Identifying and Mapping Ticks on Wild Boars from Romania
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
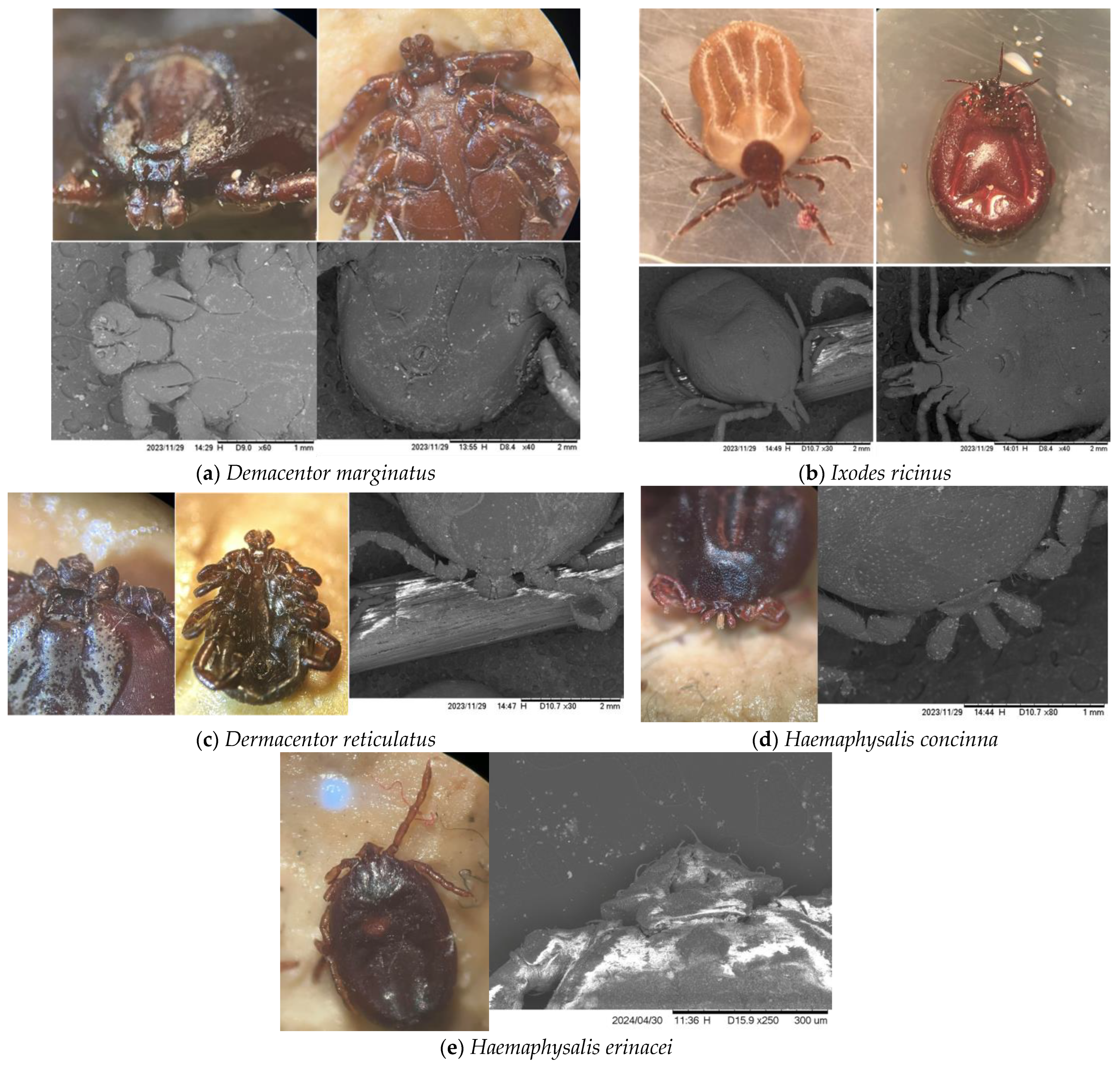
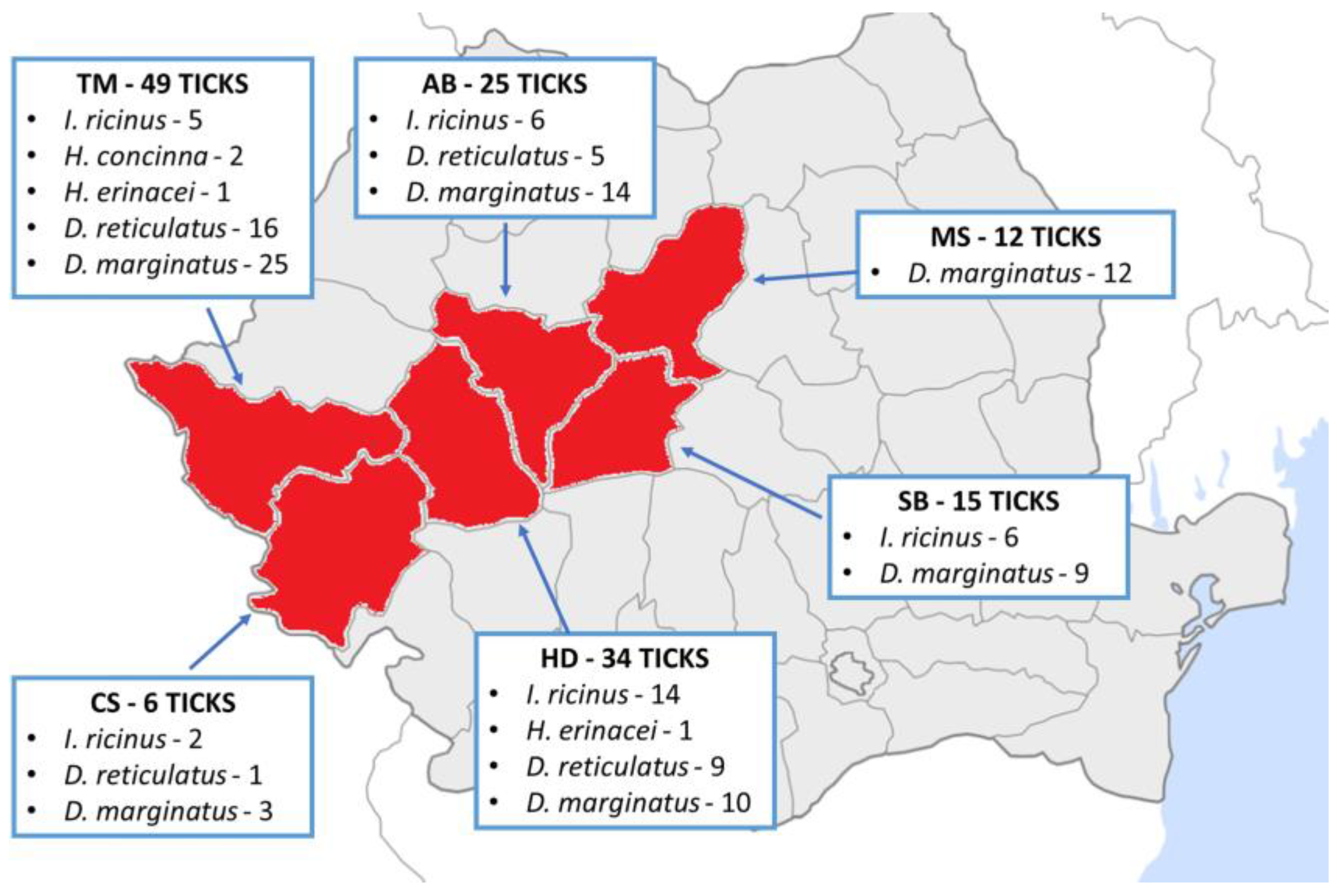
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Colwell, D.D.; Dantas-Torres, F.; Otranto, D. Vector-borne parasitic zoonoses: Emerging scenarios and new perspectives. Vet. Parasitol. 2011, 182, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- James, M.C.; Bowman, A.S.; Forbes, K.J.; Lewis, F.; McLeod, J.E.; Gilbert, L. Environmental determinants of Ixodes ricinus ticks and the incidence of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Parasitology 2013, 140, 237–246. [Google Scholar] [CrossRef]
- Randolph, S.E. Tick ecology: Processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 2004, 129, S37–S65. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; Fernández-de-Mera, I.G.; Acevedo, P.; Gortázar, C.; de la Fuente, J. Factors driving the abundance of Ixodes ricinus ticks and the prevalence of zoonotic I. ricinus-borne pathogens in natural foci. Appl. Environ. Microbiol. 2012, 78, 2669–2676. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivi. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Varela-Castro, L.; Zuddas, C.; Ortega, N.; Serrano, E.; Salinas, J.; Castellà, J.; Castillo-Contreras, R.; Carvalho, J.; Lavín, S.; Mentaberre, G. On the possible role of ticks in the eco-epidemiology of Coxiella burnetii in a Mediterranean ecosystem. Ticks Tick Borne Dis. 2018, 9, 687–694. [Google Scholar] [CrossRef]
- Bradley, C.A.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Fernández-Aguilar, X.; Gottschalk, M.; Aragon, V.; Càmara, J.; Ardanuy, C.; Velarde, R.; Galofré-Milà, N.; Castillo-Contreras, R.; López-Olvera, J.R.; Mentaberre, G.; et al. Urban wild boars and risk for zoonotic Streptococcus suis, Spain. Emerg. Infect. Dis. 2018, 24, 1083–1086. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Range expansion of tick disease vectors in north america: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; Fernández-de-Mera, I.G.; Acevedo, P.; Höfle, U.; Vicente, J.; De la Fuente, J.; Gortázar, C. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: Geographical and temporal distribution. Vet. Parasitol. 2006, 140, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kmieciak, W.; Ciszewski, M.; Szewczyk, E.M. Tick-borne diseases in Poland: Prevalence and difficulties in diagnostics. Med. Pr. 2016, 67, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Organizarea Administrativ-Teritorială A României. Available online: https://ro.wikipedia.org/wiki/Organizarea_administrativ-teritorial%C4%83_a_Rom%C3%A2niei#/media/Fi%C8%99ier:Romania_counties_blank_big.png (accessed on 7 March 2025).
- Estrada-Peña, A.; Mihalca, A.; Petney, T. Ticks of Europe and North Africa: A Guide to Species Identification. Med. Vet. Entomol. 2017, 10, 404. [Google Scholar]
- Chiţimia, L. Căpușe Ixodidae; Editura Mirton: Timișoara, Romania, 2007; pp. 44–51. [Google Scholar]
- Horak, I.G.; Jordaan, A.J.; Nel, P.J.; Van Heerden, J.; Heyne, H.; Van Dalen, E.M. Distribution of endemic and introduced tick species in Free State Province, South Africa. J. S. Afr. Vet. Assoc. 2015, 86, 1255. [Google Scholar] [CrossRef]
- Guardone, L.; Nogarol, C.; Accorsi, A.; Vitale, N.; Listorti, V.; Scala, S.; Brusadore, S.; Miceli, I.N.; Wolfsgruber, L.; Guercio, A.; et al. Ticks and Tick-Borne Pathogens: Occurrence and Host Associations over Four Years of Wildlife Surveillance in the Liguria Region (Northwest Italy). Animals 2024, 14, 2377. [Google Scholar] [CrossRef]
- Sevestre, J.; Diarra, A.Z.; Oumarou, H.A.; Durant, J.; Delaunay, P.; Parola, P. Detection of emerging tick-borne disease agents in the Alpes-Maritimes region, southeastern France. Ticks Tick-Borne Dis. 2021, 12, 101800. [Google Scholar] [CrossRef]
- Mysterud, A.; Hügli, C.; Viljugrein, H. Tick infestation on medium–large-sized mammalian hosts: Are all equally suitable to Ixodes ricinus adults? Parasit Vectors 2021, 14, 254. [Google Scholar] [CrossRef]
- Perez, G.; Bournez, L.; Boulanger, N.; Fite, J.; Livoreil, B.; McCoy, K.D.; Quillery, E.; René-Martellet, M.; Bonnet, S.I. The distribution, phenology, host range and pathogen prevalence of Ixodes ricinus in France: A systematic map and narrative review. Peer Community J. 2023, 3, e81. [Google Scholar] [CrossRef]
- Bertola, M.; Montarsi, F.; Obber, F.; Da Rold, G.; Carlin, S.; Toniolo, F.; Porcellato, E.; Falcaro, C.; Mondardini, V.; Ormelli, S. Occurrence and Identification of Ixodes ricinus Borne Pathogens in Northeastern Italy. Pathogens 2021, 10, 1181. [Google Scholar] [CrossRef]
- Accorsi, A.; Schiavetti, I.; Listorti, V.; Dellepiane, M.; Masotti, C.; Ercolini, C.; Guardone, L.; Razzuoli, E. Hard Ticks (Ixodidae) from Wildlife in Liguria, Northwest Italy: Tick Species Diversity and Tick-Host Associations. Insects 2022, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Selmi, M.; Ballardini, M.; Salvato, L.; Ricci, E. Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host-vector-pathogen interactions in a northern Mediterranean area. Exp. Appl. Acarol. 2017, 72, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, G.; Iatta, R.; Lia, R.P.; D’Alessio, N.; Manoj RR, S.; Veneziano, V.; Otranto, D. Spotted fever group rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound. Emerg. Dis. 2021, 68, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Belova, O.A.; Kholodilov, I.S.; Didyk, Y.M.; Kurzrock, L.; García-Pérez, A.L.; Kahl, O. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 2020, 82, 95–123. [Google Scholar] [CrossRef]
- Defaye, B.; Moutailler, S.; Pasqualini, V.; Quilichini, Y. A Systematic Review of the Distribution of Tick-Borne Pathogens in Wild Animals and Their Ticks in the Mediterranean Rim between 2000 and 2021. Microorganisms 2022, 10, 1858. [Google Scholar] [CrossRef]
- Hrazdilová, K.; Lesiczka, P.M.; Bardoň, J.; Vyroubalová, Š.; Šimek, B.; Zurek, L.; Modrý, D. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef]
- Hornok, S.; Szekeres, S.; Horváth, G.; Takács, N.; Bekő, K.; Kontschán, J.; Gyuranecz, M.; Tóth, B.; Sándor, A.D.; Juhász, A.; et al. Diversity of tick species and associated pathogens on peri-urban wild boars—First report of the zoonotic Babesia cf. crassa from Hungary. Ticks Tick Borne Dis. 2022, 13, 101936. [Google Scholar] [CrossRef]
- Matei, I.A.; Kalmár, Z.; Balea, A.; Mihaiu, M.; Sándor, A.D.; Cocian, A.; Crăciun, S.; Bouari, C.; Briciu, V.T.; Fiț, N. The Role of Wild Boars in the Circulation of Tick-Borne Pathogens: The First Evidence of Rickettsia monacensis Presence. Animals 2023, 13, 1743. [Google Scholar] [CrossRef]
- Sousa, A.C.P.; Suzin, A.; da Silva Rodrigues, V.; Rezende, L.M.; da Costa Maia, R.; Vieira, R.B.K.; Szabó, M.P.J. Ticks (Acari: Ixodidae) and rickettsiae associated with wild boars in a rural area of Minas Gerais, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2024, 50, 101016. [Google Scholar] [CrossRef]
- Paddock, C.D.; Lane, R.S.; Staples, J.E. Changing paradigms for tick-borne diseases in the Americas. In Forum on Microbial Threats; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Global Health Impacts of Vector-Borne Diseases: Workshop Summary; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Sonenshine, D.E.; Kocan, K.M.; de la Fuente, D. Tick control: Further thought on a research agenda. Trends Parasitol. 2006, 22, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Rodo, A. Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasites Vectors 2021, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Kiewra, D.; Czulowska, A. Evidence for an increased distribution range of Dermacentor reticulatus in south-west Poland. Exp. Appl. Acarol. 2013, 59, 501–506. [Google Scholar] [CrossRef][Green Version]
- Masala, G.; Chisu, V.; Satta, G.; Socolovschi, C.; Raoult, D.; Parola, P. Rickettsia slovaca from Dermacentor marginatus ticks in Sardinia, Italy. Ticks Tick Borne Dis. 2012, 3, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Lia, R.P.; Dantas-Torres, F.; Mallia, E.; Ravagnan, S.; Capelli, G.; Otranto, D. Ixodid ticks of road-killed wildlife species in southern Italy: New tick-host associations and locality records. Exp. Appl. Acarol. 2011, 55, 293–300. [Google Scholar] [CrossRef]
- Bonnet, S.; de la Fuente, J.; Nicollet, P.; Liu, X.; Madani, N.; Blanchard, B.; Joncour, G. Prevalence of Tick-Borne Pathogens in Adult Dermacentor spp. Ticks from Nine Collection Sites in France. Vector Borne Zoonotic Dis. 2013, 13, 226–236. [Google Scholar] [CrossRef]
- Leschnik, M.W.; Khanakah, G.; Duscher, G.; Wille-Piazzai, W.; Hörweg, C.; Joachim, A.; Stanek, G. Species, developmental stage and infection with microbial pathogens of engorged ticks removed from dogs and questing ticks. Med. Vet. Entomol. 2012, 26, 440–446. [Google Scholar] [CrossRef]
- Duscher, G.G.; Feiler, A.; Leschnik, M.; Joachim, A. Seasonal and spatial distribution of ixodid tick species feeding on naturally infested dogs from Eastern Austria and the influence of acaricides/repellents on these parameters. Parasit Vectors 2013, 6, 76. [Google Scholar] [CrossRef]
- Sixl, W. Occurrence of Dermacentor marginatus and Dermacentor reticulatus in Austria (Arachnida, Acari, Ixodidae). Mitt. Abt. Zool. Landesmus. Joanneum 1975, 4, 7–10. [Google Scholar]
- Thaler, K. Fragmenta Faunistica Tirolensia—XV (Arachnida: Araneae, Acari [Ixodida]; Diplopoda; Insecta: Archaeognatha, Zygentoma, Blattariae). Ber. Nat.-Med. Verein Innsbruck. 2003, 90, 151–163. [Google Scholar]
- Reye, A.L.; Stegniy, V.; Mishaeva, N.P.; Velhin, S.; Hübschen, J.M.; Ignatyev, G.; Muller, C.P. Prevalence of tick-borne pathogens in Ixodes ricinus and Dermacentor reticulatus ticks from different geographical locations in Belarus. PLoS ONE 2013, 8, e54476. [Google Scholar] [CrossRef] [PubMed]
- Obsomer, V.; Wirtgen, M.; Linden, A.; Claerebout, E.; Heyman, P.; Heylen, D.; Madder, M.; Maris, J.; Lebrun, M.; Tack, W.; et al. Spatial disaggregation of tick occurrence and ecology at a local scale as a preliminary step for spatial surveillance of tick-borne diseases: General framework and health implications in Belgium. Parasites Vectors 2013, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Arnaudov, D.Y.; Arnaudov, A.D.; Kirin, D.A.; Gospodinova, S.G. Ixodidae ticks of small ruminants in the region of Parvomai, Southern Bulgaria. Bulg. J. Agric. Sci. 2014, 20, 590–594. [Google Scholar]
- Kanchev, K.; Kamenov, Y.; Atanassova, I.; Davidova, R.; Tomov, R. Parasitic alien terrestrial arthropods on small mammals in northeast and south Bulgaria. Bulg. J. Agric. Sci. 2012, 18, 965–970. [Google Scholar]
- Krčmar, S.; Vereš, M.; Trilar, T. Fauna of hard ticks (Acari: Ixodidae) in different habitats in Croatian part of Baranja. Šumarski List. 2014, 5–6, 309–314. [Google Scholar]
- Hubálek, Z.; Halouzka, J.; Juricova, Z. Host-seeking activity of ixodid ticks in relation to weather variables. J. Vector Ecol. 2003, 28, 159–165. [Google Scholar]
- Siroky, P.; Kubelová, M.; Bednár, M.; Modry, D.; Hubálek, Z.; Tkadlec, E. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding. Vet. Parasitol. 2011, 183, 130–135. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Monazahian, M.; Habedank, B.; Dautel, H.; Leverenz, S.; Kahl, O. The first German map of georeferenced ixodid tick locations. Parasites Vectors 2014, 7, 477. [Google Scholar] [CrossRef]
- Dautel, H.; Dippel, C.; Oehme, R.; Hartelt, K.; Schettler, E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int. J. Med. Microbiol. 2006, 296 (Suppl. 40), 149–156. [Google Scholar] [CrossRef]
- Petney, T.; Pfäffle, M.; Littwin, N.; Norra, S.; Böhnke, D.; Hogewind, F.; Gebhardt, R.; Oehme, R.; Sebastian, P.; Steidle, J.; et al. Untersuchung der Ökologie von Zecken als Überträger von Krankheitserregern in Baden-Württemberg in Bezug auf Habitat, Landnutzung, Wirtstiere und Klima; BWPLUS Project Report; Karlsruhe Institute of Technology: Karlsruhe, Germany, 2013; Volume 13. [Google Scholar]
- Pluta, S.; Hartelt, K.; Oehme, R.; Mackenstedt, U.; Kimmig, P. Prevalence of Coxiella burnetii and Rickettsia spp. in ticks and rodents in southern Germany. Ticks Tick Borne Dis. 2010, 1, 145–147. [Google Scholar] [CrossRef]
- Tijsse-Klasen, E.; Hansford, K.M.; Jahfari, S.; Phipps, S.; Sprong, H.; Medlock, J.M. Spotted fever group rickettsiae in Dermacentor reticulatus and Haemaphysalis punctata ticks in the UK. Parasites Vectors 2013, 6, 212. [Google Scholar] [CrossRef]
- Papadopoulos, B.; Morel, P.C.; Aeschlimann, A. Ticks of domestic animals in the Macedonia region of Greece. Vet. Parasitol. 1996, 63, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Farkas, R. Ixodid tick species attaching to dogs in Hungary. Vet. Parasitol. 2005, 129, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hornok, S.; Farkas, R. Influence of biotope on the distribution and peak activity of questing ixodid ticks in Hungary. Med. Vet. Entomol. 2009, 23, 41–46. [Google Scholar] [CrossRef]
- Pintér, R.; Madai, M.; Vadkerti, E.; Németh, V.; Oldal, M.; Kemenesi, G.; Dallos, B.; Gyuranecz, M.; Kiss, G.; Bányai, K.; et al. Identification of tick-borne encephalitis virus in ticks collected in southeastern Hungary. Ticks Tick Borne Dis. 2013, 4, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Otranto, D. Species diversity and abundance of ticks in three habitats in southern Italy. Ticks Tick Borne Dis. 2013, 4, 251–255. [Google Scholar] [CrossRef]
- Martello, E.; Selmi, M.; Ragagli, C.; Ambrogi, C.; Stella, M.C.; Mannelli, A.; Tomassone, L. Rickettsia slovaca in immature Dermacentor marginatus and tissues from Apodemus spp. in the northern Apennines, Italy. Ticks Tick Borne Dis. 2013, 4, 518–521. [Google Scholar] [CrossRef]
- Ceballos, A.L.; Pintore, M.D.; Tomassone, L.; Pautasso, A.; Bisanzio, D.; Mignone, W.; Casalone, C.; Mannelli, A. Habitat and occurrence of ixodid ticks in the Liguria region, northwest Italy. Exp. Appl. Acarol. 2014, 64, 121–135. [Google Scholar] [CrossRef]
- Paulauskas, A.; Radzijevskaja, J.; Mardosaite-Busaitiene, D.; Aleksandravičienė, A.; Galdikas, M.; Krikstolaitis, R. New localities of Dermacentor reticulatus ticks in the Baltic countries. Ticks Tick Borne Dis. 2015, 6, 630–635. [Google Scholar] [CrossRef]
- Paulauskas, A.; Radzijevskaja, J.; Turčinavičienė, J.; Ambrasienė, D.; Galdikaitė, E. Data on some ixodid tick species (Acari, Ixodidae) in the Baltic countries. New Rare Lith. Insect Species 2010, 22, 43–51. [Google Scholar]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector-Borne Zoon. Dis. 2007, 7, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, F.; Ringenier, M.; Putting, M.; Berger, L.; Burgers, S.; Kortekaas, R.; Lenssen, J.; van Roessel, M.; Wijnveld, M.; Madder, M. Novel foci of Dermacentor reticulatus ticks infected with Babesia canis and Babesia caballi in the Netherlands and in Belgium. Parasites Vectors 2015, 8, 232. [Google Scholar] [CrossRef]
- Stanczak, J. Detection of spotted fever group (SFG) rickettsiae in Dermacentor reticulatus (Acari: Ixodidae) in Poland. Int. J. Med. Microbiol. 2006, 296 (Suppl. 1), 144–148. [Google Scholar] [CrossRef] [PubMed]
- Biernat, B.; Karbowiak, G.; Werszko, J.; Stanczak, J. Prevalence of tick-borne encephalitis virus (TBEV) RNA in Dermacentor reticulatus ticks from natural and urban environment, Poland. Exp. Appl. Acarol. 2014, 64, 543–551. [Google Scholar] [CrossRef]
- Nowak, M. Discovery of Dermacentor reticulatus (Acari, Amblyommidae) populations in the Lubuskie Province (Western Poland). Exp. Appl. Acarol. 2011, 54, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, E.J.; Estrada-Peña, A.; Alsarraf, M.; Kowalec, M.; Bajer, A. Mapping of Dermacentor reticulatus expansion in Poland in 2012-2014. Ticks Tick Borne Dis. 2016, 7, 94–106. [Google Scholar] [CrossRef]
- Wójcik-Fatla, A.; Cisak, E.; Zajac, V.; Sawczyn, A.; Dutkiewicz, J. Study on tick-borne rickettsiae in eastern Poland. I. Prevalence in Dermacentor reticulatus (Acari: Amblyommidae). Ann. Agric. Environ. Med. 2013, 20, 276–279. [Google Scholar]
- Biaduń, W. New habitat of Dermacentor reticulatus (Fabricius, 1794) in the Lublin region. Polish J. Environ. Stud. 2011, 20, 263–266. [Google Scholar]
- Kadulski, S.; Izdebska, J.N. New data on distribution of Dermacentor reticulatus (Fabr.) (Acari, Ixodidae) in Poland. In Inwazje i ich Ograniczanie; Stawonogi; Buczek, A., Blaszak, C., Eds.; Akapit: Lublin, Poland, 2009; pp. 53–58. [Google Scholar]
- Norte, A.C.; Carvalho, I.L.; Ramos, J.A.; Goncalves, M.; Gern, L.; Núncio, M.S. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp. Appl. Acarol. 2012, 58, 327–339. [Google Scholar] [CrossRef]
- Santos-Silvia, M.; Sousa, R.; Santos, A.S.; Lopes, D.; Queijo, E.; Doreta, A.; Vitorino, L.; Bacellar, F. Ticks and tick-borne rickettsiae surveillance in Montesinho Natural Park, Portugal. Ann. N. Y. Acad. Sci. 2006, 1078, 137–142. [Google Scholar] [CrossRef]
- Mihalca, A.D.; Dumitrache, M.O.; Magdas, C.; Gherman, C.M.; Domsa, C.; Mircean, V.; Ghira, I.V.; Pocora, V.; Ionescu, D.T.; Barabási, S.S.; et al. Synopsis of the hard ticks (Acari, Ixodidae) of Romania with update on host associations and geographical distribution. Exp. Appl. Acarol. 2012, 58, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Chitimia-Dobler, L. Spatial distribution of Dermacentor reticulatus in Romania. Vet. Parasitol. 2015, 214, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Mihaljica, D.; Radulović, Z.; Tomanović, S.; Cakić, S.; Penezić, A.; Milutinović, M. Molecular detection of Babesia spp. in ticks in northern Serbia. Arch. Biol. Sci. 2012, 64, 1591–1598. [Google Scholar] [CrossRef]
- Nosek, J. The ecology and public health importance of Dermacentor marginatus and D. reticulatus ticks in Central Europe. Folia Parasitol. 1972, 19, 93–102. [Google Scholar]
- Kubelová, M.; Tkadlec, E.; Bednár, M.; Roubalová, E.; Siroky, P. West-to-east differences of Babesia canis canis prevalence in Dermacentor reticulatus ticks in Slovakia. Vet. Parasitol. 2011, 180, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Bullova, E.; Lukán, M.; Stanko, M.; Pet’ko, B. Spatial distribution of Dermacentor reticulatus tick in Slovakia in the beginning of the 21st century. Vet. Parasitol. 2009, 165, 357–360. [Google Scholar] [CrossRef]
- Immler, R.; Aeschlimann, A.; Büttiker, W.; Diehl, P.A.; Eichenberger, G.; Weiss, N. Über das Vorkommen von Dermacentor-Zecken (Ixodoidea) in der Schweiz. Bull. Soc. Entomol. Suisse. 1970, 43, 99–110. [Google Scholar]
- Schaarschmidt, D.; Gilli, U.; Gottstein, B.; Marreros, N.; Kuhnert, P.; Daeppen, J.A.; Rosenberg, G.; Hirt, D.; Frey, C.F. Questing Dermacentor reticulatus harbouring Babesia canis DNA associated with outbreaks of canine babesiosis in the Swiss Midlands. Ticks Tick Borne Dis. 2013, 4, 334–340. [Google Scholar] [CrossRef][Green Version]
- Porchet, M.J.; Sager, H.; Muggli, L.; Oppliger, A.; Müller, N.; Frey, C.; Gottstein, B. A descriptive epidemiological study on canine babesiosis in the Lake Geneva region (in French). Schweiz. Arch. Tierheilk. 2007, 149, 457–465. [Google Scholar] [CrossRef]
- Hekimoglu, O.; Ozer, N.; Ergunay, K.; Ozkul, A. Species distribution and detection of Crimean Congo Hemorrhagic Fever Virus (CCHFV) in field-collected ticks in Ankara Province, Central Anatolia, Turkey. Exp. Appl. Acarol. 2011, 56, 75–84. [Google Scholar] [CrossRef]
- Bakirci, S.; Sarali, H.; Aydin, L.; Eren, H.; Karagenc, T. Distribution and seasonal activity of tick species on cattle in the West Aegean region of Turkey. Exp. Appl. Acarol. 2011, 56, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Yesilbag, K.; Aydin, L.; Dincer, E.; Alpay, G.; Girisgin, A.O.; Tuncer, P.; Ozkul, A. Tick survey and detection of Crimean-Congo hemorrhagic fever virus in tick species from a non-endemic area, South Marmara region, Turkey. Exp. Appl. Acarol. 2013, 60, 253–261. [Google Scholar] [CrossRef]
- Aydin, M.F.; Aktas, M.; Dumanli, N. Tick infestations on sheep and goats in the Black Sea region of Trkiye. Kafkas Univ. Vet. Fak. Derg. 2012, 18, A17–A22. [Google Scholar]
- Akimov, I.A.; Nebogatkin, I.V. Distribution of the ticks of the genus Dermacentor (Acari, Ixodidae) in Ukraine. Vestnik Zoologii. 2011, 45, e1–e6. [Google Scholar] [CrossRef]
- Karbowiak, G.; Vichová, B.; Slivinska, K.; Werszko, J.; Didyk, J.; Pet’ko, B.; Stanko, M.; Akimov, I. The infection of questing Dermacentor reticulatus ticks with Babesia canis and Anaplasma phagocytophilum in the Chernobyl exclusion zone. Vet. Parasitol. 2014, 204, 372–375. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Pfefferb, M.; Chitimia-Dobler, L.; Didykd, Y.M.; Leverenze, S.; Dautele, H.; Kahle, O. Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks Tick Borne Dis. 2016, 7, 224–233. [Google Scholar] [CrossRef]
- Tsatsaris, A.; Chochlakis, D.; Papadopoulos, B.; Petsa, A.; Georgalis, L.; Angelakis, E.; Ioannou, I.; Tselentis, Y.; Psaroulaki, A. Species composition, distribution, ecological preference and host association of ticks in Cyprus. Exp. Appl. Acarol. 2016, 70, 523–542. [Google Scholar] [CrossRef]
- Brugger, K.; Rubel, F. Tick maps on the virtual globe: First results using the example of Dermacentor reticulatus. Ticks Tick-borne Dis. 2023, 14, 102102. [Google Scholar] [CrossRef]


| County | Hunting Grounds Total/Positive/% | Tails Sampled | % Ticks Found on Tails | ||
|---|---|---|---|---|---|
| Total | With Ticks | ||||
| No. (%) | Average No. Ticks/Tail | ||||
| Timiș | 18 (7) 38.89% | 92 | 25 (27.174) | 1.96 | 34.75% (49/141) |
| Hunedoara | 20 (9) 45% | 57 | 13 (22.807) | 2.62 | 24.11% (34/141) |
| Alba | 9 (6) 66.67% | 32 | 11 (34.375) | 2.27 | 17.73% (25/141) |
| Mureș | 2 (2) 100% | 16 | 4 (25) | 3 | 8.51% (12/141) |
| Caraș-Severin | 10 (3) 30% | 16 | 3 (18.750) | 2 | 4.26% (6/141) |
| Sibiu | 21 (6) 28.57% | 56 | 7 (12.5) | 2.14 | 10.64% (15/141) |
| Total | 80 (33) 41.25% | 270 | 63 (23.333) | 2.24 | 141 |
| Epidemiological Factors | No. of Ticks | (%) |
|---|---|---|
| Sex | ||
| Male | 37 | 26% |
| Female | 104 | 74% |
| Stage of development | ||
| Adult | 141/141 | 100% |
| Species | ||
| Ixodes ricinus | 33 | 23% |
| Dermacentor reticulatus | 31 | 21% |
| Dermacentor marginatus | 73 | 52% |
| Haemaphysalis concinna | 2 | 2% |
| Haemaphysalis erinacei | 2 | 2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreghiciu, I.C.; Imre, M.; Hoffman, D.; Oprescu, I.; Iorgoni, V.; Giubega, S.; Morariu, S.; Ilie, M.S. Identifying and Mapping Ticks on Wild Boars from Romania. Animals 2025, 15, 1092. https://doi.org/10.3390/ani15081092
Dreghiciu IC, Imre M, Hoffman D, Oprescu I, Iorgoni V, Giubega S, Morariu S, Ilie MS. Identifying and Mapping Ticks on Wild Boars from Romania. Animals. 2025; 15(8):1092. https://doi.org/10.3390/ani15081092
Chicago/Turabian StyleDreghiciu, Ioan Cristian, Mirela Imre, Diana Hoffman, Ion Oprescu, Vlad Iorgoni, Simona Giubega, Sorin Morariu, and Marius Stelian Ilie. 2025. "Identifying and Mapping Ticks on Wild Boars from Romania" Animals 15, no. 8: 1092. https://doi.org/10.3390/ani15081092
APA StyleDreghiciu, I. C., Imre, M., Hoffman, D., Oprescu, I., Iorgoni, V., Giubega, S., Morariu, S., & Ilie, M. S. (2025). Identifying and Mapping Ticks on Wild Boars from Romania. Animals, 15(8), 1092. https://doi.org/10.3390/ani15081092









