Length–Weight Relationship, Age, and Growth of Invasive Carassius auratus in Lugu Lake, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
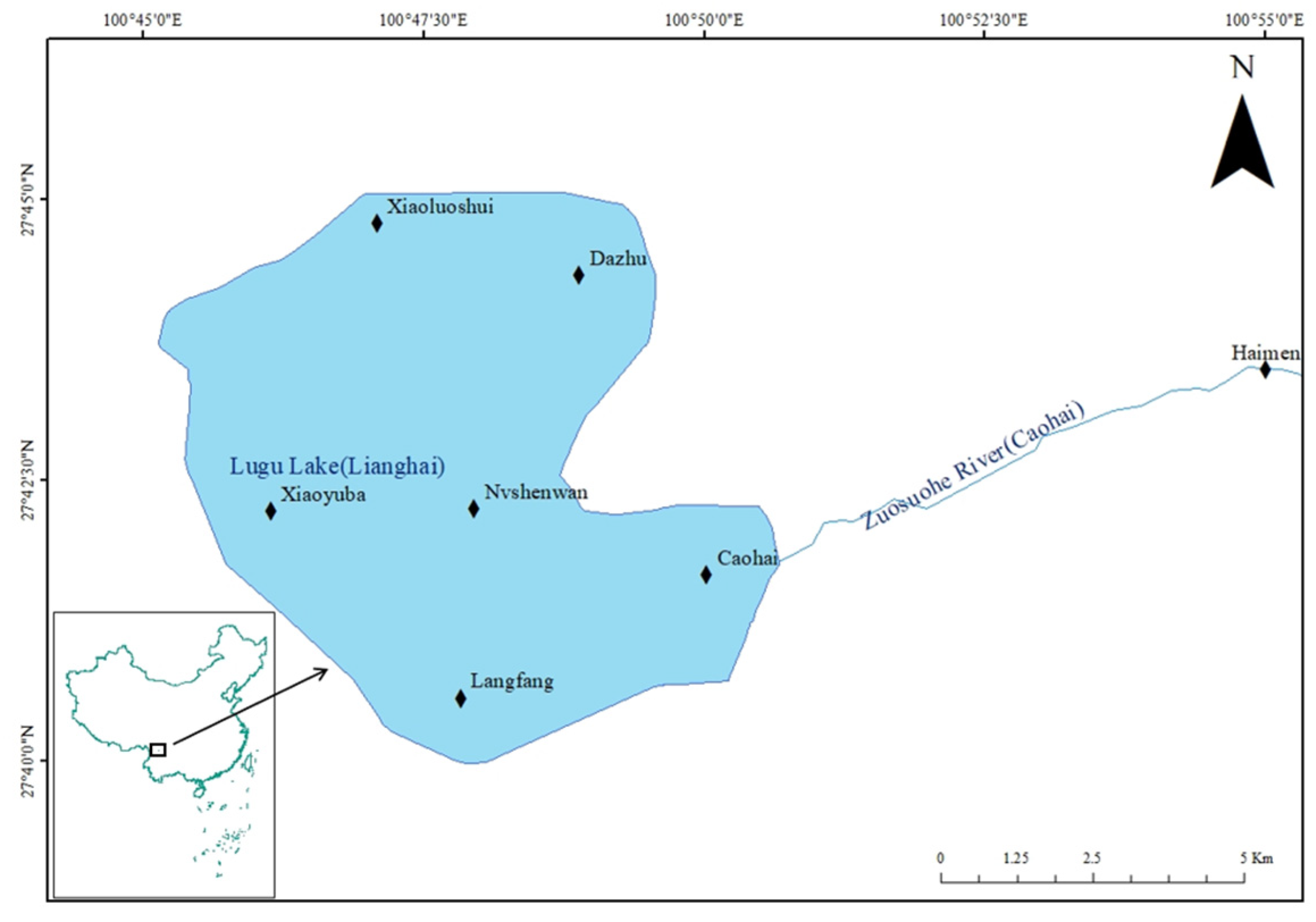
2.1. Sampling Point Settings
2.2. Sample Collection and Processing
2.3. Data Analysis
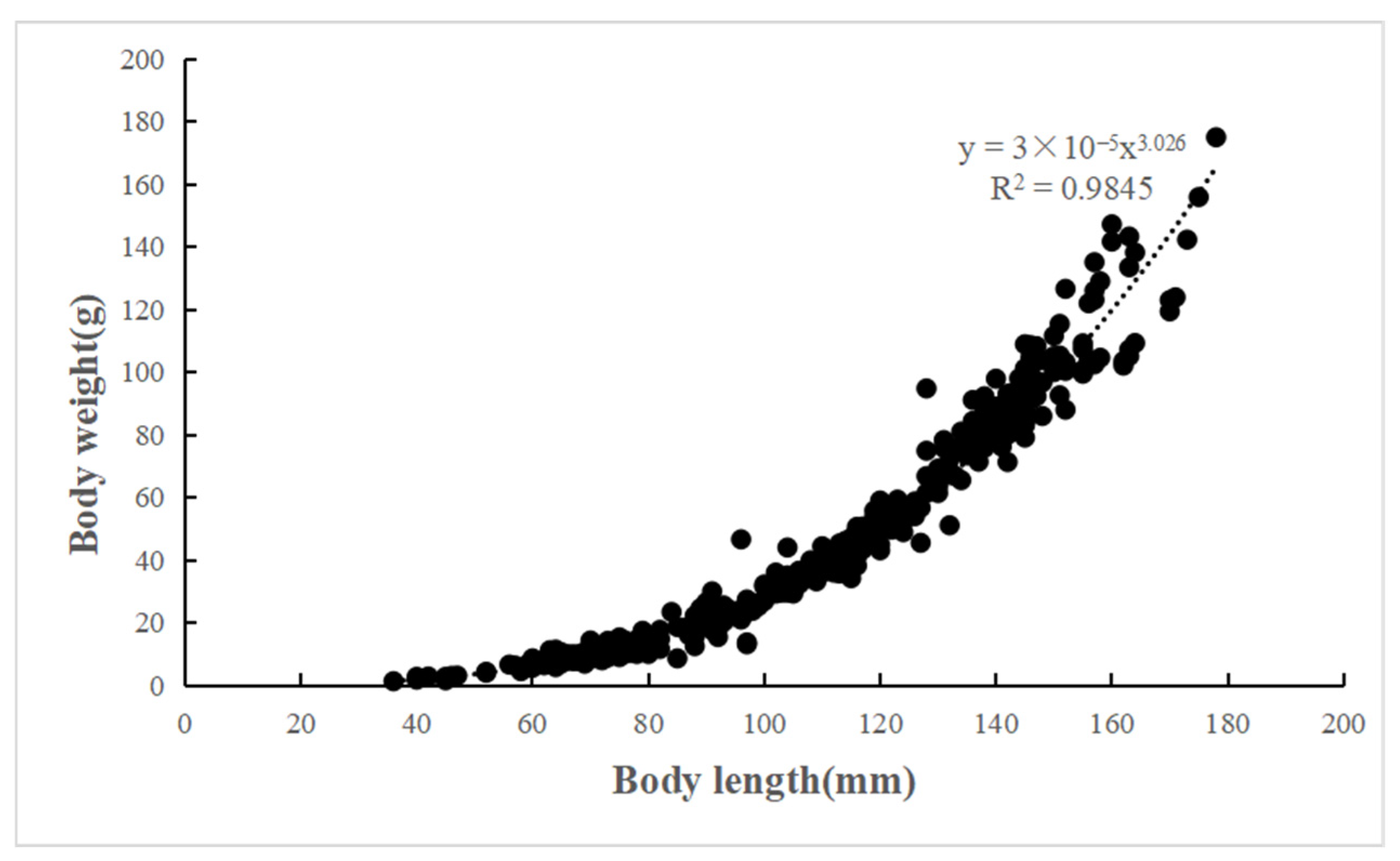
2.3.1. Length–Weight Relationships (LWRs)
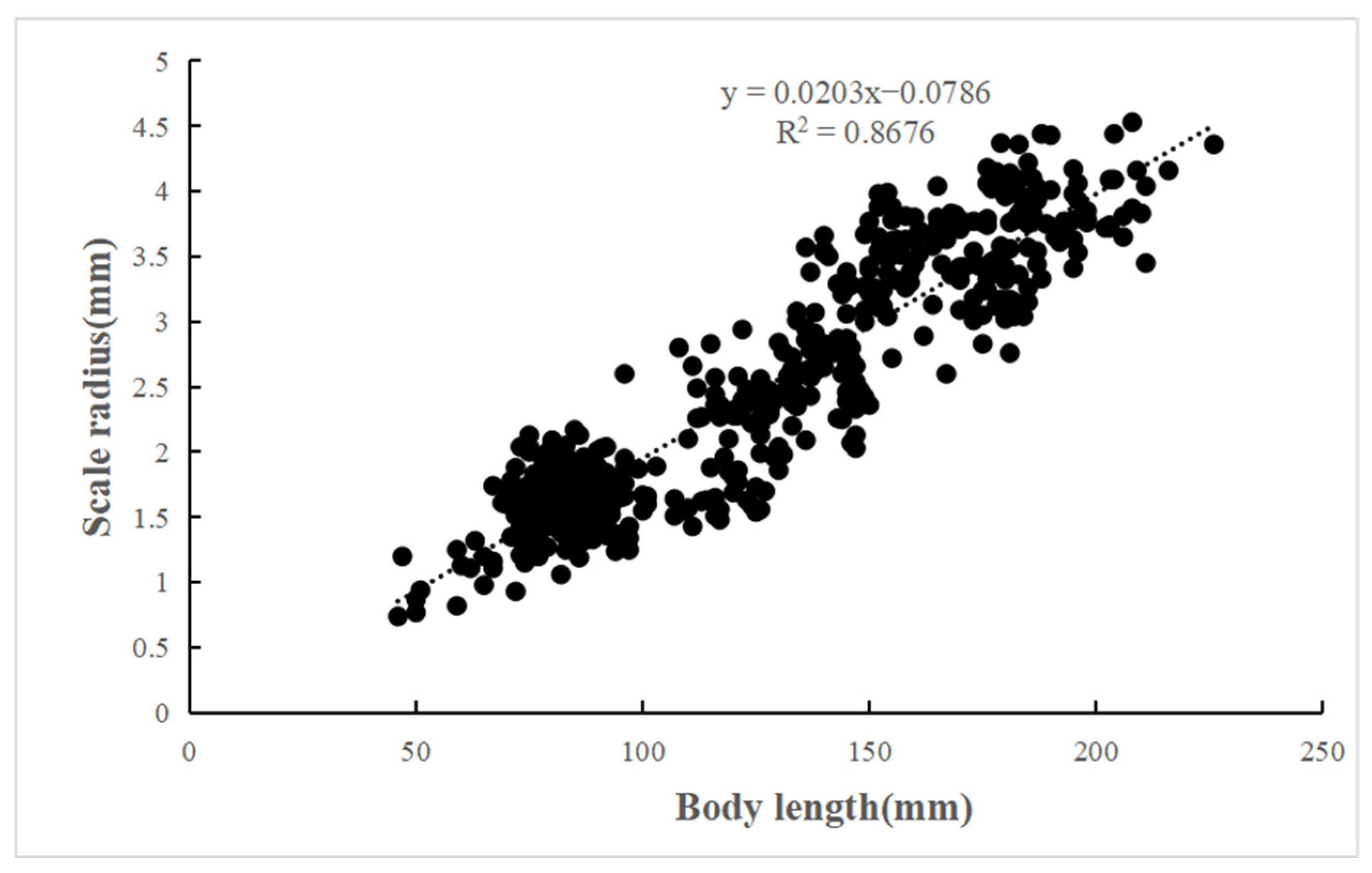
2.3.2. Relationship Between Body Length and Scale Radius
2.3.3. Growth Equation
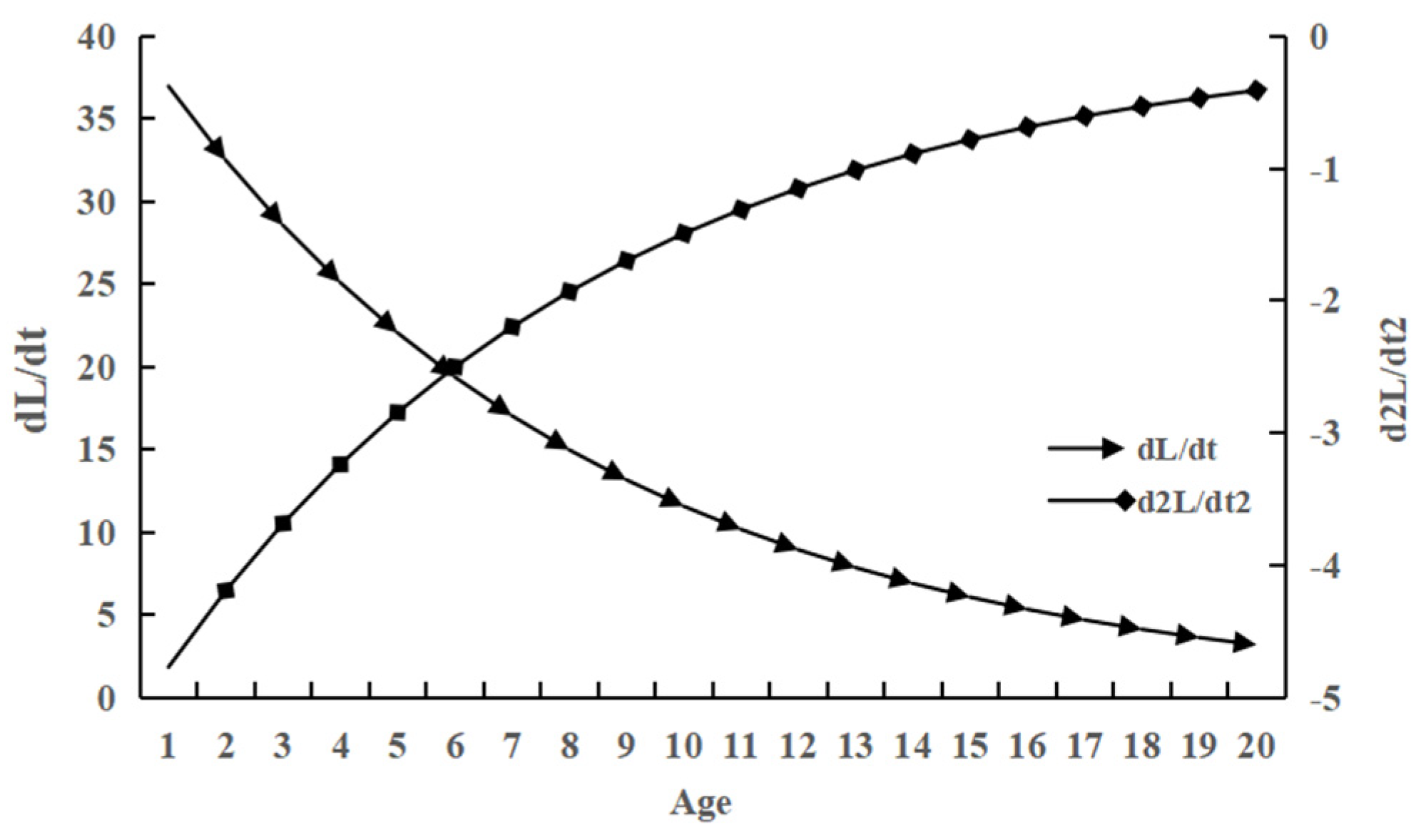
2.3.4. Growth Rate, Acceleration, and Growth Inflection Point
3. Results
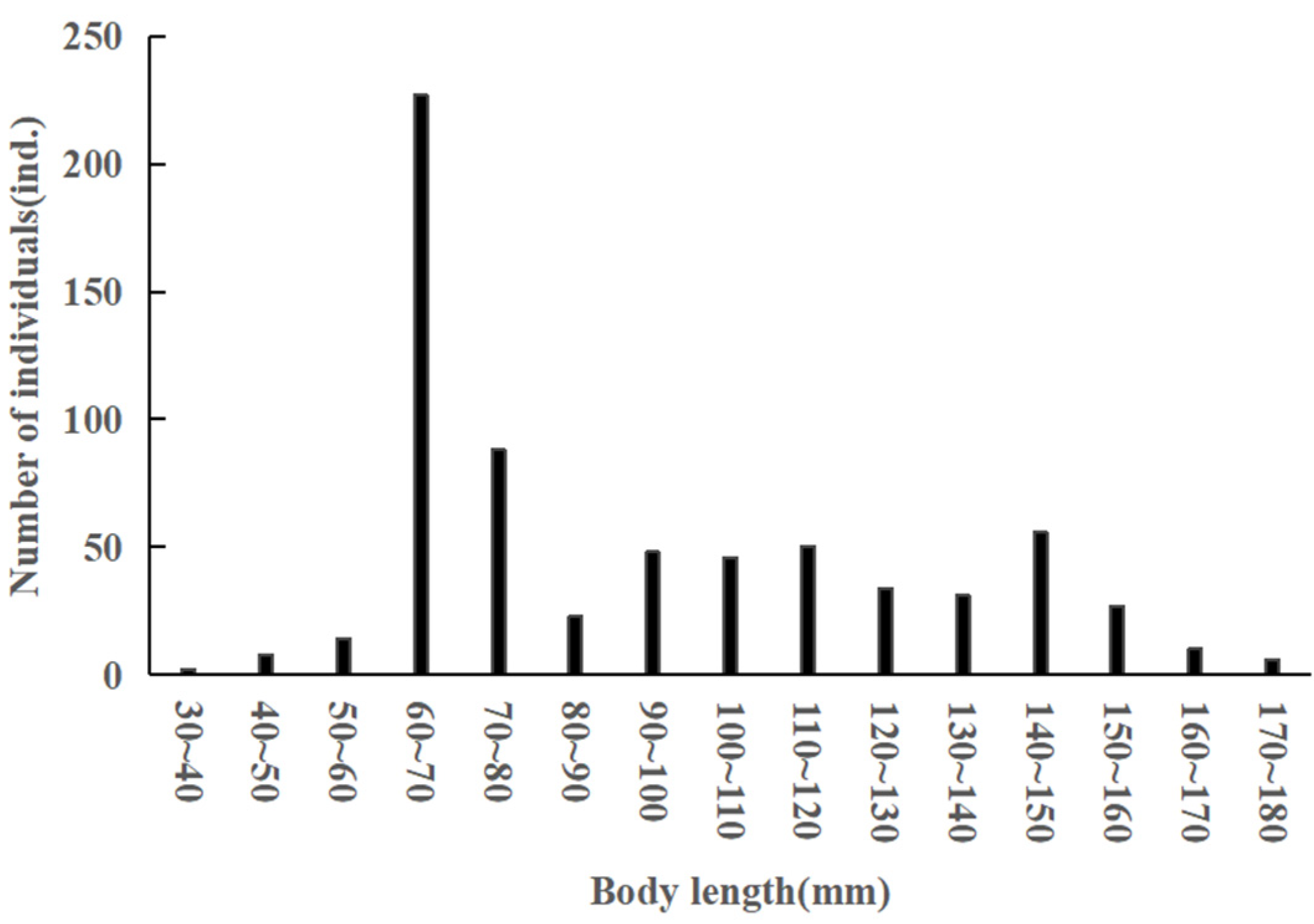
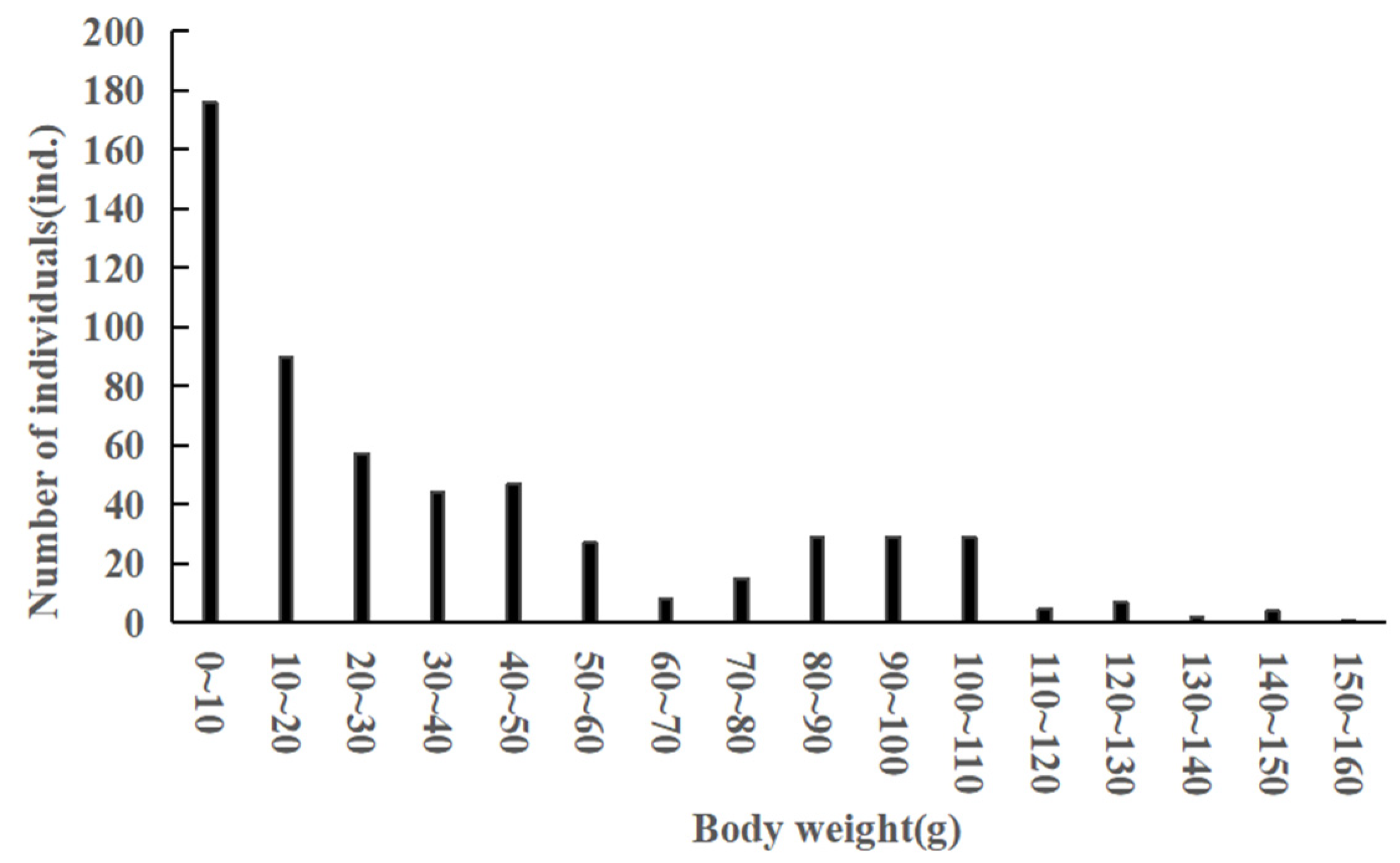
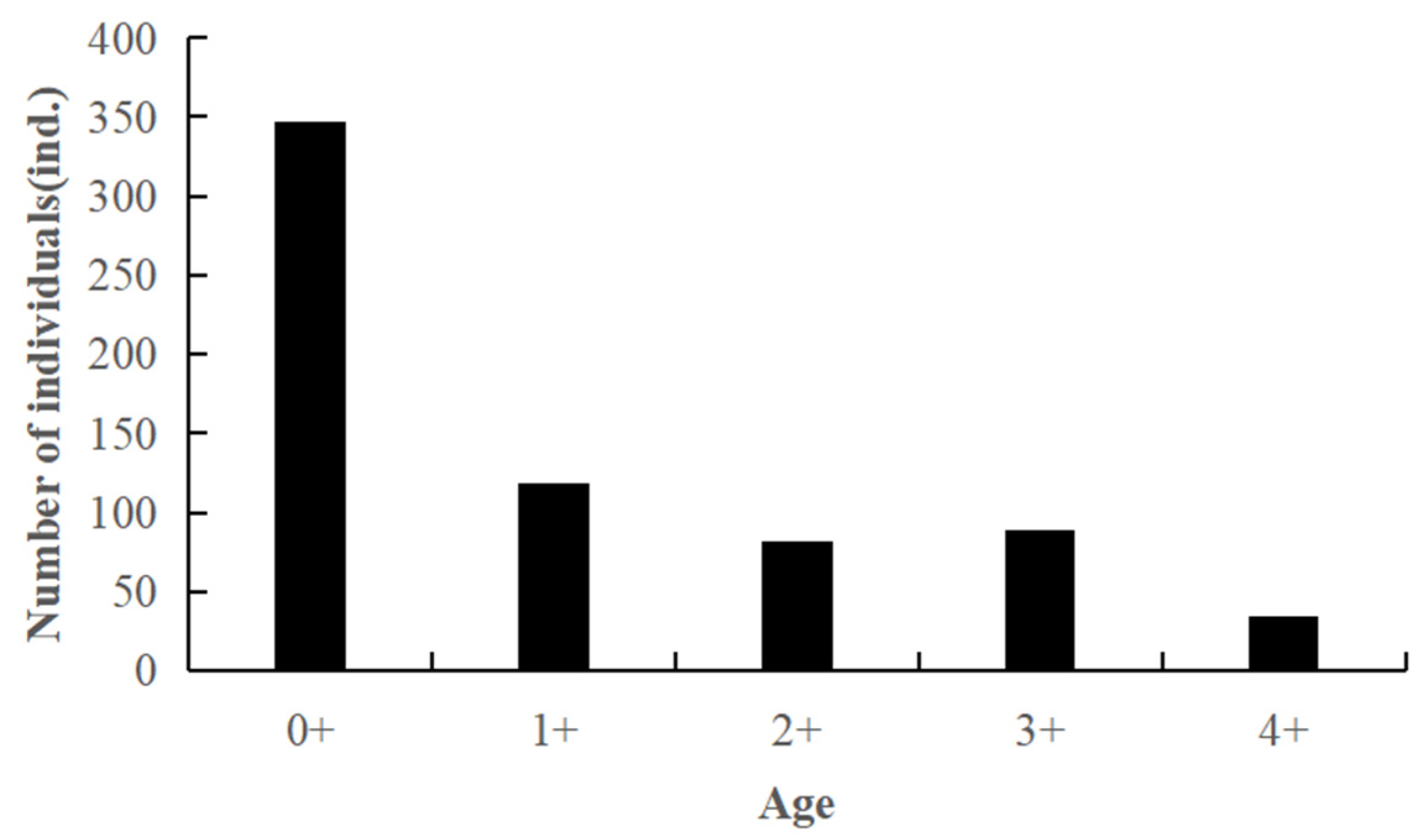
3.1. Body Size and Age Structure
3.2. Length–Weight Relationships (LWRs) and Scale Radius Relationships
3.3. Back-Calculated Body Length
3.4. Growth Equation and Growth Parameters
4. Discussion
4.1. Age Structure of C. auratus in Lugu Lake
4.2. Growth Characteristics of C. auratus in Lugu Lake
4.3. The Impact of C. auratus Invasion in Lugu Lake and Recommendations for Population Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.L.; Wang, Z.P.; Lang, X.B.; Guan, M.L.; Feng, S.X. Spatial-temporal distribution of dissolved oxygen in Lugu Lake. Environ. Sci. Technol. 2020, 43, 135–140. [Google Scholar] [CrossRef]
- Xu, M.W. Investigation on Fish Diversity in Luguhu Provincial Nature Reserve. For. Surv. Plan. 2020, 45, 54–59+157. [Google Scholar]
- Huang, S.; Li, L.X.; Dao, W.; Wang, Y.; Li, J. Spatial distribution characteristics analysis and resources assessment of fish in Lugu Lake. South Aquat. Sci. 2020, 16, 53–61. (In Chinese) [Google Scholar] [CrossRef]
- Peng, X.; Xu, D.Y.; Dong, Y.; Li, C.; Zhou, M. Current status of fish resources in Lugu Lake and conservation countermeasures. J. Xichang Coll. (Nat. Sci. Ed.) 2015, 29, 1–4. (In Chinese) [Google Scholar]
- Lorenzoni, M.; Corboli, M.; Ghetti, L.; Dörr, A.J.M.; Pedicillo, G. Growth and reproduction of the goldfish Carassius auratus: A case study from Italy. In Biological Invaders in Inland Waters; Gherardi, F., Ed.; Springer: New York, NY, USA, 2007; pp. 259–273. [Google Scholar] [CrossRef]
- Tang, G.W.; Cao, Z.D.; Fu, S.J. The relationship between temperature, intraspecific standard metabolic differences and excess post-exercise oxygen consumption in juvenile Carassius auratus. Chin. J. Ecol. 2013, 32, 3255–3260. (In Chinese) [Google Scholar] [CrossRef]
- Chen, R.; Ma, H.H.; Fu, S.J.; Liu, Y.; Zhang, Q. Effect of heating rate on upper temperature tolerance and group behavior of juvenile Carassius auratus. J. Chongqing Norm. Univ. (Nat. Sci. Ed.) 2022, 39, 31–37. (In Chinese) [Google Scholar]
- Yang, H.Y.; Huang, D.M.; Xie, S.; Zhao, Y.; Wang, L. Status quo of fishery resources in the middle reach of Yarlung Zangbo River. J. Hydroecol. 2010, 31, 120–126. (In Chinese) [Google Scholar] [CrossRef]
- Yang, H.Y.; Huang, D.M. Preliminary investigation on fish fauna and resource status in the middle and upper reaches of the Yarlung Zangbo River. J. Cent. China Norm. Univ. (Nat. Sci. Ed.) 2011, 45, 629–633. (In Chinese) [Google Scholar]
- Chen, F. Study on Life History Strategies of Exotic Carassius auratus in the Yarlung Zangbo River. Ph.D. Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China, 2009. (In Chinese). [Google Scholar]
- Chen, F.; Chen, Y.F. Investigation and protection of fish in the Lhasa River. Acta Hydrobiol. Sin. 2010, 34, 278–284. (In Chinese) [Google Scholar] [CrossRef]
- Shen, H.G.; Guo, L. Investigation and analysis of fish composition in the Niyang River of Tibet. Hebei Fish. 2008, 5, 51–54. (In Chinese) [Google Scholar]
- Li, F. Research on Aquatic Organisms in the Nyang River Basin of Tibet and Prediction and Evaluation of the Impact of Hydropower Projects. Master’s Thesis, Northwest University, Xi’an, China, 2009. (In Chinese). [Google Scholar]
- Pu, B.; La, D.; Ba, S.; Wang, X.; Li, Z. Study on the diversity of vertebrate species in the Lhasa Larung Wetland National Nature Reserve. Tibet. J. Tibet Univ. (Nat. Sci. Ed.) 2010, 25, 1–7. (In Chinese) [Google Scholar] [CrossRef]
- Ding, H.P.; Qin, J.H.; Lin, S.Q.; Liu, Y.; Zhang, T. Exotic fish in the Chabalong Wetland of Lhasa City. J. Hydroecol. 2014, 35, 49–55. (In Chinese) [Google Scholar] [CrossRef]
- Ding, H.P. Biology of Invasive Fish in Chabalong Wetland and Their Stress on Native Fish. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2014. (In Chinese). [Google Scholar]
- Kong, D.P.; Chen, X.Y.; Yang, J.X. Fish fauna status in the Lugu Lake with preliminary analysis on cause and effect of human impacts. Zool. Res. 2006, 27, 94–97. (In Chinese) [Google Scholar] [CrossRef]
- Hu, F.F.; Li, J.Y.; Li, X.M.; Zhou, Y.; Wang, H. Age and growth characteristics of Chinese hook snout carp Opsariichthys bidens in Duhe Cascade Reservoirs of the Hanjiang River. Fish. Sci. 2023, 42, 424–431. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, L.B. Age and Growth of Acrossocheilus parallens in the Beijiang River. Chin. J. Zool. 2015, 4, 518–528. [Google Scholar]
- Liang, X.X.; Qing, N.; Yang, K.L.; Wan, C.X.; Zhao, J.; Chen, X.L. Population Genetic Variation and Phylogeography of Zacco platypus in Guangdong Region; Acta Hydrobiologica Sinica: Guangzhou, China, 2010. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Gozlan, R.E.; Britton, J.R.; Cowx, I.; Copp, G.H. Current knowledge on non-native freshwater fish introductions. Fish Fish. 2010, 11, 315–340. [Google Scholar] [CrossRef]
- Yin, M.N. Fish Ecology; China Agricultural Press: Beijing, China, 2007; pp. 30–31. (In Chinese) [Google Scholar]
- Li, Z.L.; Ma, J.; Lei, H.M.; Deng, X. Age structure, growth characteristics and resource utilization of cuttail catfish in Foding Mountain of Middle Reaches of the Wujiang River. J. Dalian Ocean Univ. 2019, 34, 718–724. (In Chinese) [Google Scholar]
- Yang.T.H. Study on Fish Community Structure and Population Dynamics of Culter oxycephaloides in Dongting Lake. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2024. (In Chinese). [CrossRef]
- Liu, F.; Wei, H.; Hu, Y.C.; Li, Z.; Wang, T. Age and growth characteristic of invasive fish Pterygoplichthys pardalis in the Liuxi River. Freshw. Fish. 2019, 49, 22–30. (In Chinese) [Google Scholar] [CrossRef]
- Li, F.; Yang, D.G.; He, Y.F.; Wang, L.; Zhang, H. Age and growth of Ptychobarbus kaznakovi in the Zengqu River. Freshw. Fish. 2016, 46, 39–44, 63. (In Chinese) [Google Scholar] [CrossRef]
- Pauly, D. Theory and management of tropical multispecies stocks: A review with emphasis on the Southeast Asian demersal fisheries. ICES J. Mar. Sci. 1979, 39, 175–192. [Google Scholar] [CrossRef]
- Zhang, X.J.; Cheng, J.H. Survey on study of the fish age determination. Mar. Fish. 2009, 31, 92–99. (In Chinese) [Google Scholar]
- Zhang, H.; Xie, S.; Wang, S. Weight–Length Relationship and Condition Factor of Gibel Carp at Different Growth Stages. Fishes 2023, 8, 439. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, D.Z.; Chen, Z.X.; Li, W.; Wang, Y. Study on the age and growth of crucian carp from Yellow River in Ningxia. J. Ningxia Agric. Coll. 1997, 18, 62–66. (In Chinese) [Google Scholar]
- Zhang, L.; Meng, H.P.; Li, Z.M.; Peng, B.C.; Yuan, M.C.; Du, S.H.; An, M.; Han, G.C.; Gao, Y.K.; Liu, Y.H. Population Characteristics of Golden (chinese) Carp Carassius Auratus (Linnaeus) in Dali Lake. Acta Agric. Boreali-Sin. 2007, 22, 128–130. [Google Scholar] [CrossRef]
- Shi, L.N.; Zhang, L.P.; Wu, X.D. Study on the Growth, Development, and Reproductive Biology of the Lanzhou Catfish. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2007. (In Chinese). [Google Scholar]
- Yang, Z.F.; Liu, B.; Ge, C.P.; Song, C.Y.; Zhang, H.M.; Shan, F. Research progress of aquacultureenvironmental stress on epigenetic regulation in fish: A review. J. Dalian Ocean Univ. 2018, 33, 270–282. (In Chinese) [Google Scholar] [CrossRef]
- Uiuiu, P.; Constantinescu, R.; Păpuc, T.; Muntean, G.-C.; Matei-Lațiu, M.C.; Becze, A.; Cocan, D.; Lațiu, C.; Martonoș, C.O. Influence of Longitudinal Fragmentation on Length–Weight Relationships of Fishes in the Someșul Cald River, Romania. Fishes 2024, 9, 420. [Google Scholar] [CrossRef]
- Morey, G.; Moranta, J.; Massutí, E.; Grau, A.M.; Linde, M.; Riera, F.; Morales-Nin, B. Weight–length relationships of littoral to lower slope fishes from the western Mediterranean. Fish. Res. 2003, 62, 89–96. [Google Scholar] [CrossRef]
- Duan, Z.H.; Sun, J.Y.; Chang, J.B.; Li, H.; Wang, X. Growth and stock assessment of Carassius auratus in Wanghu Lake. J. Lake Sci. 1994, 6, 257–269. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Li, H.J.; Li, K.T.; Chu, Z.J. Studies on age and growth of crucian carp from Nanwan. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2006, 19, 188–190. (In Chinese) [Google Scholar]
- Zhu, Q.G.; Wu, Z.Q.; Liu, H.Z. Age and growth characteristics of the common carp in Poyang Lake. Jiangxi Aquat. Sci. Technol. 2010, 4, 25–29. (In Chinese) [Google Scholar]
- Wang, J.N.; Zhou, Q.C.; An, M.; Zhang, Y.; Liu, H. Age and growth of Carassius auratus in Caohai Lake. Fish. Sci. 2014, 33, 9. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhuang, D.D.; Zhang, K.X.; Gao, L.C. Three new species of Schizothorax fish in Lugu Lake, Yunnan Plateau. Acta Zootaxonomica Sin. 1981, 27, 328–333. [Google Scholar]
- Strong, D.R.; Ayres, D.R. Ecological and evolutionary misadventures of Spartina. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 523–548. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L.; Bestgen, K.R. Life-history strategies predict fish invasions and extirpations in the Colorado River Basin. Trends Ecol. Evol. 2004, 19, 586–591. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, S.; Liu, Z. Invasion history of goldfish (Carassius auratus) in Yunnan lakes. Aquat. Invasions 2018, 13, 141–154. [Google Scholar] [CrossRef]
- Tapkir, S.; Boukal, D.; Kalous, L.; Bartoň, D.; Souza, A.T.; Kolar, V.; Soukalová, K.; Duchet, C.; Gottwald, M.; Šmejkal, M. Invasive gibel carp (Carassius gibelio) outperforms threatened native crucian carp (Carassius carassius) in growth rate and effectiveness of resource use: Field and experimental evidence. Aquat. Conserv. 2022, 32, 12. [Google Scholar] [CrossRef]


| Sampling Site | Temp (°C) | pH | DO (mg/L) | Conductivity (μS/cm) | TN (mg/L) | TP (mg/L) | TSS (mg/L) | COD (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Dazu | 17.43 | 8.27 | 7.19 | 238.5 | 1.1 | 0.34 | 1.9 | 24.75 |
| Xiaoluoshui | 17.03 | 8.43 | 7.24 | 239.8 | 1.08 | 0.19 | 1.5 | 15.75 |
| Xiaoyuba | 17.33 | 8.37 | 7.15 | 238.5 | 0.6 | 0.19 | 1.75 | 33.75 |
| Nvshenwan | 17.03 | 8.27 | 7.21 | 239.8 | 0.63 | 0.12 | 1.5 | 23.75 |
| Langfang | 18.35 | 8.28 | 7.16 | 238.8 | 1.5 | 0.14 | 1.25 | 45.25 |
| Caohai | 17.15 | 8.4 | 7.31 | 236.3 | 1.2 | 0.26 | 2.5 | 3.88 |
| Haimen | 16.8 | 7.19 | 3.47 | 247.5 | 1.08 | 0.24 | 3 | 30.5 |
| Age Group | Calculate the Body Length/mm | Sample Size | |||
|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | ||
| 0+ | 347 | ||||
| 1+ | 67.41 | 118 | |||
| 2+ | 67.91 | 112.25 | 82 | ||
| 3+ | 66.43 | 110.77 | 147.22 | 89 | |
| 4+ | 63.47 | 104.86 | 141.80 | 173.82 | 34 |
| Average | 66.31 | 109.29 | 144.51 | 173.82 | 670 |
| Location | Lake Network | Lake Baikal | South Bay Reservoir | Poyang Lake | Caohai Lake | Lugu Lake |
|---|---|---|---|---|---|---|
| Growth coefficient | 0.2640 | 0.1797 | 0.1990 | 0.1424 | 0.1537 | 0.0720 |
| Growth rate | 2.9274 | 2.8179 | 4.0060 | 2.8871 | 2.9024 | 2.9113 |
| Inflection point age | 3.95 | 5.60 | 7.90 | 7.13 | 6.28 | 6.66 |
| Asymptotic body, length/mm | 285.82 | 301.00 | 301.20 | 294.90 | 259.67 | 401.57 |
| Asymptotic body, mass/g | 653.30 | 832.30 | 1097.32 | 776.96 | 481.94 | 484.38 |
| References | [37] | [30] | [38] | [39] | [40] | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Gong, J.; Hu, F.; Guo, Z.; Lu, Z.; Luo, M.; Zhu, T. Length–Weight Relationship, Age, and Growth of Invasive Carassius auratus in Lugu Lake, China. Animals 2025, 15, 1091. https://doi.org/10.3390/ani15081091
Li K, Gong J, Hu F, Guo Z, Lu Z, Luo M, Zhu T. Length–Weight Relationship, Age, and Growth of Invasive Carassius auratus in Lugu Lake, China. Animals. 2025; 15(8):1091. https://doi.org/10.3390/ani15081091
Chicago/Turabian StyleLi, Kaifei, Jinling Gong, Feifei Hu, Zhibin Guo, Zhaoyuan Lu, Mingzhong Luo, and Tingbing Zhu. 2025. "Length–Weight Relationship, Age, and Growth of Invasive Carassius auratus in Lugu Lake, China" Animals 15, no. 8: 1091. https://doi.org/10.3390/ani15081091
APA StyleLi, K., Gong, J., Hu, F., Guo, Z., Lu, Z., Luo, M., & Zhu, T. (2025). Length–Weight Relationship, Age, and Growth of Invasive Carassius auratus in Lugu Lake, China. Animals, 15(8), 1091. https://doi.org/10.3390/ani15081091





