Role of Seagrass as a Food Source for Benthos in Tidal Flats: Toward Conservation and Restoration of Resilient Ecosystems
Simple Summary
Abstract
1. Introduction
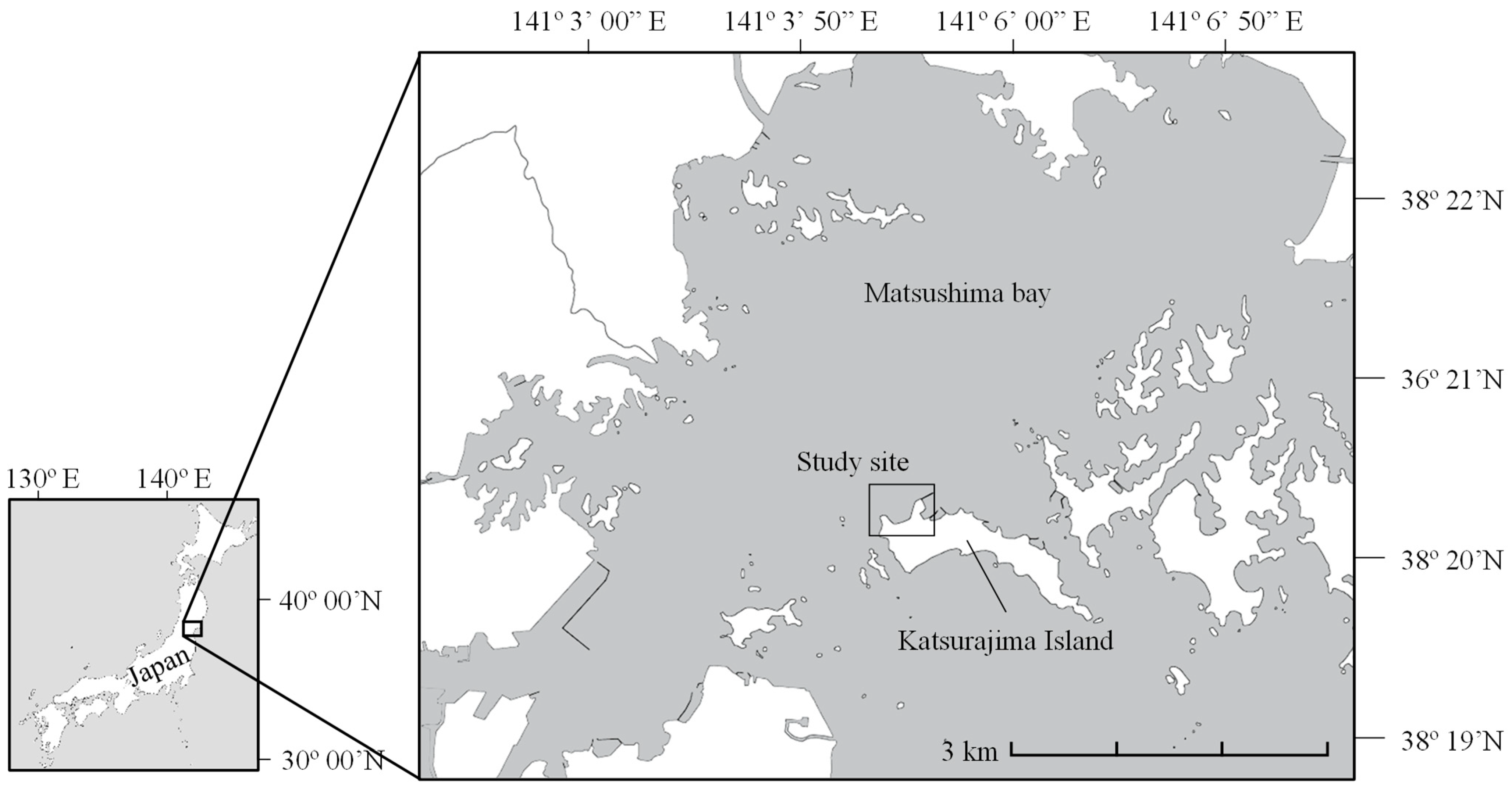
2. Materials and Methods
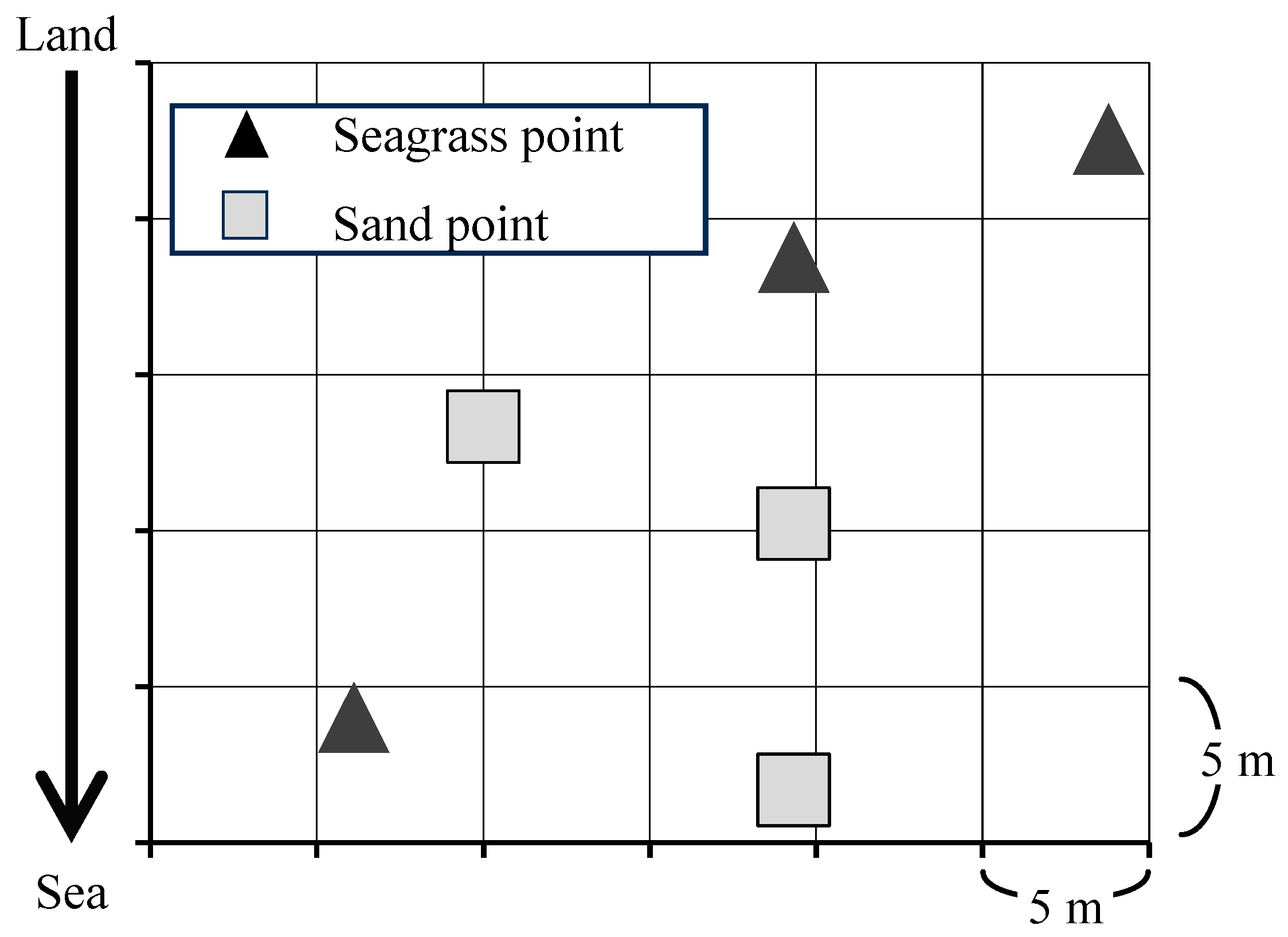
2.1. Sample Collection
2.2. Carbon and Nitrogen Stable Isotope Ratio Analyses
2.3. Fatty Acid Composition Analysis
2.4. Biomarkers of Organic Matter Sources in the Tidal Flat Ecosystem
2.5. Statistical Analysis
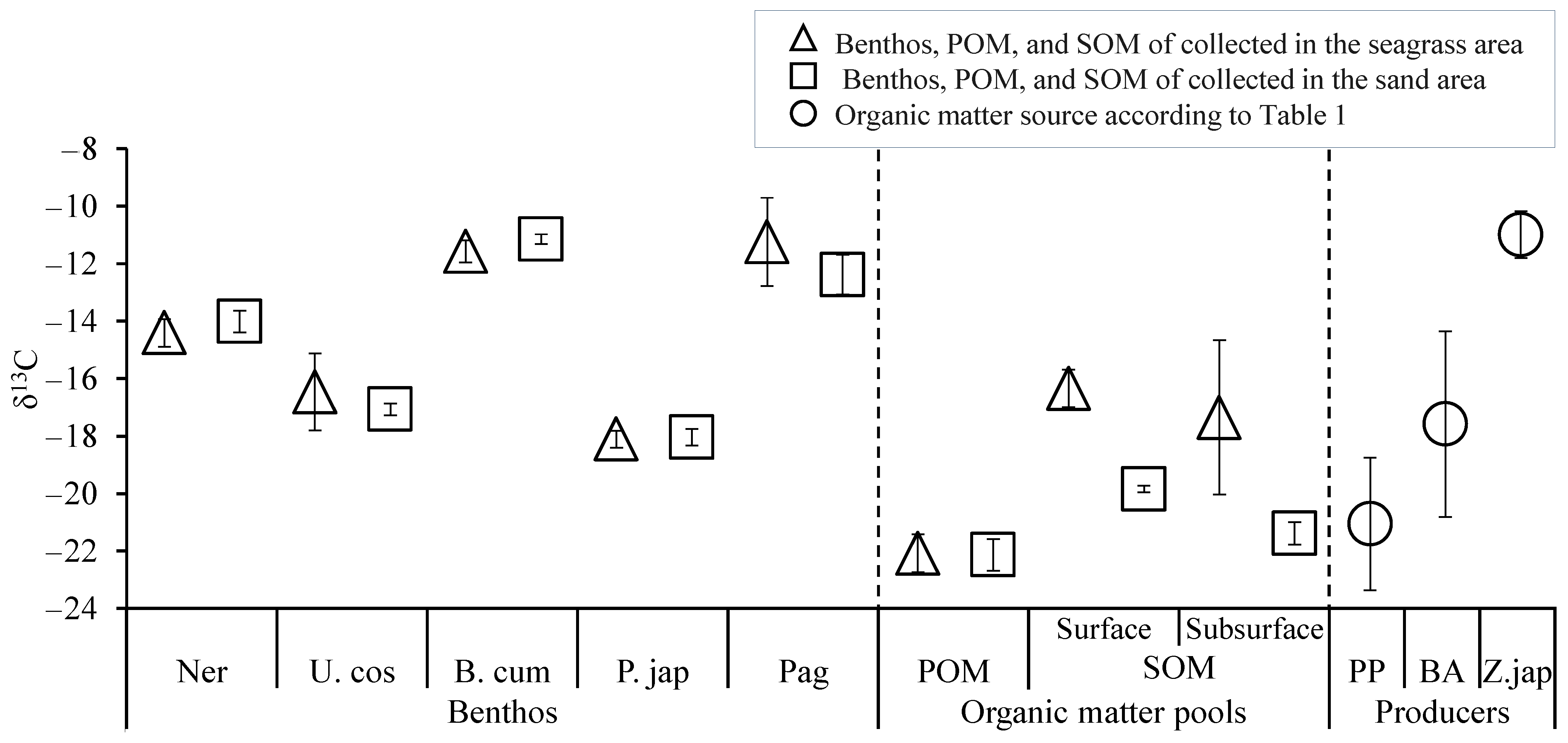
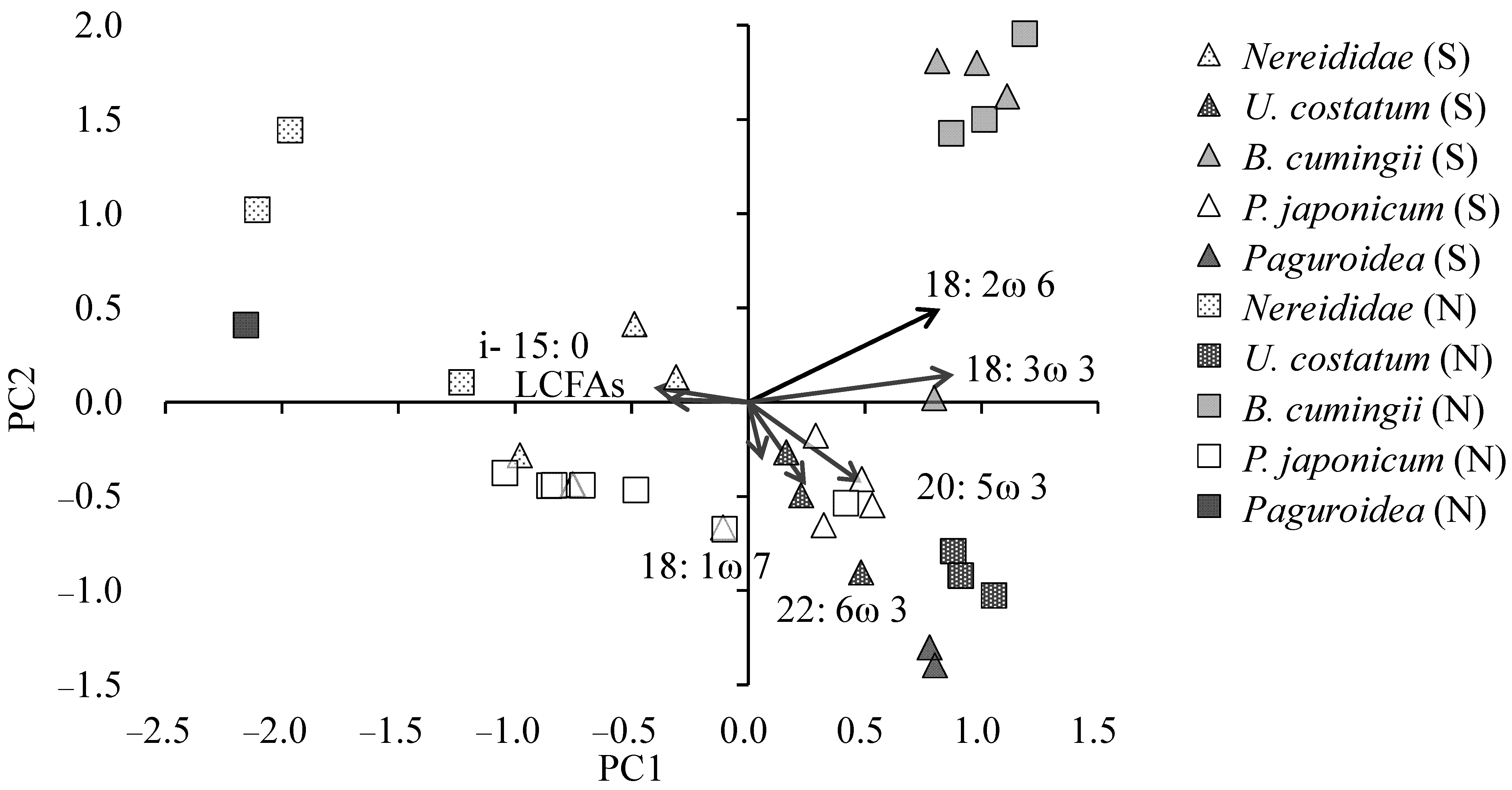
3. Results
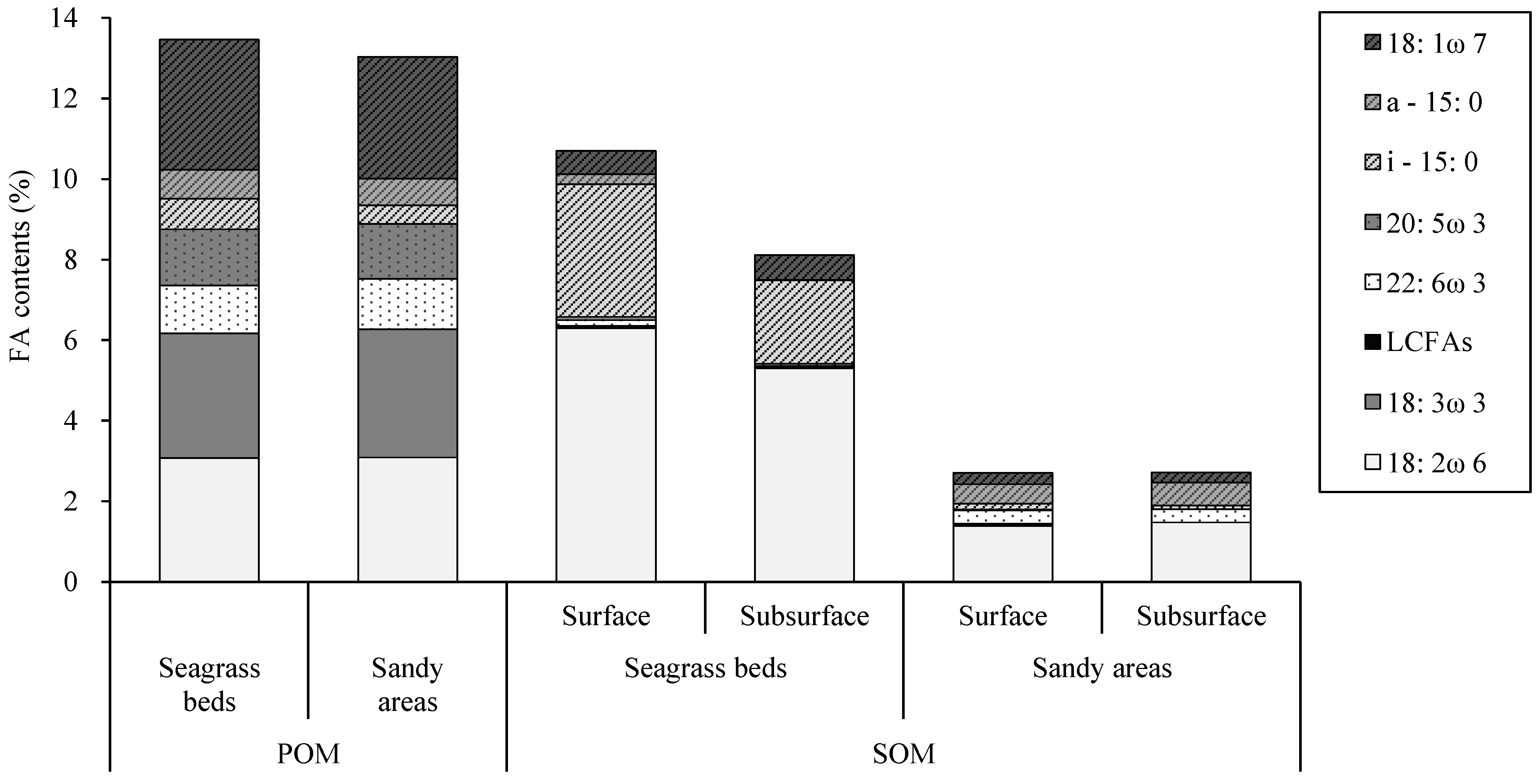
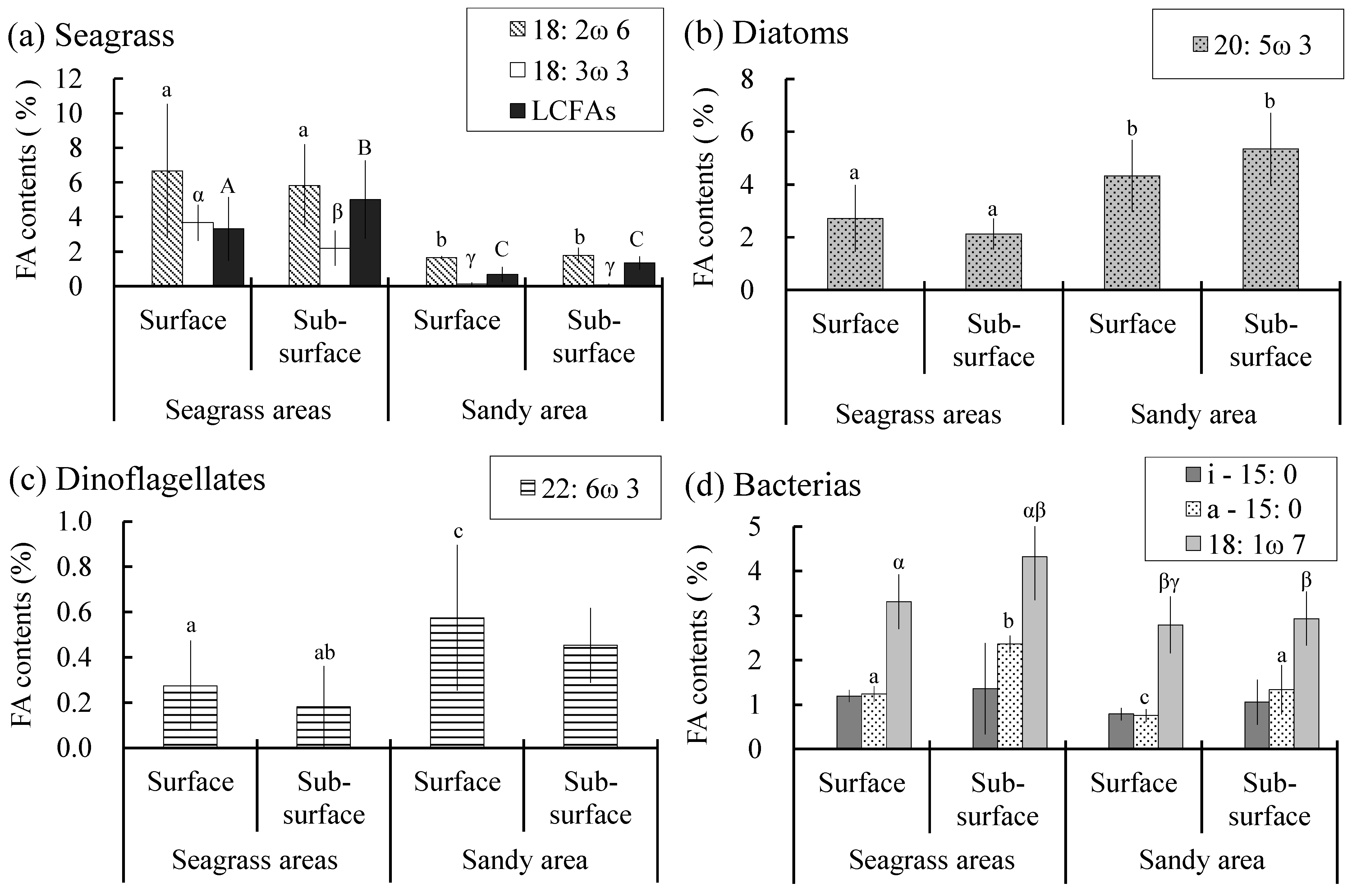
3.1. POM and SOM
3.2. Benthic Fauna and Target Benthos
4. Discussion
5. Conclusions
- (1)
- Batillaria cumingii, an epifaunal mud snail inhabiting intertidal flats, actively consumes organic matter derived from intertidal seagrass Z. japonica. Z. japonica-derived organic matter was present not only in the surface sediments of seagrass beds, but also in sandy areas. Therefore, the presence of nearby intertidal seagrass beds provides a favorable habitat within the intertidal flats of B. cumingii.
- (2)
- Nereididae, which inhabit intertidal flats, have been suggested to primarily consume bacteria and seagrass detritus. A significant correlation was observed between Z. japonica and bacteria, with bacterial abundance being higher in the intertidal seagrass beds. This indicates that intertidal seagrass beds serve as suitable habitats for Nereididae within intertidal flats.
- (3)
- Although Z. japonica-derived organic matter was present in the POM of both the intertidal seagrass beds and nearby bare sandy areas, Umbonium costatum and Phacosoma japonicum, filter feeders inhabiting intertidal flats, showed little utilization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, N.J.; Phinn, S.R.; DeWitt, M.; Ferrari, R.; Johnston, R.; Lyons, M.B.; Clinton, N.; Thau, D.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.K.; Woodworth, B.K.; Phinn, S.R.; Murray, N.J.; Fuller, R.A. Global protected-area coverage and human pressure on tidal flats. Conserv. Biol. 2021, 35, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Zhou, G. Spatio-temporal changes of mangrove-covered tidal flats over 35 years using satellite remote sensing imageries: A case study of Beibu Gulf, China. Remote Sens. 2023, 15, 1928. [Google Scholar] [CrossRef]
- Sasaki, K. The Problem about Reviving tidal Flats. Proc. Civ. Eng. Ocean 2002, 18, 49–54. [Google Scholar] [CrossRef]
- Pennekamp, F.; Pontarp, M.; Tabi, A.; Altermatt, F.; Alther, R.; Choffat, Y.; Fronhofer, E.A.; Ganesanandamoorthy, P.; Garnier, A.; Griffiths, J.I.; et al. Biodiversity increases and decreases ecosystem stability. Nature 2018, 563, 109–112. [Google Scholar] [CrossRef]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.; Bruelheide, H.; de Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 2015, 526, 574–577. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Sheridan, P. Benthos of adjacent mangrove, seagrass and non-vegetated habitats in Rookery Bay, Florida, USA. Estuarine Coast. Shelf Sci. 1997, 44, 455–469. [Google Scholar] [CrossRef]
- Alfaro, A.C. Benthic macro-invertebrate community composition within a mangrove/seagrass estuary in northern New Zealand. Estuarine Coast. Shelf Sci. 2006, 66, 97–110. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fong, C.W.; Wu, R.S.S. The effects of seagrass (Zostera japonica) canopy structure on associated fauna: A study using artificial seagrass units and sampling of natural beds. J. Exp. Mar. Biol. Ecol. 2001, 259, 23–50. [Google Scholar] [CrossRef]
- Nagahama, Y.; Nomura, M.; Nakano, K.; Kimura, K.; Nishimura, O. Characterization of seagrass bed habitat and its effect on benthic fauna. JSCE J. Environ. Syst. Eng. 2007, 63, 233–240. (In Japanese) [Google Scholar] [CrossRef]
- Kharlamenko, V.I.; Kiyashko, S.I.; Imbs, A.B.; Vyshkvartzev, D.I. Identification of food sources of invertebrates from the seagrass Zostera marina community using carbon and sulfur stable isotope ratio and fatty acid analyses. Mar. Ecol. Prog. Ser. 2001, 220, 103–117. [Google Scholar] [CrossRef]
- Brun, F.G.; Cobo-Díaz, J.F.; González-Ortiz, V.; Varela, J.L.; Pérez-Lloréns, J.L.; Vergara, J.J. Seagrass patch complexity affects macroinfaunal community structure in intertidal areas: An in situ experiment using seagrass mimics. Diversity 2021, 13, 572. [Google Scholar] [CrossRef]
- Wajima, T.; Arimatsu, T.; Ito, N.; Toyohara, T.; Yoshizawa, S.; Fukushima, T. Tokyo-Wan Moba Bunpu Chousa–Amamo-Ba Chousa No Matome (Survey on the Distribution of Seagrass Beds in Tokyo Bay–Summary of Zostera Bed Survey) Marine Biological Research Institute of Japan Annual Report 2004. Volume 2004, pp. 31–37. Available online: https://mbrij.co.jp/wp-content/uploads/2024/04/2004_k4.pdf (accessed on 6 April 2025).
- Meziane, T.; Tsuchiya, M. Fatty acids as tracers of organic matter in the sediment and food web of a mangrove intertidal flat ecosystem, Okinawa, Japan. Mar. Ecol. Prog. Ser. 2000, 200, 49–57. [Google Scholar] [CrossRef]
- Nagahama, Y.; Nomura, M.; Fujibayashi, M.; Shin, W.S.; Nishimura, O. Characterization of the carbon stable isotope ratio and fatty acid structure of Zostera japonica in coastal areas. J. Water Environ. Technol. 2011, 9, 101–109. [Google Scholar] [CrossRef]
- Abrantes, K.; Sheaves, M. Food web structure in a near-pristine mangrove area of the Australian Wet Tropics. Estuarine Coast. Shelf Sci. 2009, 82, 597–607. [Google Scholar] [CrossRef]
- Svensson, C.J.; Hyndes, G.A.; Lavery, P.S. Food web analysis in two permanently open temperate estuaries: Consequences of saltmarsh loss? Mar. Environ. Res. 2007, 64, 286–304. [Google Scholar] [CrossRef]
- Yokoyama, H. Food sources of consumers in temperate estuaries and coastal waters: Achievements and potential problems of isotopic studies. Jpn. J. Ecol. 2008, 58, 23–36. (In Japanese) [Google Scholar]
- Jaschinski, S.; Brepohl, D.C.; Sommer, U. Carbon sources and trophic structure in an eelgrass Zostera marina bed, based on stable isotope and fatty acid analyses. Mar. Ecol. Prog. Ser. 2008, 358, 103–114. [Google Scholar] [CrossRef]
- Matsuo, H.; Ariyama, H.; Ikemoto, T.; Omori, K.; Takeuchi, I. Analysis of food web structure in an artificial tidal flat in Osaka Bay using stable isotopes of carbon and nitrogen. J. Jpn. Soc. Water Environ. 2009, 32, 99–104. [Google Scholar] [CrossRef]
- Schaal, G.; Riera, P.; Leroux, C. Trophic coupling between two adjacent benthic food webs within a man-made intertidal area: A stable isotopes evidence. Estuarine Coast. Shelf Sci. 2008, 77, 523–534. [Google Scholar] [CrossRef]
- Arts, M.T.; Wainman, B.C. Lipids in Freshwater Ecosystems; Springer: New York, NY, USA, 1998. [Google Scholar] [CrossRef]
- Koike, H.; Nakajima, T.; Nakai, N. Stable carbon isotopic and gut content analyses of a tidal flat food web. Benthos Res. 1989, 1989, 1–10. (In Japanese) [Google Scholar] [CrossRef]
- Yamada, S.B. Growth and longevity of the mud snail Batillaria attramentaria. Mar. Biol. 1982, 67, 187–192. [Google Scholar] [CrossRef]
- Byers, J.E. Competition between two estuarine snails: Implications for invasions of exotic species. Ecology 2000, 81, 1225–1239. [Google Scholar] [CrossRef]
- Schembri, P.J. Feeding behaviour of fifteen species of hermit crabs (Crustacea: Decapoda: Anomura) from the Otago region, southeastern New Zealand. J. Nat. Hist. 1982, 16, 859–878. [Google Scholar] [CrossRef]
- Cúcio, C.; Engelen, A.H.; Costa, R.; Muyzer, G. Rhizosphere microbiomes of European + seagrasses are selected by the plant, but are not species specific. Front. Microbiol. 2016, 7, 440. [Google Scholar] [CrossRef]
- Cúcio, C.; Overmars, L.; Engelen, A.H.; Muyzer, G. Metagenomic analysis shows the presence of bacteria related to free-living forms of sulfur-oxidizing chemolithoautotrophic symbionts in the rhizosphere of the seagrass Zostera marina. Front. Mar. Sci. 2018, 5, 171. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Harley, M.B. Occurrence of a filter-feeding mechanism in the polychaete Nereis diversicolor. Nature 1950, 165, 734–735. [Google Scholar] [CrossRef]
- Pardo, E.V.; Dauer, D.M. Particle size selection in individuals from epifaunal versus infaunal populations of the nereidid polychaete Neanthes succinea (Polychaeta: Nereididae). Hydrobiologia 2003, 496, 355–360. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Kurihara, Y. Effect of the feeding behaviour of macrobenthos on changes in environmental conditions of intertidal flats. J. Exp. Mar. Biol. Ecol. 1980, 44, 85–94. [Google Scholar] [CrossRef]
- Noda, T.; Nakao, S.; Goshima, S. Life history of the temperate subtidal gastropod Umbonium costatum. Mar. Biol. 1995, 122, 73–78. [Google Scholar] [CrossRef]
- Miyaji, T.; Tanabe, K.; Schöne, B.R. Environmental controls on daily shell growth of Phacosoma japonicum (Bivalvia: Veneridae) from Japan. Mar. Ecol. Prog. Ser. 2007, 336, 141–150. [Google Scholar] [CrossRef]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Tilman, D. Plant diversity and the stability of foodwebs. Ecol. Lett. 2011, 14, 42–46. [Google Scholar] [CrossRef]


| Organic Matter Source | Producer | δ13C (SD) | δ15N (SD) | Ref. | Fatty Acid | Ref. |
|---|---|---|---|---|---|---|
| Seagrass | Z. japonica | −11.0 (0.8) | 5.9 (0.4) | This research | 18: 2ω6 18: 3ω3 LCFAs | [17] |
| Benthic algae | Diatoms | −17.6 (3.2) | 6.2 (4.7) | [18,19,20] | 20: 5ω3 | [10,13,21] |
| Phytoplankton | Diatoms and Dinoflagellates | −21.1 (2.3) | 5.7 (3.7) | [13,20,22,23] | Diatom: 20: 5ω3 | [10,13,21] |
| Dinoflagellates: 22: 6ω3 | [10,13,24] | |||||
| Soil bacteria | i-15:0 a-15:0 18: 1ω7 | [10,13,16,21] |
| Seagrass Points | Sand Points | Seagrass Points | Sand Points | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | mean | SD | ||
| Polychaeta | Gastropoda | ||||||||
| Nereididae | 485 | (743) | Trochidae | 76 | (68) | 466 | (317) | ||
| Phyllodocidae | 13 | (22) | Batillariidae | 32 | (55) | ||||
| Nephtyiclae | 25 | (11) | Naticidae | 6 | (11) | ||||
| Glyceridae | 6 | (11) | Nassariidae | 139 | (95) | 13 | (22) | ||
| Lumbrineridae | 32 | (22) | 13 | (11) | Cylichnidae | 6 | (11) | ||
| Onuphidae | 6 | (11) | 19 | (33) | Bivalvia | ||||
| Spionidae | 63 | (58) | Tellinidae | 25 | (44) | 19 | (19) | ||
| Cirratulidae | 25 | (11) | Veneridae | 271 | (39) | 170 | (168) | ||
| Capitellidae | 50 | (39) | 32 | (55) | Myidae | 44 | (76) | ||
| Maldanidae | 6 | (11) | Malacostraca | ||||||
| Terebellidae | 32 | (55) | 6 | (11) | Gammaridae | 50 | (39) | 13 | (11) |
| Cirolanidae | 25 | (44) | |||||||
| Paguroidea | 687 | (398) | |||||||
| Barchyuran | 6 | (11) | |||||||
| Seagrass Area | Sandy Area | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ner | U.cos | B.cum | P.jap | Pag | Ner | B.cum | U.cos | P.jap | N_Pag | |||||||||||
| n = 3 | n = 4 | n = 3 | n = 6 | n = 2 | n = 3 | n = 3 | n = 3 | n = 7 | n = 1 | |||||||||||
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| 18:2n6c | - | - | 1.7 | (0.3) | 3.6 | (0.4) | 0.8 | (0.6) | 2.1 | (0.0) | - | - | 4.3 | (0.9) | 1.8 | (0.5) | 0.5 | (0.6) | - | - |
| 18:3n3 | - | - | 1.4 | (0.4) | 1.6 | (0.3) | 0.6 | (0.7) | 1.3 | (0.0) | - | - | 2.1 | (0.2) | 1.8 | (0.1) | 0.4 | (0.6) | - | - |
| LCFAs | 3.2 | (5.6) | 0.8 | (1.4) | 0.3 | (0.6) | - | - | - | - | 2.5 | (1.6) | - | - | - | - | - | - | - | - |
| 22:6n3 | 0.9 | (0.9) | 5.8 | (0.5) | 4.9 | (1.5) | 17.6 | (2.8) | 6.8 | (0.7) | 0.4 | (0.7) | 3.5 | (0.3) | 5.6 | (0.3) | 11.0 | (5.6) | - | - |
| 20:5n3 | 17.8 | (3.8) | 14.0 | (0.4) | 11.0 | (0.1) | 13.7 | (1.3) | 15.7 | (0.4) | 9.2 | (7.1) | 10.3 | (1.0) | 16.2 | (0.2) | 9.8 | (4.1) | - | - |
| i-15:0 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.3 | - |
| a-15:0 | 1.6 | (0.1) | - | - | 2.7 | (1.2) | - | - | 1.3 | (0.1) | 3.7 | (1.6) | 1.3 | (0.1) | - | - | - | - | 2.5 | - |
| 18:1n7 | 11.9 | (1.9) | 8.5 | (0.9) | 4.7 | (1.5) | 3.4 | (0.6) | 6.8 | (0.3) | 6.0 | (3.5) | 3.9 | (0.7) | 9.3 | (0.3) | 4.6 | (0.6) | 1.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagahama, Y.; Nomura, M.; Nishimura, O. Role of Seagrass as a Food Source for Benthos in Tidal Flats: Toward Conservation and Restoration of Resilient Ecosystems. Animals 2025, 15, 1098. https://doi.org/10.3390/ani15081098
Nagahama Y, Nomura M, Nishimura O. Role of Seagrass as a Food Source for Benthos in Tidal Flats: Toward Conservation and Restoration of Resilient Ecosystems. Animals. 2025; 15(8):1098. https://doi.org/10.3390/ani15081098
Chicago/Turabian StyleNagahama, Yumi, Munehiro Nomura, and Osamu Nishimura. 2025. "Role of Seagrass as a Food Source for Benthos in Tidal Flats: Toward Conservation and Restoration of Resilient Ecosystems" Animals 15, no. 8: 1098. https://doi.org/10.3390/ani15081098
APA StyleNagahama, Y., Nomura, M., & Nishimura, O. (2025). Role of Seagrass as a Food Source for Benthos in Tidal Flats: Toward Conservation and Restoration of Resilient Ecosystems. Animals, 15(8), 1098. https://doi.org/10.3390/ani15081098





