Es Colomer, a Unique Population of the Lilford’s Wall Lizard, Podarcis lilfordi (Squamata: Lacertidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
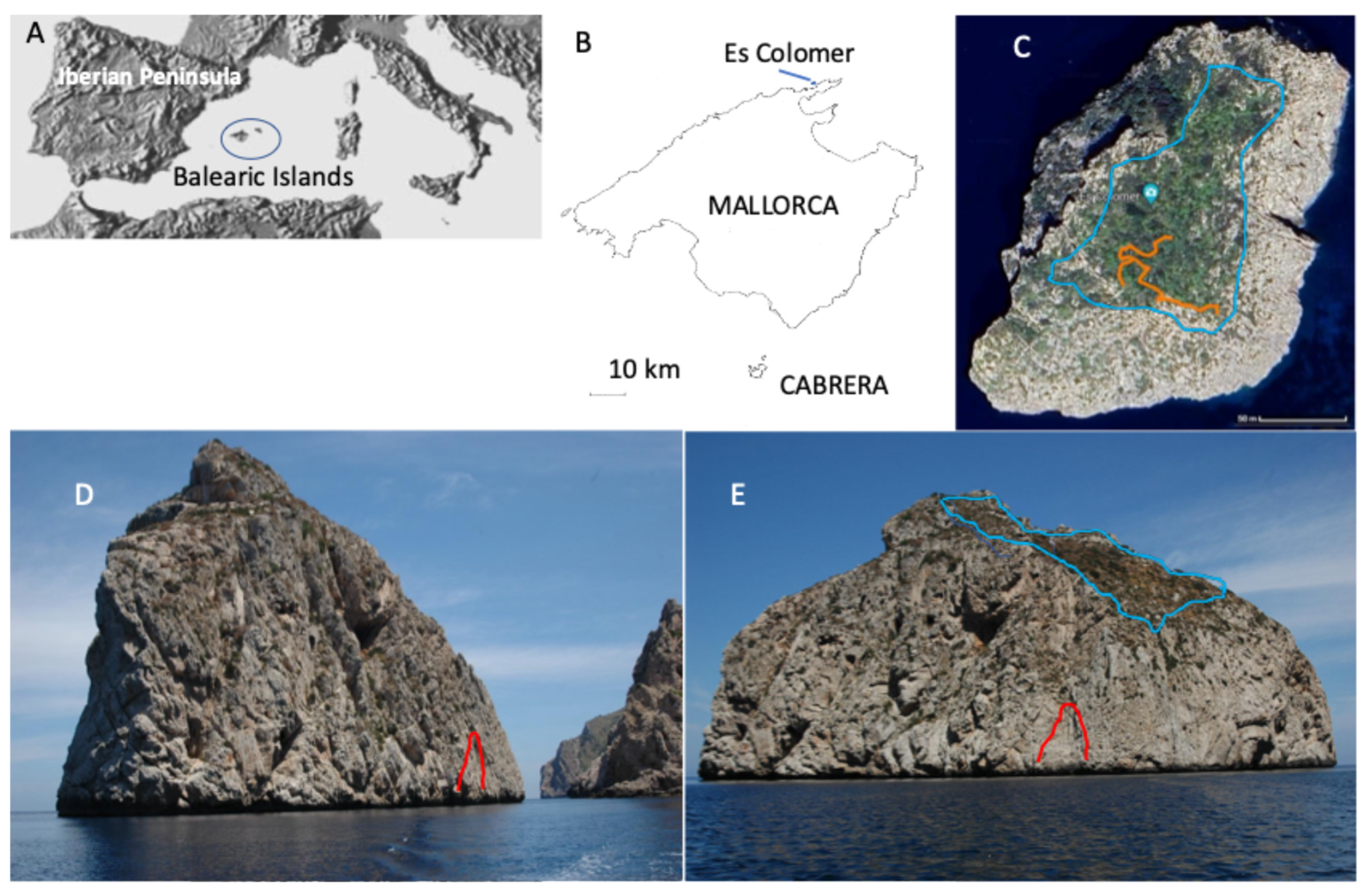
2.1. Study Area
2.2. Study Period
2.3. Lizards
2.4. Morphological Analysis, Body Condition, and Sex-Ratio
2.5. Mites and Blood Parasites
2.6. Lizard Abundance
2.7. Diet
3. Results
3.1. Morphometry, Sexual Dimorphism, and Sex-Ratio
3.2. Parasite Load
3.3. Abundance
3.4. Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESU | Evolutionarily Significant Unit |
References
- Boulenger, G.A. Monograph of the Lacertidae; British Museum (Natural History): London, UK, 1920; Volume 1, p. 352. [Google Scholar]
- Salvador, A. Una nueva subespecie melánica de lagartija balear (Lacerta lilfordi). Bol. R. Soc. Esp. Hist. Nat. (Biol.) 1979, 77, 491–492. [Google Scholar]
- Salvador, A. Podarcis lilfordi (GÜNTHER, 1874)—Balearen Eidechse. In Handbuch der Amphibien und Reptilien Europas. Echsen III (Podarcis); Böhme, W., Ed.; Aula-Verlag: Wiesbaden, Germany, 1986; pp. 83–110. [Google Scholar]
- Pérez-Mellado, V. Podarcis lilfordi (Günther, 1874). In Reptiles. Fauna Ibérica; Salvador, A., Ed.; Museo Nacional de Ciencias Naturales, CSIC: Madrid, Spain, 1998; Volume 10, pp. 272–282. [Google Scholar]
- Colom, G. Hallazgo de una colonia de Lacerta lilfordi en la costa norte de Mallorca: Islote d’Es Colomé (Formentor). Bol. Soc. Hist. Nat. Baleares 1962, 7, 61–67. [Google Scholar]
- Alomar, G. Aproximació a la flora vascular del Colomer de Formentor (Mallorca, Illes Balears). Boll. Soc. Hist. Nat. Balears 2020, 63, 35–51. [Google Scholar]
- Bibiloni, G.; Mayol, J. Illes de l’oest, nord I est de Mallorca. In Atles de les Petites illes i Els Illots de les Balears; Pim, S.H.N.B., Mayol, J., Eds.; Monografies de la Societat d’Historia Natural de les Balears, Ed. Perifèrics: Palma, Mallorca, Spain, 2020; Volume 29, pp. 83–101. [Google Scholar]
- Rayó, M. Pròleg 1. Illes, entre els somnis i l’oblit. In Atles de les Petites illes i els Illots de les Balears; Mayol, J., Ed.; Monografies de la Societat d’Historia Natural de les Balears, 29; Perifèrics: Palma, Spain, 2020; p. 10. [Google Scholar]
- Pérez-Mellado, V.; Hernández-Estévez, J.A.; García-Díez, T.; Terrassa, B.; Ramón, M.M.; Castro, J.A.; Picornell, A.; Martín-Vallejo, F.J.; Brown, R.P. Population density in Podarcis lilfordi (Squamata, Lacertidae), a lizard species endemic to small islets in the Balearic Islands (Spain). Amphibia-Reptilia 2008, 29, 49–60. [Google Scholar] [CrossRef]
- Pérez-Mellado, V. Les Sargantanes de les Balears; de Natura de les Balears, Q., Ed.; Documenta Balear: Palma, Spain, 2009; p. 96. [Google Scholar]
- Terrasa, B.; Pérez-Mellado, V.; Brown, R.P.; Picornell, A.; Castro, J.A.; Ramon, M.M. Foundations for conservation of intraspecific genetic diversity revealed by analysis of phylogeographical structure in the endangered endemic lizard, Podarcis lilfordi. Divers. Distrib. 2009, 15, 207–221. [Google Scholar] [CrossRef]
- Terrasa, B.; Rodríguez, V.; Pérez-Mellado, V.; Picornell, A.; Brown, R.P.; Castro, J.A.; Ramon, M.M. Use of NCPA to understanding genetic sub-structuring of Podarcis lilfordi from the Balearic archipelago. Amphibia-Reptilia 2009, 30, 505–514. [Google Scholar] [CrossRef]
- Brown, R.P.; Terrasa, B.; Pérez-Mellado, V.; Castro, J.A.; Hoskisson, P.A.; Picornell, A.; Ramon, M.M. Bayesian estimation of Post-Messinian Divergence Times in Balearic Island lizards. Mol. Phylogenet. Evol. 2008, 48, 350–358. [Google Scholar] [CrossRef]
- Bassitta, M.; Brown, R.P.; Buades, J.M.; Pérez-Cembranos, A.; Pérez-Mellado, V.; Picornell, A.; Ramon, C. Genomic signatures of drift and selection driven by predation and human pressure in an insular lizard. Sci. Rep. 2021, 11, 6136. [Google Scholar] [CrossRef]
- Damgaard, C. Estimating mean plant cover from different types of cover data: A coherent statistical framework. Ecosphere 2014, 5, 20. [Google Scholar] [CrossRef]
- Pérez-Mellado, V.; Corti, C. Dietary adaptations and herbivory in lacertid lizards of the genus Podarcis from western Mediterranean islands (Reptilia: Sauria). Bonn. Zool. Beitr. 1993, 44, 193–220. [Google Scholar]
- Pérez-Cembranos, A.; León, A.; Pérez-Mellado, V. Omnivory of an Insular Lizard: Sources of Variation in the Diet of Podarcis lilfordi (Squamata, Lacertidae). PLoS ONE 2016, 11, e0148947. [Google Scholar] [CrossRef]
- Pérez-Mellado, V.; Gosá, A. Biometría y Folidosis en Lacertidae (Sauria, Reptilia). Algunos aspectos metodológicos. Rev. Esp. Herpetol. 1988, 3, 97–104. [Google Scholar]
- Crawley, M.J. The R Book, 2nd ed.; Wiley: Chichester, UK, 2013; p. 1051. [Google Scholar]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alterna tive method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Pérez-Cembranos, A.; Pérez-Mellado, V.; Alemany, I.; Bassitta, M.; Terrasa, B.; Picornell, A.; Castro, J.A.; Brown, R.P.; Ramon, C. Morphological and genetic diversity of the Balearic lizard, Podarcis lilfordi (Günther. 1874). Is it relevant for its conservation? Divers. Distrib. 2020, 26, 1122–1141. [Google Scholar] [CrossRef]
- Barnard, S.M.; Upton, S.J. A Veterinary Guide to the Parasites of Reptiles; Krieger: Malabar, FL, USA, 1994; Volume 1, Protozoa, p. 154. [Google Scholar]
- Maia, J.P.M.C.; Perera, A.; Harris, D.J. Molecular survey and microscopic examination of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina) in lacertid lizards from the western Mediterranean. Folia Parasitol. 2012, 59, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Mazerolle, M.J.; Bailey, L.L.; Kendall, W.L.; Royle, J.A.; Converse, S.J.; Nichols, J.D. Making Great Leaps Forwards: Accounting fro Detectability in Herpetological Field Studies. J. Herpetol. 2007, 41, 672–689. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Barzaghi, B.; Melotto, A.; Muraro, M.; Lunghi, E.; Canedoli, C.; Parrino, E.L.; Nanni, V.; Silva-Rocha, I.; Urso, A.; et al. N-mixture models reliably estimate the abundance of small vertebrates. Sci. Rep. 2018, 8, 10357. [Google Scholar] [CrossRef]
- Link, W.A.; Schofield, M.R.; Barker, R.J.; Sauer, J.R. On the robustness of N-mixture models. Ecology 2018, 99, 1547–1551. [Google Scholar] [CrossRef]
- Fiske, I.; Chandler, R. Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 13 September 2024).
- Royle, J.A.; Dawson, D.K.; Bates, S. Modeling abundance effects in distance sampling. Ecology 2004, 85, 1591–1597. [Google Scholar] [CrossRef]
- Pérez-Mellado, V.; Pérez-Cembranos, A.; Garrido, M.; Corti, C.; Luiselli, L. Using faecal samples in lizard dietary studies. Amphibia-Reptilia 2011, 32, 1–7. [Google Scholar] [CrossRef]
- Teerink, B.J. Hair of West European Mammals: Atlas and Identification; Cambridge University Press: Cambridge, UK, 1991; p. 236. [Google Scholar]
- Oksanen, J.; Guillaume-Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B. vegan: Community Ecology Package. R package Version 2.2–1. 2015. Available online: http://CRAN.R-project.org/package=vegan (accessed on 16 September 2024).
- Pallmann, P.; Schaarschmidt, F.; Hothorn, L.A.; Fischer, C.; Nacke, H.; Priesnitz, K.U. Assessing group differences in biodiversity by simultaneously testing a user-defined selection of diversity indices. Mol. Ecol. Resour. 2012, 12, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Malden, UK, 2004; p. 200. [Google Scholar]
- Scherer, R.; Pallmann, P. simboot: Simultaneous Inference for Diversity Indices. R Package Version 0.2–5. 2014. Available online: http://CRAN.Rproject.org/package=simboot (accessed on 16 September 2024).
- Pérez-Cembranos, A.; Pérez-Mellado, V. La Lagartija Balear Podarcis lilfordi (Günther, 1874). Abundancia y Conservación; Documents Técnics de Conservació, IIª època, 14; Conselleria d’Agricultura, Pesca i Medi Natural: Palma, Spain, 2024; 238p. [Google Scholar]
- Vervust, B.; Van Dongen, S.; Grbac, I.; Van Damme, R. The mystery of the missing toes: Extreme levels of natural mutilation in island lizard populations. Funct. Ecol. 2009, 23, 996–1003. [Google Scholar] [CrossRef]
- Ferreira, A.I.; Damas-Moreira, I.; Marshall, K.L.A.; Perera, A.; Harris, D.J. What influences the prevalence and intensity of haemoparasites and ecoparasites in an insular lizard? Animals 2023, 13, 723. [Google Scholar] [CrossRef]
- Harris, D.J.; Maia, J.P.M.C.; Perera, A. Molecular survey of Apicomplexa in Podarcis wall lizards detects Hepatozoon, Sarcocystis, and Eimeria species. J. Parasitol. 2012, 98, 592–597. [Google Scholar] [CrossRef]
- Pérez-Cembranos, A.; Pérez-Mellado, V.; Terrasa, B.; Alemany, I.; Bassitta, M.; Ramon, C. La sargantana Balear: Un experiment evolutiu. In Arxipèlag de Cabrera: Història Natural; Grau, A.M., Fornós, J.J., Mateu, G., Oliver, P.A., Terrasa, B., Eds.; Monografies de la Societat d’Història Natural de les Balears: Palma, Spain, 2020; Volume 30, pp. 635–662. [Google Scholar]
- García-Ramirez, A.; Delgado-García, J.D.; Foronda-Rodríguez, P.; Abreu-Acosta, N. Haematozoans, mites and body condition in the oceanic island lizard Gallotia atlantica (Peters and Doria, 1882) (Reptilia: Lacertidae). J. Nat. Hist. 2005, 39, 1299–1305. [Google Scholar] [CrossRef]
- Roberts, M.L.; Buchanan, K.L.; Evans, M.R. Testing the inmunocompetence handicap hypothesis: A review of the evidence. Anim. Behav. 2004, 68, 227–239. [Google Scholar] [CrossRef]
- Amo, L.; López, P.; Martín, J. Prevalence and intensity of haemogregarine blood parasites and their mite vectors in the common wall lizard, Podarcis muralis. Parasitol. Res. 2005, 96, 378–381. [Google Scholar] [CrossRef]
- Drechsler, R.M.; Belliure, J.; Megía-Palma, R. Phenological and intrinsic predictors of mite and haemacoccidian infection d namics in a Mediterranean community of lizards. Parasitology 2021, 148, 1328–1338. [Google Scholar] [CrossRef]
- López-González, G.A. Los Árboles y Arbustos de la Península Ibérica y e Islas Baleares. (Especies silvestres y las Principales cultivadas); Ediciones Mundi-Prensa: Madrid, Spain, 2001; Volume 1, p. 861. [Google Scholar]
- Neghme, C.; Santamaría, L.; Calviño-Cancela, M. Strong dependence of a pioneer shrub on seed dispersal services provided by an endemic endangered lizard in a Mediterranean island ecosystem. PLoS ONE 2017, 12, e0183072. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, J.; Larrinaga, A.R.; Santamaría, L. Effects of Frugivore Preferences and Habitat Heterogeneity on Seed Rain: A Multi-Scale Analysis. PLoS ONE 2012, 7, e33246. [Google Scholar] [CrossRef] [PubMed]
- Salvi, D. Climbing on the La Canna Volcanic Sea Stack to Obtain First-Hand Data on the Tiniest Population of the Critically Endangered Aeolian Wall Lizard Podarcis raffonei. Animals 2023, 13, 2289. [Google Scholar] [CrossRef] [PubMed]




| Trait | Males Mean ± SE (n) | Females Mean ± SE (n) | F-Value | d.f. | p-Value | F-Value of Interaction | p-Value of Interaction |
|---|---|---|---|---|---|---|---|
| SVL | 74.32 ± 0.53 (48) | 66.86 ± 0.89 (28) | 59.85 | 1.74 | 4.08 × 10−11 | ||
| Tail | 130.75 ± 2.18 (8) | 116.21 ± 4.63 (7) | 10.76 | 3.11 | 0.007 | 1.67 | 0.22 |
| Weigth | 11.62 ± 0.31 (23) | 6.84 ± 0.42 (21) | 141.58 | 3.39 | 1.49 × 10−14 | 1.44 | 0.24 |
| PL | 18.04 ± 0.14 (46) | 15.31 ± 0.19 (21) | 180.30 | 3. 62 | 2.2 × 10−16 | 1.16 | 0.28 |
| HH | 8.74 ± 0.11 (47) | 7.06 ± 0.1 (21) | 92.10 | 3.63 | 6.09 × 10−14 | 0.09 | 0.77 |
| HW | 8.3 ± 0.07 (46) | 7.29 ± 0.25 (21) | 39.86 | 3.62 | 3.26 × 10−8 | 0.34 | 0.56 |
| HLL | 39.43 ± 0.28 (42) | 34.47 ± 0.5 (20) | 89.36 | 3.57 | 2.83 × 10−13 | 0.85 | 0.36 |
| LAM | 30.88 ± 0.42 (34) | 30.67 ± 0.4 (12) | 0.04 | 3.42 | 0.84 | 0.20 | 0.66 |
| FEM | 22.92 ± 0.4 (38) | 22.36 ± 0.75 (11) | 0.37 | 3.44 | 0.55 | 0.27 | 0.60 |
| GUL | 35.72 ± 0.77 (25) | 32.83 ± 1.25 (6) | 2.83 | 3.27 | 0.10 | 0.57 | 0.45 |
| DOR | 93.23 ± 1.62 (26) | 84.83 ± 1.4 (6) | 7.51 | 3.27 | 0.01 | 0.0001 | 0.99 |
| VENT | 23.96 ± 0.23 (26) | 26 ± 0.26 (6) | 14.93 | 3.27 | 0.0006 | 0.46 | 0.50 |
| COLL | 11.68 ± 0.23 (25) | 10.67 ± 0.61 (6) | 3.95 | 3.26 | 0.057 | 5.46 | 0.02 |
| Date | N | l (m) | Density (log) ± SE | z | p | AIC | Density (ind./ha) ± SE | g(x) |
|---|---|---|---|---|---|---|---|---|
| 2008 | 54 | 140 | 8.3 ± 0.169 | 49.2 | 0 | 130.06 | 4007 ± 675 | 0.5129 |
| 2022 | 19 | 79.3 | 7.45 ± 0.288 | 25.9 | 1.11 × 10−147 | 24.07 | 1723 ± 496 | 0.3476 |
| 2024 | 113 | 108.5 | 8.56 ± 0.12 | 71.4 | 0 | 225.31 | 5195 ± 623 | 0.5012 |
| Taxa | Overall Diet | Spring Diet | Summer Diet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Presence | Frequency | Presence | Frequency | Presence | |||||||
| n | n% | np | %p | n | n% | np | %p | n | n% | np | %p | |
| Gastropoda | 26 | 3.7735 | 26 | 12.50 | 7 | 2.7777 | 7 | 6.1946 | 19 | 4.3478 | 19 | 20.000 |
| Pseudoscorpionida | 2 | 0.2902 | 2 | 0.9615 | 1 | 0.3968 | 1 | 0.8849 | 1 | 0.2288 | 1 | 1.0526 |
| Araneae | 4 | 0.5805 | 4 | 1.9230 | 3 | 1.1904 | 3 | 2.6548 | 1 | 0.2288 | 1 | 1.0526 |
| Acari | 1 | 0.1451 | 1 | 0.4807 | 0 | 0 | 0 | 0 | 1 | 0.2288 | 1 | 1.0526 |
| Isopoda | 43 | 6.2409 | 42 | 20.1923 | 30 | 11.9047 | 29 | 25.6637 | 13 | 2.9748 | 13 | 13.6842 |
| Dictyoptera | 1 | 0.1451 | 1 | 0.4807 | 1 | 0.3968 | 1 | 0.8849 | 0 | 0 | 0 | 0 |
| Isoptera | 5 | 0.7256 | 5 | 2.4038 | 1 | 0.3968 | 1 | 0.8849 | 4 | 0.9153 | 4 | 4.2105 |
| Homoptera | 6 | 0.8708 | 6 | 2.8846 | 4 | 1.5873 | 4 | 3.5398 | 2 | 0.4576 | 2 | 2.1052 |
| Heteroptera | 9 | 1.3062 | 9 | 4.3269 | 7 | 2.7777 | 7 | 6.1946 | 2 | 0.4576 | 2 | 2.1052 |
| Diptera | 3 | 0.4354 | 3 | 1.4423 | 3 | 1.1904 | 3 | 2.6548 | 0 | 0 | 0 | 0 |
| Lepidoptera | 8 | 1.1611 | 7 | 3.3653 | 8 | 3.1746 | 7 | 6.1946 | 0 | 0 | 0 | 0 |
| Coleoptera | 68 | 9.8693 | 53 | 25.4807 | 35 | 13.8888 | 29 | 25.6637 | 33 | 7.5514 | 24 | 25.2631 |
| Hymenoptera | 12 | 1.7416 | 10 | 4.8076 | 8 | 3.1746 | 6 | 5.3097 | 4 | 0.9153 | 4 | 4.2105 |
| Formicidae | 391 | 56.7489 | 125 | 60.0961 | 126 | 50.0000 | 65 | 57.5221 | 265 | 60.6407 | 60 | 63.1578 |
| Arthropoda undet. | 9 | 1.3062 | 9 | 4.3269 | 8 | 3.1746 | 8 | 7.0796 | 1 | 0.2288 | 1 | 1.0526 |
| Larvae | 13 | 1.8867 | 13 | 6.2500 | 6 | 2.3809 | 6 | 5.3097 | 7 | 1.6018 | 7 | 7.3684 |
| Birds | 1 | 0.1451 | 1 | 0.4807 | 1 | 0.3968 | 1 | 0.8849 | 0 | 0 | 0 | 0 |
| Lizards | 2 | 0.2902 | 2 | 0.9615 | 2 | 0.7936 | 2 | 1.7699 | 0 | 0 | 0 | 0 |
| Mammals | 1 | 0.1451 | 1 | 0.4807 | 1 | 0.3968 | 1 | 0.8849 | 0 | 0 | 0 | 0 |
| Seeds | 84 | 12.1915 | 38 | 18.2692 | 0 | 0 | 0 | 0 | 84 | 19.2219 | 38 | 40.00 |
| Total | 689 | 100 | 252 | 100 | 437 | 100 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cembranos, A.; Pérez-Mellado, V. Es Colomer, a Unique Population of the Lilford’s Wall Lizard, Podarcis lilfordi (Squamata: Lacertidae). Animals 2025, 15, 1093. https://doi.org/10.3390/ani15081093
Pérez-Cembranos A, Pérez-Mellado V. Es Colomer, a Unique Population of the Lilford’s Wall Lizard, Podarcis lilfordi (Squamata: Lacertidae). Animals. 2025; 15(8):1093. https://doi.org/10.3390/ani15081093
Chicago/Turabian StylePérez-Cembranos, Ana, and Valentín Pérez-Mellado. 2025. "Es Colomer, a Unique Population of the Lilford’s Wall Lizard, Podarcis lilfordi (Squamata: Lacertidae)" Animals 15, no. 8: 1093. https://doi.org/10.3390/ani15081093
APA StylePérez-Cembranos, A., & Pérez-Mellado, V. (2025). Es Colomer, a Unique Population of the Lilford’s Wall Lizard, Podarcis lilfordi (Squamata: Lacertidae). Animals, 15(8), 1093. https://doi.org/10.3390/ani15081093






