Pilot Study on the Profiling and Functional Analysis of mRNA, miRNA, and lncRNA in the Skeletal Muscle of Mongolian Horses, Xilingol Horses, and Grassland-Thoroughbreds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Library Construction
2.3. Mapping and Initial Assembly of RNA-Seq Reads
2.4. Functional Enrichment Analysis
2.5. Co-Expression Network Construction
2.6. Expression Trend Analysis
2.7. Quantitative Real-Time PCR
3. Results
3.1. Muscle Fiber Types in Three Horse Breeds
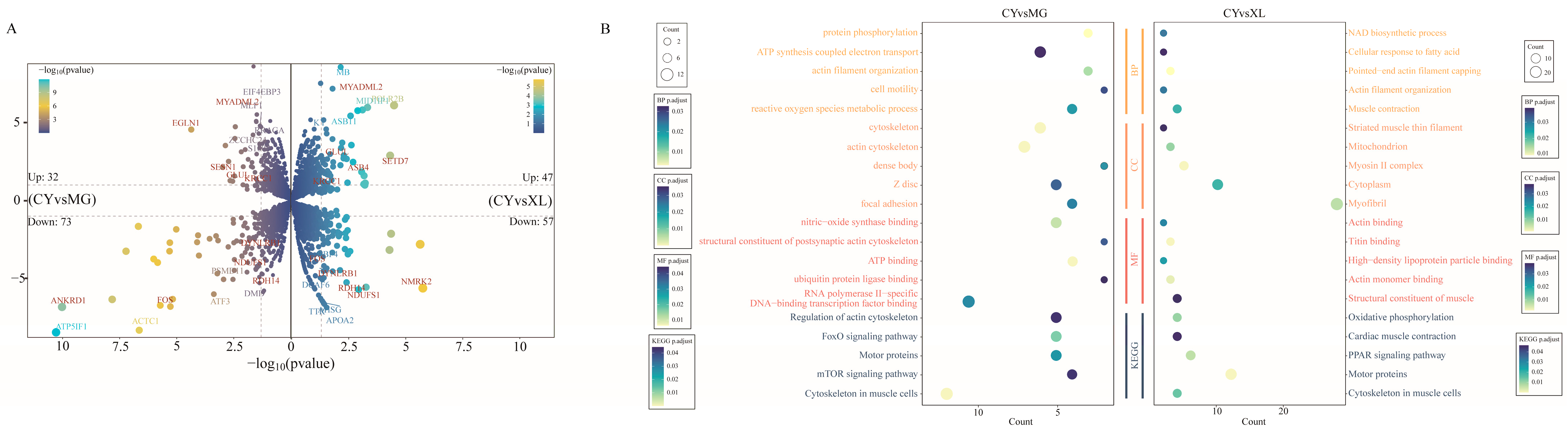
3.2. Differential Expression Analysis of mRNA
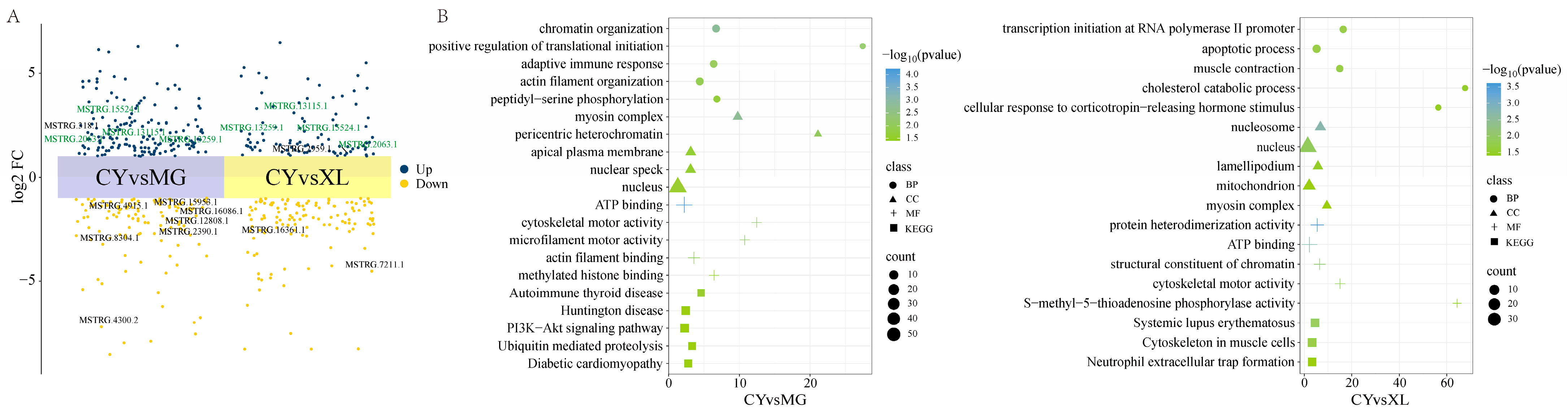
3.3. The Role of lncRNA Changes
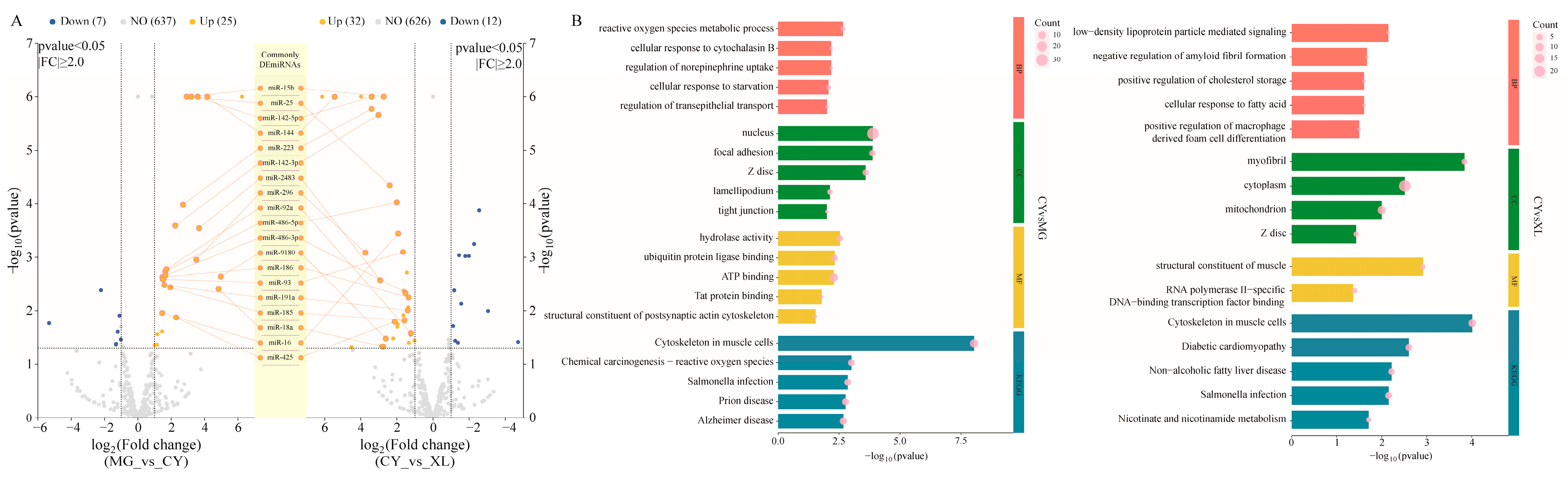
3.4. The Role of MicroRNA Changes
3.5. Generation of the lncRNA-mRNA Co-Expression Network
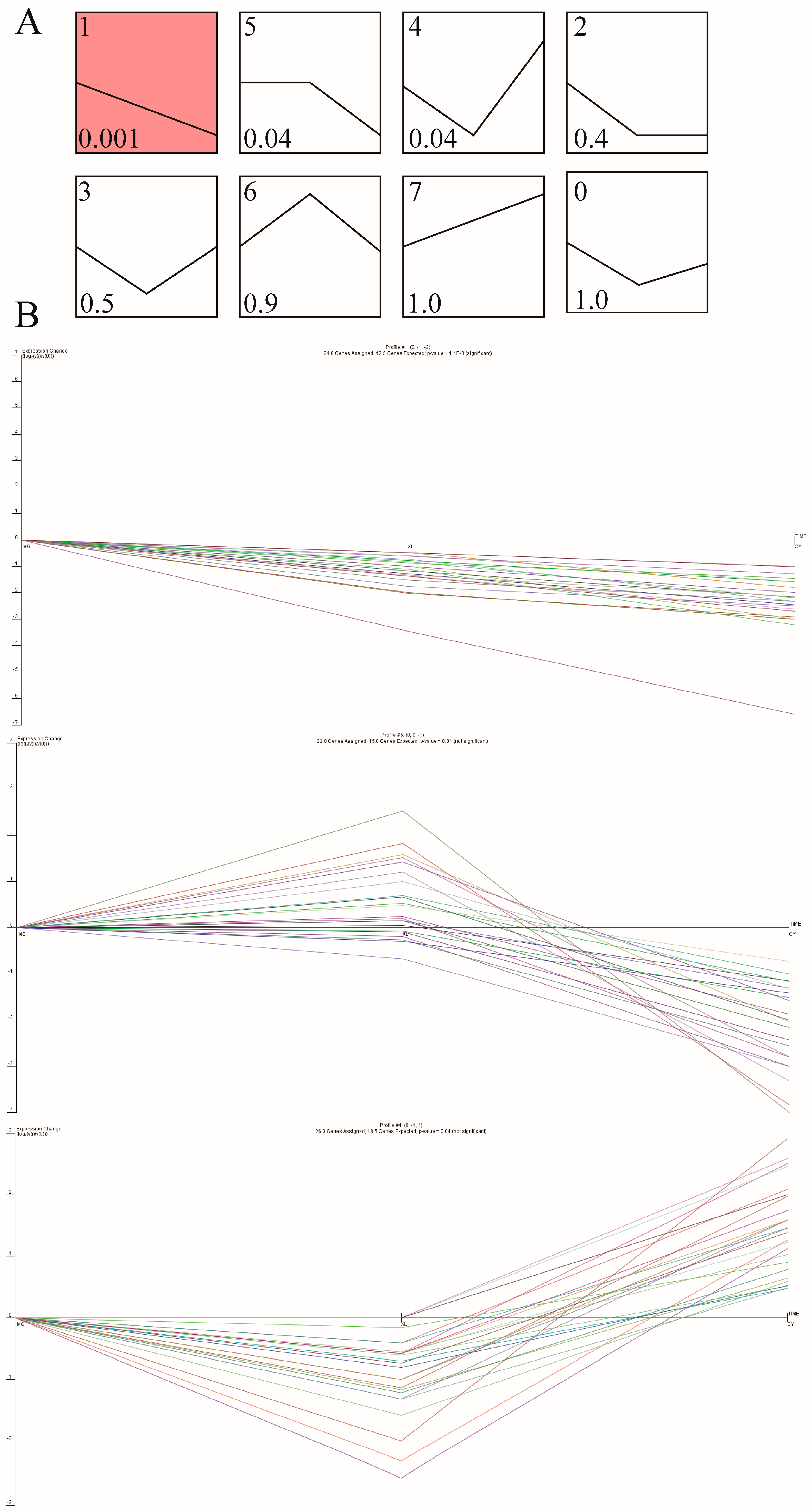
3.6. Expression Trend Analysis
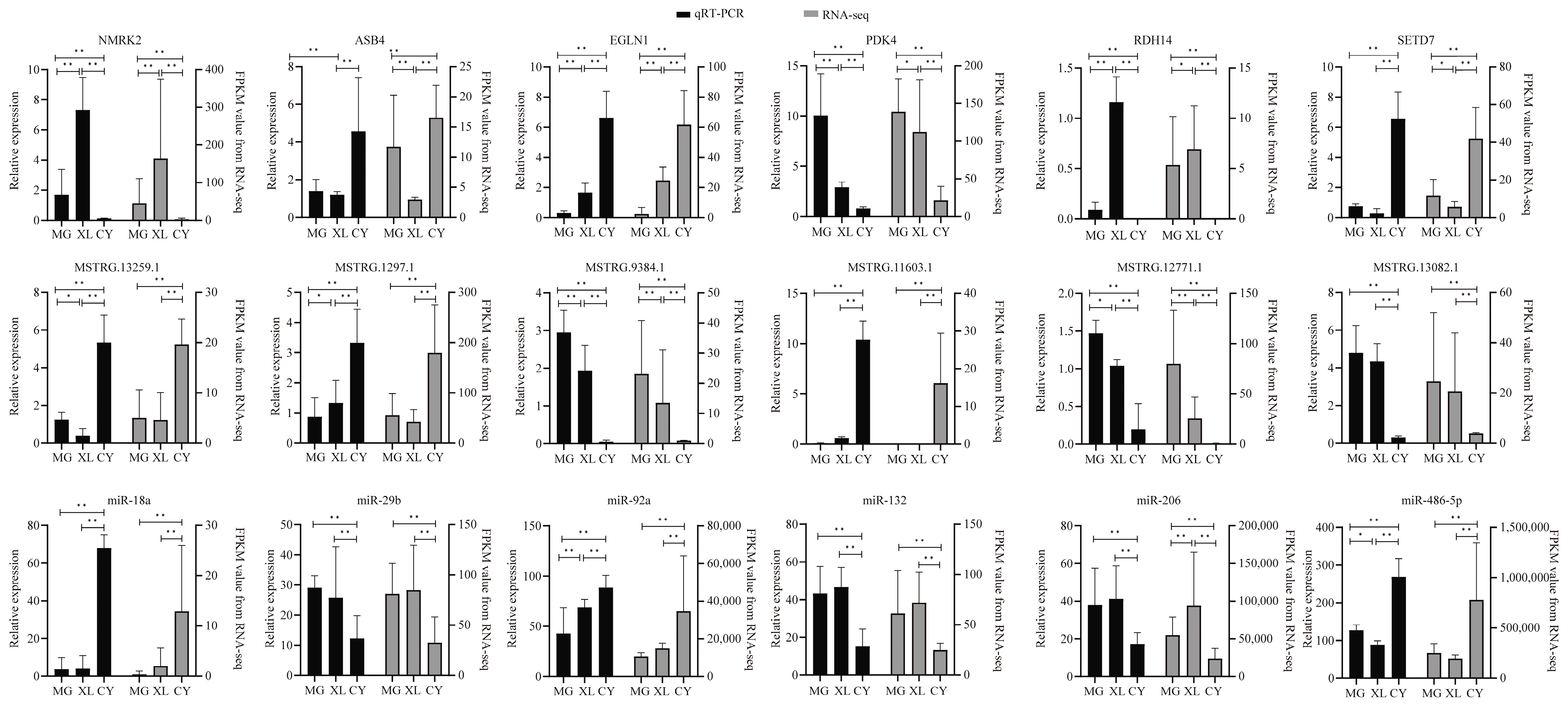
3.7. Validation of DE Coding RNAs and DE Non-Coding RNAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thiruvenkadan, A.; Kandasamy, N.; Panneerselvam, S. Inheritance of racing performance of Thoroughbred horses. Livest. Sci. 2009, 121, 308–326. [Google Scholar] [CrossRef]
- IFHA. International Federation of Horseracing Authorities; Article 6; IFHA: Paris, France, 2022; pp. 1–110. [Google Scholar]
- Davis, M. When Things Get Dark: A Mongolian Winter’s Tale; Macmillan: New York, NY, USA, 2010. [Google Scholar]
- Qi, B. Xilin Gol League Animal Husbandry Chronicle; Inner Mongolia People’s Publishing House: Hohhot, China, 2002. [Google Scholar]
- Cassidy, R. The Sport of Kings: Kinship, Class and Thoroughbred Breeding in Newmarket; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Kay, J.; Vamplew, W. Encyclopedia of British Horse Racing; Routledge: Oxford, UK, 2012. [Google Scholar]
- de Rezende, A.S.C.; Fonseca, M.G.; de Rezende Jordão, L.; de Freitas D’Angelis, F.H.; de Almeida, M.L.M.; de Queiroz Neto, A.; de Camargo Ferraz, G.; Rivero, J.-L.L. Skeletal muscle fiber composition of untrained Mangalarga Marchador fillies. J. Equine Vet. Sci. 2016, 36, 101–104. [Google Scholar] [CrossRef][Green Version]
- Bassel-Duby, R.; Olson, E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Fochi, S.; Giuriato, G.; De Simone, T.; Gomez-Lira, M.; Tamburin, S.; Del Piccolo, L.; Schena, F.; Venturelli, M.; Romanelli, M.G. Regulation of microRNAs in Satellite Cell Renewal, Muscle Function, Sarcopenia and the Role of Exercise. Int. J. Mol. Sci. 2020, 21, 6732. [Google Scholar] [CrossRef]
- Wu, W. Changes in expression of specific miRNAs and their target genes in repair of exercise-induced muscle injury in rats. Genet. Mol. Res. 2016, 15, gmr.15038698. [Google Scholar] [CrossRef]
- Mooren, F.C.; Viereck, J.; Krüger, K.; Thum, T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H557–H563. [Google Scholar] [CrossRef]
- Denham, J.; Prestes, P.R. Muscle-enriched microRNAs isolated from whole blood are regulated by exercise and are potential biomarkers of cardiorespiratory fitness. Front. Genet. 2016, 7, 196. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Al-Muhtaresh, H.A.; Salem, A.H. Expression and clinical significance of miR-1 and miR-133 in pre-diabetes. Biomed. Rep. 2021, 14, 33. [Google Scholar] [CrossRef]
- Deniz, E.; Erman, B. Long noncoding RNA (lincRNA), a new paradigm in gene expression control. Funct. Integr. Genom. 2017, 17, 135–143. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, biology and functioning. In Non-Coding RNAs in Colorectal Cancer; Springer: Cham, Switzerland, 2016; pp. 3–17. [Google Scholar]
- Latos, P.A.; Pauler, F.M.; Koerner, M.V.; Şenergin, H.B.; Hudson, Q.J.; Stocsits, R.R.; Allhoff, W.; Stricker, S.H.; Klement, R.M.; Warczok, K.E. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 2012, 338, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Cesana, M.; Cacchiarelli, D.; Legnini, I.; Santini, T.; Sthandier, O.; Chinappi, M.; Tramontano, A.; Bozzoni, I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011, 147, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Kallen, A.N.; Zhou, X.B.; Xu, J.; Qiao, C.; Ma, J.; Yan, L.; Lu, L.; Liu, C.; Yi, J.S.; Zhang, H.; et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell 2013, 52, 101–112. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1. [Google Scholar] [CrossRef]
- Archacka, K.; Ciemerych, M.A.; Florkowska, A.; Romanczuk, K. Non-Coding RNAs as Regulators of Myogenesis and Postexercise Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 11568. [Google Scholar] [CrossRef] [PubMed]
- Ricard, A.; Robert, C.; Blouin, C.; Baste, F.; Torquet, G.; Morgenthaler, C.; Rivière, J.; Mach, N.; Mata, X.; Schibler, L. Endurance exercise ability in the horse: A trait with complex polygenic determinism. Front. Genet. 2017, 8, 89. [Google Scholar] [CrossRef]
- Bou, T.; Ding, W.; Ren, X.; Liu, H.; Gong, W.; Jia, Z.; Zhang, X.; Dugarjaviin, M.; Bai, D. Muscle fibre transition and transcriptional changes of horse skeletal muscles during traditional Mongolian endurance training. Equine Vet. J. 2024, 56, 178–192. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Kovaka, S.; Zimin, A.V.; Pertea, G.M.; Razaghi, R.; Salzberg, S.L.; Pertea, M. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019, 20, 278. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from highthroughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef]
- Aleman, M.; Nieto, J.E. Gene expression of proteolytic systems and growth regulators of skeletal muscle in horses with myopathy associated with pituitary pars intermedia dysfunction. Am. J. Vet. Res. 2010, 71, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fang, C.; Sun, Y.; Liu, W. Evaluation of key miRNAs during early pregnancy in Kazakh horse using RNA sequencing. PeerJ 2021, 9, e10796. [Google Scholar] [CrossRef]
- Gu, Y.; Li, M.; Zhang, K.; Chen, L.; Jiang, A.A.; Wang, J.; Lv, X.; Li, X. Identification of suitable endogenous control microRNA genes in normal pig tissues. Anim. Sci. J. 2011, 82, 722–728. [Google Scholar] [CrossRef]
- Albertson, R.C.; Kocher, T.D. Genetic architecture sets limits on transgressive segregation in hybrid cichlid fishes. Evolution 2005, 59, 686–690. [Google Scholar] [CrossRef]
- Kang, O.D.; Ryu, Y.C.; Yun, Y.M.; Kang, M.S. Physiological changes in jeju crossbred riding horses by swim training. Asian-Australas. J. Anim. Sci. 2012, 25, 200–206. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Wang, J.; Wang, T.; Li, X.; Shen, Z.; Meng, J.; Yao, X. Estimation of Genetic Parameters of Body Conformation and Racing Performance Traits in Yili Horses. J. Equine Vet. Sci. 2025, 146, 105378. [Google Scholar] [CrossRef] [PubMed]
- Rivero, J.L.; Talmadge, R.J.; Edgerton, V.R. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in equine skeletal muscle and the influence of training. Anat. Rec. 1996, 246, 195–207. [Google Scholar] [CrossRef]
- Zhao, X.; Mo, D.; Li, A.; Gong, W.; Xiao, S.; Zhang, Y.; Qin, L.; Niu, Y.; Guo, Y.; Liu, X. Comparative analyses by sequencing of transcriptomes during skeletal muscle development between pig breeds differing in muscle growth rate and fatness. PLoS ONE 2011, 6, e19774. [Google Scholar] [CrossRef]
- Townley-Tilson, W.H.; Wu, Y.; Ferguson III, J.E.; Patterson, C. The ubiquitin ligase ASB4 promotes trophoblast differentiation through the degradation of ID2. PLoS ONE 2014, 9, e89451. [Google Scholar] [CrossRef]
- Lin, S.; Xian, M.; Ren, T.; Mo, G.; Zhang, L.; Zhang, X. Mining of chicken muscle growth genes and the function of important candidate gene RPL3L in muscle development. Front. Physiol. 2022, 13, 1033075. [Google Scholar] [CrossRef]
- Kim, M.; Kowalsky, A.H.; Lee, J.H. Sestrins in Physiological Stress Responses. Annu. Rev. Physiol. 2021, 83, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Dey, S. Protective response of Sestrin under stressful conditions in aging. Ageing Res. Rev. 2020, 64, 101186. [Google Scholar] [CrossRef]
- Ho, A.; Cho, C.S.; Namkoong, S.; Cho, U.S.; Lee, J.H. Biochemical Basis of Sestrin Physiological Activities. Trends Biochem. Sci. 2016, 41, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, T.; Yu, Z.; Yu, Q.; Wang, Y.; Hu, J.; Shi, J.; Yang, G. The functions and roles of sestrins in regulating human diseases. Cell. Mol. Biol. Lett. 2022, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Perdiguero, E.; Serrano, A.L.; Sousa-Victor, P.; Ortet, L.; Jardí, M.; Budanov, A.V.; Garcia-Prat, L.; Sandri, M.; Thomson, D.M. Sestrin prevents atrophy of disused and aging muscles by integrating anabolic and catabolic signals. Nat. Commun. 2020, 11, 189. [Google Scholar] [CrossRef]
- Crisol, B.M.; Lenhare, L.; Gaspar, R.S.; Gaspar, R.C.; Muñoz, V.R.; da Silva, A.S.; Cintra, D.E.; de Moura, L.P.; Pauli, J.R.; Ropelle, E.R. The role of physical exercise on Sestrin1 and 2 accumulations in the skeletal muscle of mice. Life Sci. 2018, 194, 98–103. [Google Scholar] [CrossRef]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.S.; Semple, I.; Ro, S.H.; et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 2020, 11, 190. [Google Scholar] [CrossRef]
- Jing, Y.; Zuo, Y.; Sun, L.; Yu, Z.R.; Ma, S.; Hu, H.; Zhao, Q.; Huang, D.; Zhang, W.; Belmonte, J.C.I.; et al. SESN1 is a FOXO3 effector that counteracts human skeletal muscle ageing. Cell Prolif. 2023, 56, e13455. [Google Scholar] [CrossRef]
- Kallio, P.J.; Wilson, W.J.; O’Brien, S.; Makino, Y.; Poellinger, L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J. Biol. Chem. 1999, 274, 6519–6525. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Chen, Z.; Liu, Y.; Li, Y.; Ye, J.; Lu, L. Zebrafish mutants in egln1 display a hypoxic response and develop polycythemia. Life Sci. 2024, 344, 122564. [Google Scholar] [CrossRef] [PubMed]
- Lappin, T.R.; Lee, F.S. Update on mutations in the HIF: EPO pathway and their role in erythrocytosis. Blood Rev. 2019, 37, 100590. [Google Scholar] [CrossRef]
- Malekan, M.; Ebrahimzadeh, M.A.; Sheida, F. The role of Hypoxia-Inducible Factor-1alpha and its signaling in melanoma. Biomed. Pharmacother. 2021, 141, 111873. [Google Scholar] [CrossRef] [PubMed]
- Brutsaert, T.D.; Kiyamu, M.; Elias Revollendo, G.; Isherwood, J.L.; Lee, F.S.; Rivera-Ch, M.; Leon-Velarde, F.; Ghosh, S.; Bigham, A.W. Association of EGLN1 gene with high aerobic capacity of Peruvian Quechua at high altitude. Proc. Natl. Acad. Sci. USA 2019, 116, 24006–24011. [Google Scholar] [CrossRef]
- Shin, J.; Nunomiya, A.; Kitajima, Y.; Dan, T.; Miyata, T.; Nagatomi, R. Prolyl hydroxylase domain 2 deficiency promotes skeletal muscle fiber-type transition via a calcineurin/NFATc1-dependent pathway. Skelet. Muscle 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Dautova, A.; Valeeva, E.; Semenova, E.; Mavliev, F.; Zverev, A.; Nazarenko, A.; Larin, A.; Generozov, E.; Ahmetov, I. Association of the Polymorphic Marker rs1614148 of the EGLN1 Gene with Aerobic Capacity of Athletes. Fiziol. Čeloveka 2024, 50, 52–60. [Google Scholar]
- Rissanen, E.; Tranberg, H.K.; Sollid, J.; Nilsson, G.r.E.; Nikinmaa, M. Temperature regulates hypoxia-inducible factor-1 (HIF-1) in a poikilothermic vertebrate, crucian carp (Carassius carassius). J. Exp. Biol. 2006, 209, 994–1003. [Google Scholar] [CrossRef]
- Maekawa, S.; Iemura, H.; Kato, T. Enhanced erythropoiesis in mice exposed to low environmental temperature. J. Exp. Biol. 2013, 216, 901–908. [Google Scholar] [CrossRef]
- Kendon, M.; McCarthy, M.; Jevrejeva, S.; Matthews, A.; Williams, J.; Sparks, T.; West, F. State of the UK Climate 2022. Int. J. Climatol. 2023, 43, S1. [Google Scholar] [CrossRef]
- Wang, K.; Cao, C.; Xie, B.; Xu, M.; Yang, X.; Guo, H.; Duerler, R.S. Analysis of the Spatial and Temporal Evolution Patterns of Grassland Health and Its Driving Factors in Xilingol. Remote Sens. 2022, 14, 5179. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Judson, R.N.; Quarta, M.; Oudhoff, M.J.; Soliman, H.; Yi, L.; Chang, C.K.; Loi, G.; Vander Werff, R.; Cait, A.; Hamer, M.; et al. Inhibition of Methyltransferase Setd7 Allows the In Vitro Expansion of Myogenic Stem Cells with Improved Therapeutic Potential. Cell Stem Cell 2018, 22, 177–190.e177. [Google Scholar] [CrossRef]
- Li, M.; Ning, J.; Wang, J.; Yan, Q.; Zhao, K.; Jia, X. SETD7 regulates chondrocyte differentiation and glycolysis via the Hippo signaling pathway and HIF-1α. Int. J. Mol. Med. 2021, 48, 210. [Google Scholar] [CrossRef] [PubMed]
- Burguière, A.C.; Nord, H.; von Hofsten, J. Alkali-like myosin light chain-1 (myl1) is an early marker for differentiating fast muscle cells in zebrafish. Dev. Dyn. 2011, 240, 1856–1863. [Google Scholar] [CrossRef]
- Margolis, L.M.; Berryman, C.E.; Murphy, N.E.; Carrigan, C.T.; Young, A.J.; Carbone, J.W.; Pasiakos, S.M. PI3K-AKT-FOXO1 pathway targeted by skeletal muscle microRNA to suppress proteolytic gene expression in response to carbohydrate intake during aerobic exercise. Physiol. Rep. 2018, 6, e13931. [Google Scholar] [CrossRef]
- Mandal, S.; Denham, M.M.; Spencer, S.J.; Denham, J. Exercise regulates shelterin genes and microRNAs implicated in ageing in Thoroughbred horses. Pflügers Arch. Eur. J. Physiol. 2022, 474, 1159–1169. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Song, Y. Cardioprotective Effects of Exercise: The Role of Irisin and Exosome. Curr. Vasc. Pharmacol. 2024, 22, 316–334. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, M.; Shan, Y.J.; Pang, L.C.; Ji, G.G.; Ju, X.J.; Tu, Y.J.; Shi, S.Y.; Bai, H.; Zou, J.M.; et al. Transcriptome sequencing analysis of the role of miR-499-5p and SOX6 in chicken skeletal myofiber specification. Front. Genet. 2022, 13, 1008649. [Google Scholar] [CrossRef]
- van Rooij, E.; Quiat, D.; Johnson, B.A.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Kelm, R.J.; Olson, E.N. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev. Cell 2009, 17, 662–673. [Google Scholar] [CrossRef]
- Gandolfi, G.; Pomponio, L.; Ertbjerg, P.; Karlsson, A.; Costa, L.N.; Lametsch, R.; Russo, V.; Davoli, R. Investigation on CAST, CAPN1 and CAPN3 porcine gene polymorphisms and expression in relation to post-mortem calpain activity in muscle and meat quality. Meat Sci. 2011, 88, 694–700. [Google Scholar] [CrossRef]
- Felício, A.M.; Boschiero, C.; Balieiro, J.C.d.C.; Ledur, M.; Ferraz, J.B.S.; Michelan Filho, T.; Moura, A.S.A.M.T.; Coutinho, L.L. Identification and association of polymorphisms in CAPN1 and CAPN3 candidate genes related to performance and meat quality traits in chickens. Genet. Mol. Res. 2013, 12, 472–482. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Zhou, Z.; Kong, S.; Zhang, J.; Zhang, X.; Nie, Q. circPTPN4 regulates myogenesis via the miR-499-3p/NAMPT axis. J. Anim. Sci. Biotechnol. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yoon, C.-S.; Park, D.R. NAMPT regulates mitochondria biogenesis via NAD metabolism and calcium binding proteins during skeletal muscle contraction. J. Exerc. Nutr. Biochem. 2014, 18, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Yao, X.; Wang, H.; Zhao, L.; Jiang, H.; Yao, X.; Zhang, S.; Ye, C.; Liu, W. miR-182 regulates metabolic homeostasis by modulating glucose utilization in muscle. Cell Rep. 2016, 16, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-L.; Gong, Y.; Zhao, D.-P. Elevated PHD2 expression might serve as a valuable biomarker of poor prognosis in lung adenocarcinoma, but no lung squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8731–8739. [Google Scholar]
- Li, Y.; Zhang, D.; Wang, X.; Yao, X.; Ye, C.; Zhang, S.; Wang, H.; Chang, C.; Xia, H.; Wang, Y.C.; et al. Hypoxia-inducible miR-182 enhances HIF1α signaling via targeting PHD2 and FIH1 in prostate cancer. Sci. Rep. 2015, 5, 12495. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Senda, T.; Nakano, T.; Nakada, C.; Hida, T.; Ishiguro, N.; Kondo, G.; Baba, T.; Sato, K.; Osaki, M. Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab. Investig. 2002, 82, 645–655. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Hijiya, N.; Yano, S.; Yokoyama, S.; Nakada, C.; Uchida, T.; Matsuura, K.; Moriyama, M. Arpp/Ankrd2, a member of the muscle ankyrin repeat proteins (MARPs), translocates from the I-band to the nucleus after muscle injury. Histochem. Cell Biol. 2008, 129, 55–64. [Google Scholar] [CrossRef]
- Boskovic, S.; Marin-Juez, R.; Jasnic, J.; Reischauer, S.; El Sammak, H.; Kojic, A.; Faulkner, G.; Radojkovic, D.; Stainier, D.Y.; Kojic, S. Characterization of zebrafish (Danio rerio) muscle ankyrin repeat proteins reveals their conserved response to endurance exercise. PLoS ONE 2018, 13, e0204312. [Google Scholar] [CrossRef]
- Macera, M.J.; Szabo, P.; Wadgaonkar, R.; Siddiqui, M.A.; Verma, R.S. Localization of the gene coding for ventricular myosin regulatory light chain (MYL2) to human chromosome 12q23-q24.3. Genomics 1992, 13, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.; Lyon, R.C.; Chen, J. Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease. Gene 2015, 569, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Späth, E.; Schüler, S.C.; Heinze, I.; Dau, T.; Minetti, A.; Hofmann, M.; Maltzahn, J.v.; Ori, A. Proteome dynamics reveal Leiomodin 1 as a key regulator of myogenic differentiation. bioRxiv 2024, 587321. [Google Scholar]
- Ling, F.; Fang, W.; Chen, Y.; Li, J.; Liu, X.; Wang, L.; Zhang, H.; Chen, S.; Mei, Y.; Du, H. Identification of novel transcripts from the porcine MYL1 gene and initial characterization of its promoters. Mol. Cell. Biochem. 2010, 343, 239–247. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Gong, W.; Bou, T.; Shi, L.; Lin, Y.; Wu, H.; Dugarjaviin, M.; Bai, D. Pilot Study on the Profiling and Functional Analysis of mRNA, miRNA, and lncRNA in the Skeletal Muscle of Mongolian Horses, Xilingol Horses, and Grassland-Thoroughbreds. Animals 2025, 15, 1123. https://doi.org/10.3390/ani15081123
Ding W, Gong W, Bou T, Shi L, Lin Y, Wu H, Dugarjaviin M, Bai D. Pilot Study on the Profiling and Functional Analysis of mRNA, miRNA, and lncRNA in the Skeletal Muscle of Mongolian Horses, Xilingol Horses, and Grassland-Thoroughbreds. Animals. 2025; 15(8):1123. https://doi.org/10.3390/ani15081123
Chicago/Turabian StyleDing, Wenqi, Wendian Gong, Tugeqin Bou, Lin Shi, Yanan Lin, Huize Wu, Manglai Dugarjaviin, and Dongyi Bai. 2025. "Pilot Study on the Profiling and Functional Analysis of mRNA, miRNA, and lncRNA in the Skeletal Muscle of Mongolian Horses, Xilingol Horses, and Grassland-Thoroughbreds" Animals 15, no. 8: 1123. https://doi.org/10.3390/ani15081123
APA StyleDing, W., Gong, W., Bou, T., Shi, L., Lin, Y., Wu, H., Dugarjaviin, M., & Bai, D. (2025). Pilot Study on the Profiling and Functional Analysis of mRNA, miRNA, and lncRNA in the Skeletal Muscle of Mongolian Horses, Xilingol Horses, and Grassland-Thoroughbreds. Animals, 15(8), 1123. https://doi.org/10.3390/ani15081123



