387–398. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, W.Z.; Sun, Z.W.; Chen, J.H.; Wu, K.G. Effects of n-3HUFA content in broodstock diet on spawning performance and fatty acid composition of eggs and larvae in Plectorhynchus cinctus. Aquaculture 2005, 245, 263–270. [Google Scholar] [CrossRef]
- Jobling, M. Fish in aquaculture environments. In Aquaculture and Behavior; Blackwell Publishing: Oxford, UK, 2012; pp. 36–64. [Google Scholar] [CrossRef]
- Syafariyah, N.K.; Sulmartiwi, L.; Budi, D.S. Incubation temperature effects on some hatching parameters of silver rasbora (Rasbora argyrotaenia) egg. J. Appl. Aquacult. 2023, 35, 16–26. [Google Scholar] [CrossRef]
- Martell, D.J.; Kieffer, J.D.; Trippel, E.A. Effects of temperature during early life history on embryonic and larval development and growth in haddock. J. Fish Biol. 2005, 66, 1558–1575. [Google Scholar] [CrossRef]
- Kupren, K.; Mamcarz, A.; Kucharczyk, D.; Prusińska, M.; Krejszeff, S. Influence of water temperature on eggs incubation time and embryonic development of fish from genus Leuciscus. Pol. J. Nat. Sci. 2008, 23, 461–481. [Google Scholar] [CrossRef]
- Saputra, A.; Suryaningrum, L.H.; Sunarno, M.T.D.; Samsudin, R.; Kholidin, E.B.; Prihadi, T.H.; Widyastuti, Y.R.; Murniasih, S.; Kontara, E.K.M.; Taukhid, T. Enhancing early weaning strategies through artificial feeding regimes for Channa striata larvae. Egypt. J. Aquat. Res. 2024, 50, 293–300. [Google Scholar] [CrossRef]
- Ma, Z.H.; Qin, J.G.; Hutchinson, W.; Chen, B.N.; Song, L. Responses of digestive enzymes and body lipids to weaning times in yellowtail kingfish Seriola lalandi (Valenciennes, 1833) larvae. Aquac. Res. 2014, 45, 973–982. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, P.; Guo, H.; Zhang, N.; Wang, L.; Jiang, S.; Qin, J.G.; Zhang, D. Effect of weaning time on the performance of Trachinotus ovatus (Linnaeus 1758) larvae. Aquacult. Nutr. 2015, 21, 670–678. [Google Scholar] [CrossRef]
- Khemis, I.B.; Gisbert, E.; Alcaraz, C.; Zouiten, D.; Besbes, R.; Zouiten, A.; Masmoudi, A.S.; Cahu, C. Allometric growth patterns and development in larvae and juveniles of thick-lipped grey mullet Chelon labrosus reared in mesocosm conditions. Aquac. Res. 2013, 44, 1872–1888. [Google Scholar] [CrossRef]
- Liu, L.M.; Zeng, J.; Zhang, Z.; Wang, J.L.; Mei, W.P.; Wang, C.W.; Liu, Z.P.; Xu, W.G. Changes in growth, morphology, and levels of digestive enzymes and growth-related hormones in early ontogeny of black scraper, Thamnaconus modestus. Front. Mar. Sci. 2024, 11, 1344844. [Google Scholar] [CrossRef]
- Song, H.J.; Liu, W.; Wang, J.L.; Tang, F.J. Allometric growth during yolk-sac larvae of chum salmon (Oncorhynchus keta Walbaum) and consequent ecological significance. Acta Hydrobiol. Sin. 2013, 37, 329–335. [Google Scholar] [CrossRef]
- Wang, R.L.; Wang, Z.B.; Jiang, H.B.; Du, R.B.; Liu, L.M. Growth and feeding characteristics of Sebastes schlegeli in specific developmental periods. J. Ocean Univ. China 2018, 48, 42–53. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, S.; Qi, K. The changes in growth, survival, food intake and body composition of Pseudogrus fulvidraco larvae and juveniles fed with Artemia nauplii. J. Fish. Sci. China 2012, 19, 1034–1042. Available online: https://www.fishscichina.com/zgsckx/article/abstract/5074?st=article_issue (accessed on 20 April 2024). [CrossRef]
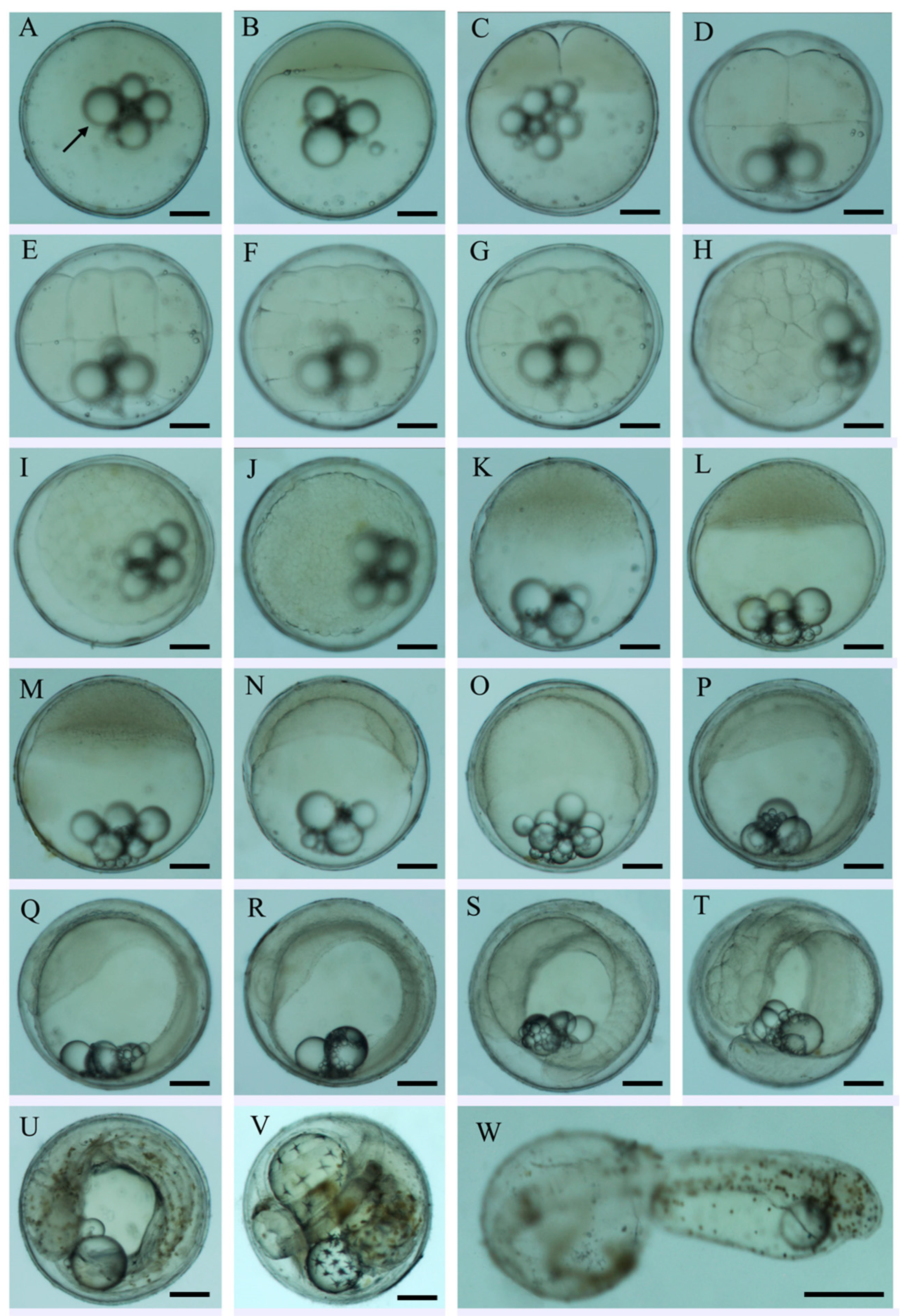
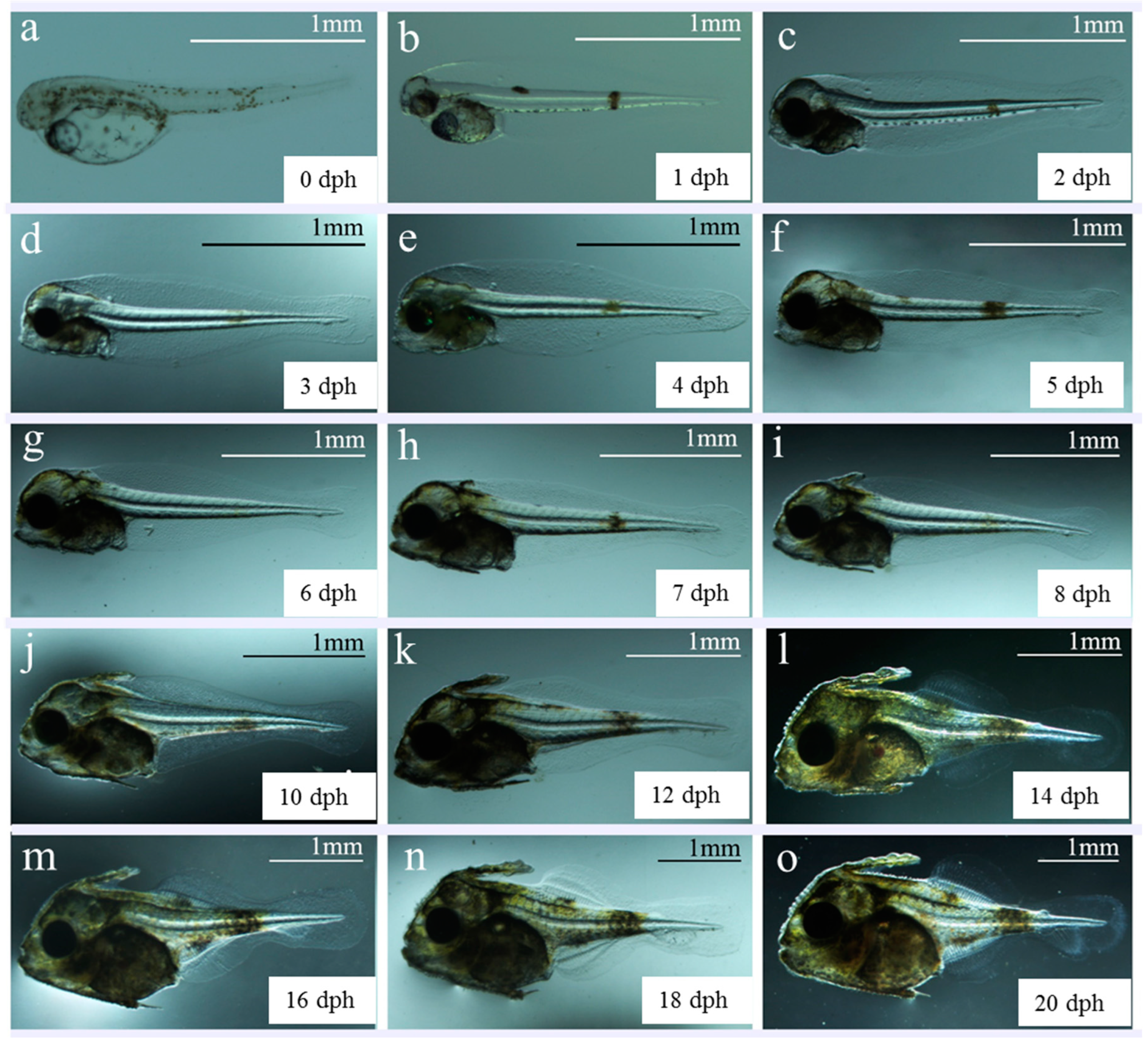
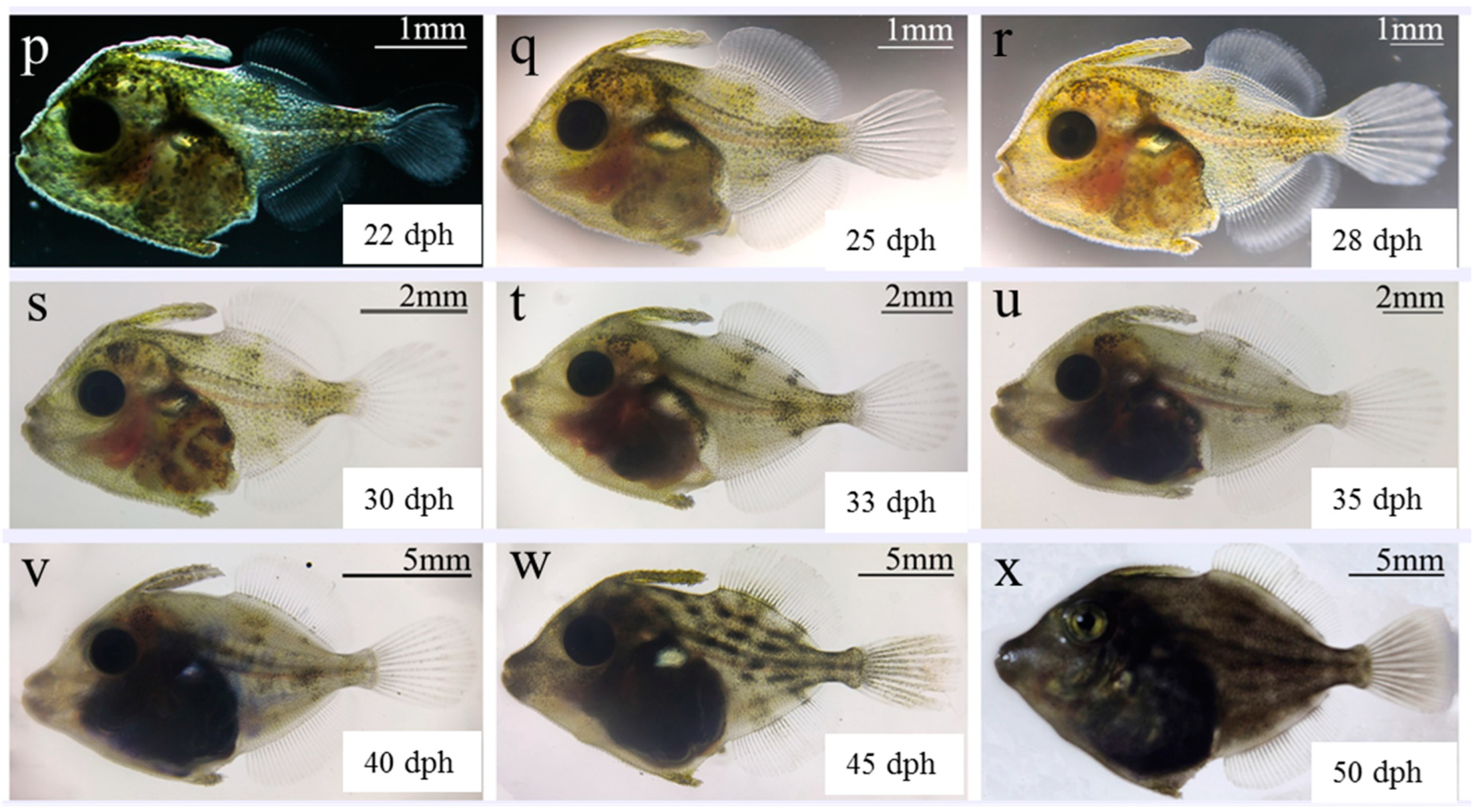

| Developmental Stage | Developmental Time (h:min) | Description | Figure Panel |
|---|---|---|---|
| Fertilized egg | 0 | Eggs spherical in shape; yolk is transparent with multiple oil globules and ooplasm is evenly distributed | Figure 1A |
| Blastodisc formation | 0:30 | Cytoplasm migrates toward the animal pole to form blastodisc. | Figure 1B |
| 2-cell | 1:10 | First cleavage: blastodisc divides via meridional cleavage to form two equal cells | Figure 1C |
| 4-cell | 1:20 | Second cleavage: 2 × 2 blastomere shape | Figure 1D |
| 8-cell | 1:50 | Third cleavage: 2 × 4 array of blastomere | Figure 1E |
| 16-cell | 2:10 | Fourth cleavage:4 × 4 blastomere shape | Figure 1F |
| 32-cell | 2:30 | Fifth cleavage: 4 × 8 blastomere shape | Figure 1G |
| 64-cell | 2:55 | Sixth cleavage: 64 blastomeres ranked irregularly | Figure 1H |
| Multi-cell | 3:25 | Variable blastomere size and shape; cleavage occured asynchronously. | Figure 1I |
| Morula | 4:30 | Multilayer cells were formed at animal pole. | Figure 1J |
| Early blastula | 5:20 | Blastoderm composed of many blastomeres with unclear border, and blastoderm hunch was high. | Figure 1K |
| Late blastula | 6:20 | Epibolic cells increased in number and blastoderm hunch gradually lowered. | Figure 1L |
| Early gastrula | 7:20 | Germ ring appeared and blastoderm epiboled toward vegetal pole to 1/3 of yolk sac. | Figure 1M |
| Middle gastrula | 9:40 | Germ ring was distinct and blastoderm epiboled 1/2 of the yolk sac; embryonic shield is formed. | Figure 1N |
| Late gastrula | 11:40 | Blastoderm epiboled 3/4 of the yolk sac; embryonic shield elongated with the involution of blastoderm cells. | Figure 1O |
| Neurula | 14:00 | Yolk plug was formed; embryonic body and neural plate was formed; optic vesicles were formed. | Figure 1P |
| Formation of somite | 15:10 | Kupffer’s vesicle was visible; somite begins to form | Figure 1Q |
| Formation of optic vesicle | 20:40 | Optic vesicles were formed. | Figure 1R |
| Formation of tail bud | 25:40 | Tail dissociated from yolk sac to form caudal bud; Kupffer’s vesicle disappeared. | Figure 1S |
| Formation of auditory capsule | 28:55 | Auditory capsules were formed bilaterally on the hindbrain. | Figure 1T |
| Muscular contraction | 32:40 | muscles contracted and tail wobbled intermittently; punctate yellow-green pigment appeared. | Figure 1U |
| Heart pulsation | 37:40 | Heart started to beat. | Figure 1V |
| Hatching | 48:00 | Embryonic body surrounded yolk sac in one lap and twisted frequently; head and tail wobbled dramatically. | Figure 1W |
| Phase | dph | Morphological Characteristics | Ecological Habits |
|---|---|---|---|
| Pre-larva a | 0 | Larvae have an oil globule in front of the yolk sac. The optic capsule and crystal are colorless and transparent. The digestive tract is attached to the upper portion of the yolk sac without opening. The anus is closed. The dorsal and gluteal fins are connected with the caudal fin membrane, without pigment distribution. There are scattered yellow-green spots on the back, abdomen, and back of the body segment. | The larvae hang upside down in the pool and are evenly distributed. |
| b | 1 | Yolk sac and oil globule are clearly reduced, and skull top is lifted upward. A small pigment appears in the optic capsule and crystal, the back and abdomen of the front end of the segment, and the rear end of the segment gathers to form three massive yellow-green spots. The pectoral fin membrane is inverted as triangular, and the digestive tract is thickened. | |
| c–d | 3 | Yolk sac disappears and the oil globule is almost invisible. The eye develops rapidly, the optic capsule and crystal are black, and the mouth fissure is initially formed. The first bend of the digestive tract occurs. The anus is formed, and digestive tract runs through. The color patches on the back and abdomen in the front body segment disappear. | Larval cluster distributed in the upper layer of the water, and some larvae feed on S-rotifers. |
| Post- larva e–f | 4 | Oil globule disappears, and the color of visceral mass deepens. The digestive tract is filled with food, and a little star-shaped pigment appears on the top of the head. The dorsal fin spine primordia appears, and the swim bladder appears and inflates. The oral fissure increases and expands, and the jaw fully opens. | |
| g–i | 6–8 | The upper and lower jaws of larvae are gradually covered by leathery skin, and the head-to-body proportion increases. The dorsal fin spines, abdominal girdle bones, and abdominal fin spines are formed, with dense distribution of melanin and yellow pigment in the head. The yellow-green spots on the body segments disappear. Three reddish-brown patches gradually grow and spread throughout the body. | Most larvae feed on L-rotifer with weak phototaxis. |
| j–k | 10–12 | Pigmentation increases in the upper and lower jaw and trunk. The dorsal, anal, and caudal fin arms thicken. | |
| l–m | 14–16 | Head appears with protruding scales. The first dorsal fin spine shows inverted spines, and the waistband bone and abdominal fin spine move downward, resulting in a substantial increase in body height. Fin differentiation occurs in the dorsal, anal, and pectoral fins, with widespread distribution of yellow and melanin on the body surface. Swim bladder is clear. | Swimming ability enhanced, resulting in obvious clustering phenomenon. |
| m–o | 16–20 | The waistband bone thickens and the abdominal fin spines atrophy. The lower lobe of the caudal fin begins to protrude, and radial elastic filaments of the caudal fin appear. The caudal vertebrae are flat. | |
| o–p | 20–22 | The spine of the abdominal fin falls off, and the girdle bone continues to thicken and differentiate into thorns. The dorsal and anal fins exhibit obvious growth, and fins begin to take shape. The lower lobe of caudal fin protrudes backward and the end of the notochord tilts upwards. Protruding scales appear on the body surface. | Larvae feeds vigorously and consume considerable amounts of Artemia nauplii. |
| p–q | 22–25 | The end of the notochord continues to tilt upwards, forming a tail fan. The trunk is evenly distributed with protruding circular punctate scales. The snout is covered with leathery epidermis, and the mouth begins to round. | |
| Juvenile q–r | 25–28 | Each fin is mostly formed, with a small amount of primitive fin membrane remaining at the end of the dorsal and anal fins. The caudal fins are segmented and not branched. | Juveniles are uniformly distributed in the water. |
| r–s | 28–30 | The scales of the trunk develop sharp thorns. | |
| t–u | 33–35 | The snout extends, the caudal fins begin to form branches, and the original membranes of each fin disappear. | |
| u–v | 35–40 | There are 12 tail fin strips, consistent with adult fish, and the ends continue to branch. | Juveniles begins to consume compound feed, and often kill each other. |
| v–w | 40–45 | Melanin increases, with horizontal dark stripes appearing on the trunk. Pigmented spots appear on the tail fins. The counts of dorsal fins and anal fins are 34–36 and 32–34, respectively. The body shape of juveniles and morphology of each fin are similar to those of adult fish. | Metamorphosis is complete, with obvious the size differentiation of juveniles. |
| Young w–x | 50 | The brown dark lines on the trunk are more prominent; the pigmented spots on the dorsal and anal fins are deepened. The tail fins are densely yellow. The fish are covered with scales. | Fish have strong swimming and avoidance abilities, and have similar ecological habits as adult fish. |
| Species | Egg Diameter (mm) | Number of Oil Globules | Diameter of Oil Globules (μm) | Incubation Temperature (°C) | Sum of Temperature (°C · h) | Hatching Time | Reference |
|---|---|---|---|---|---|---|---|
| Thamnaconus modestus | 0.59–0.63 | 18–83 | 35–206 | 20.5–21.5 | 1050 | 50 h | [32] |
| Brachaluteres ulvarum | 0.82 | 20 | 30–130 | 19–22 | 3225–3600 | 150 h | [36] |
| Paramonacanthus japonicus | 0.53 | 10–20 | 30–70 | 29.0–29.3 | 841–850 | 29 h | [37] |
| Rudarius ercodes | 0.53 | 1–3 | 120–170 | 20.7–21.3 | 1283–1311 | 62 h 39 min | [37] |
| Stephanolepis cirrhifer | 0.61–0.63 | 4–6 | 100–142 | 23.6 | 1132.8 | 48 h | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Liu, X.; Wu, Y.; Zeng, J.; Xu, W. Ontogenesis from Embryo to Juvenile in Threadsail Filefish, Stephanolepis cirrhifer. Animals 2025, 15, 1124. https://doi.org/10.3390/ani15081124
Liu L, Liu X, Wu Y, Zeng J, Xu W. Ontogenesis from Embryo to Juvenile in Threadsail Filefish, Stephanolepis cirrhifer. Animals. 2025; 15(8):1124. https://doi.org/10.3390/ani15081124
Chicago/Turabian StyleLiu, Liming, Xuanhan Liu, Yanqing Wu, Jun Zeng, and Wengang Xu. 2025. "Ontogenesis from Embryo to Juvenile in Threadsail Filefish, Stephanolepis cirrhifer" Animals 15, no. 8: 1124. https://doi.org/10.3390/ani15081124
APA StyleLiu, L., Liu, X., Wu, Y., Zeng, J., & Xu, W. (2025). Ontogenesis from Embryo to Juvenile in Threadsail Filefish, Stephanolepis cirrhifer. Animals, 15(8), 1124. https://doi.org/10.3390/ani15081124





