The Antioxidant Effect of Selenium Is Enhanced by Cortisol Through Nrf2 Pathway in Bovine Endometrial Epithelial Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. BEEC Isolation and Primary Cell Culture
2.2. Experiment Design and Treatments
2.3. Indicators of Oxidative Stress
2.3.1. Intracellular ROS, LDH, and MDA
2.3.2. Intracellular CAT, SOD, GSH-PX, and TRXR Activity and T-AOC
2.4. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.5. Protein Extraction and Western Blotting
2.6. Immunofluorescence Staining
2.7. Statistical Analysis
3. Results
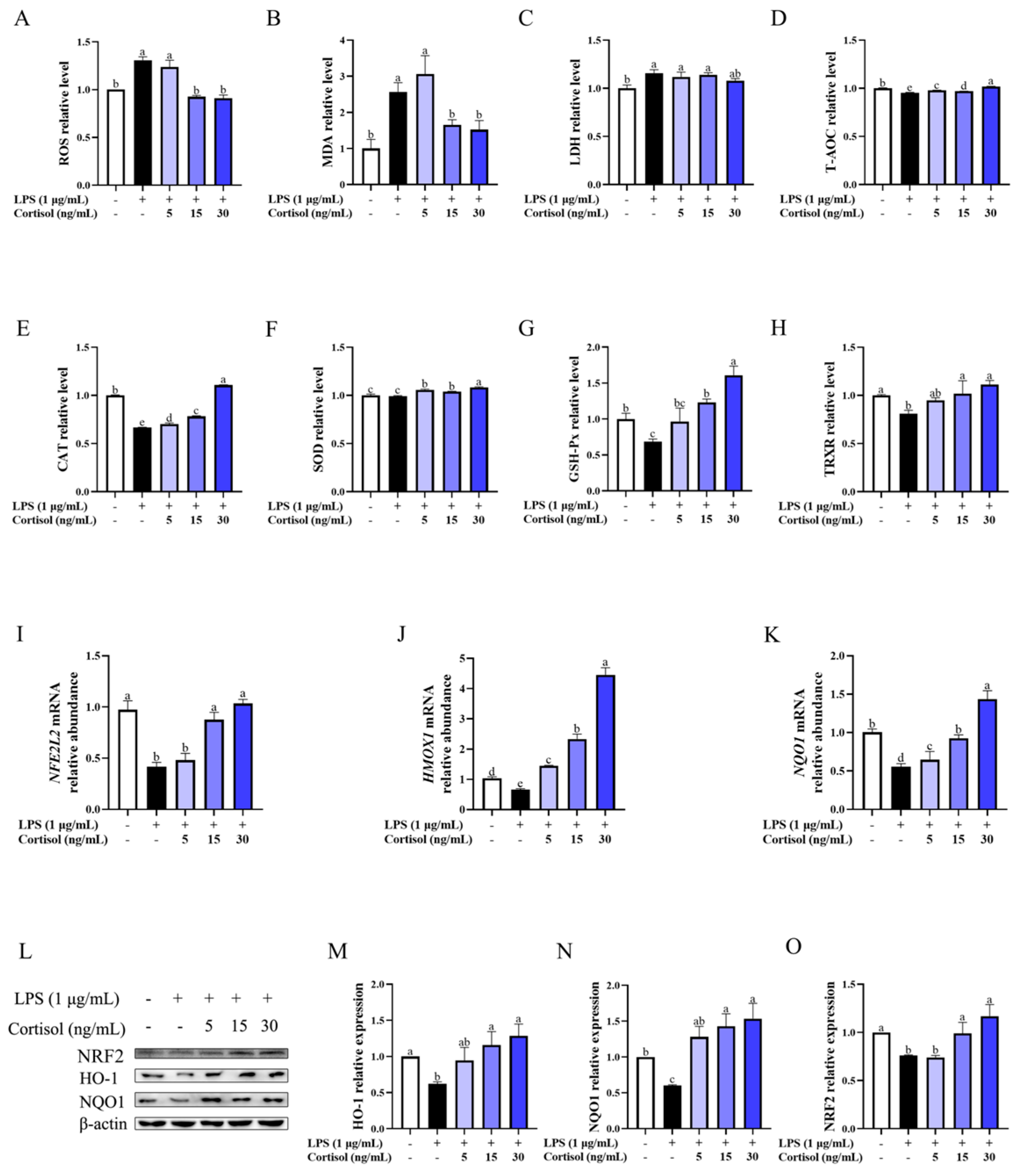
3.1. Cortisol Enhanced the Antioxidant Capacity of BEEC
3.2. Cortisol Alleviated LPS-Induced Oxidative Stress
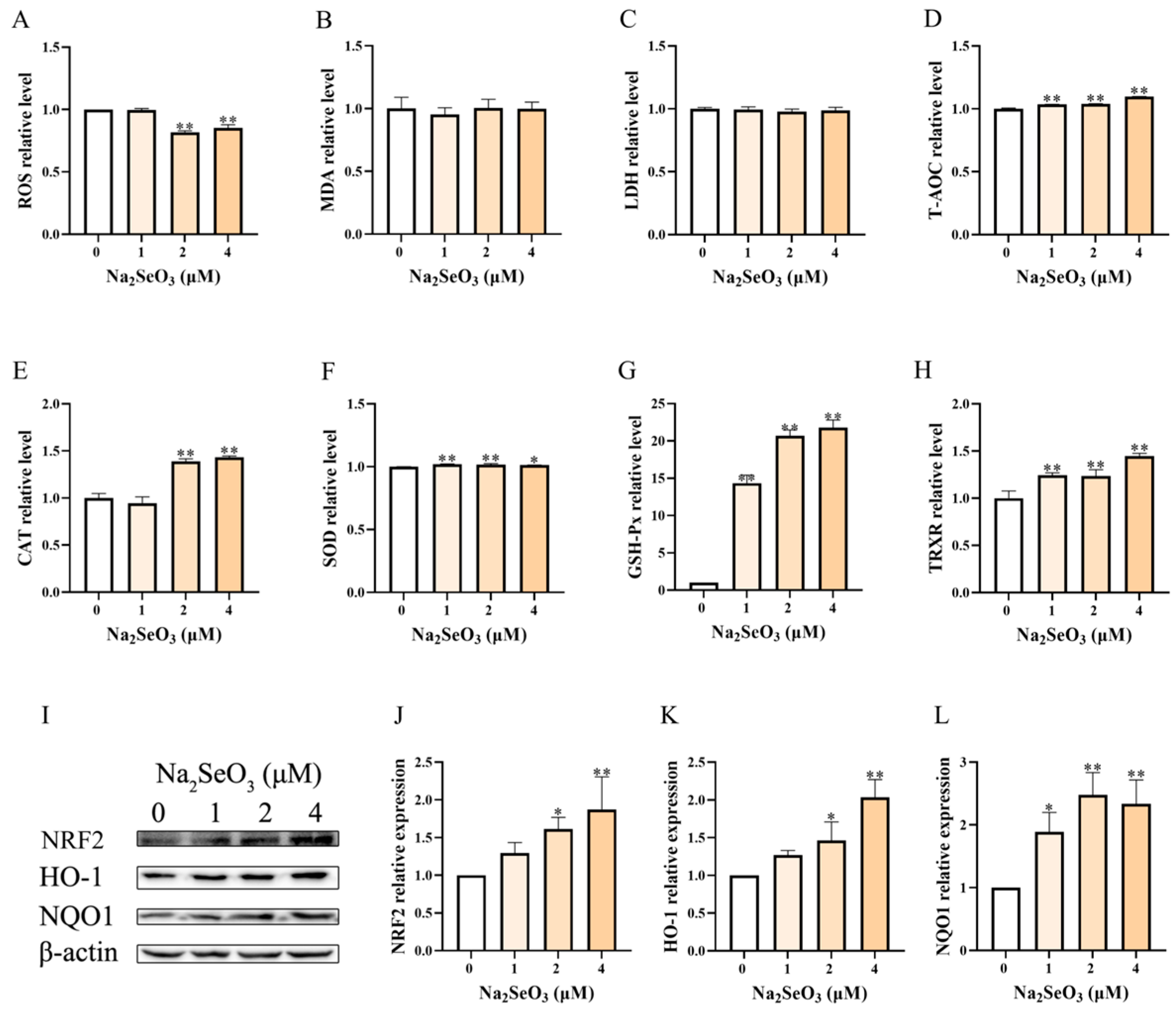
3.3. Se Alone Enhanced the Antioxidant Capacity of BEEC
3.4. Se Alleviated LPS-Induced Oxidative Stress of BEEC in the Presence of Cortisol
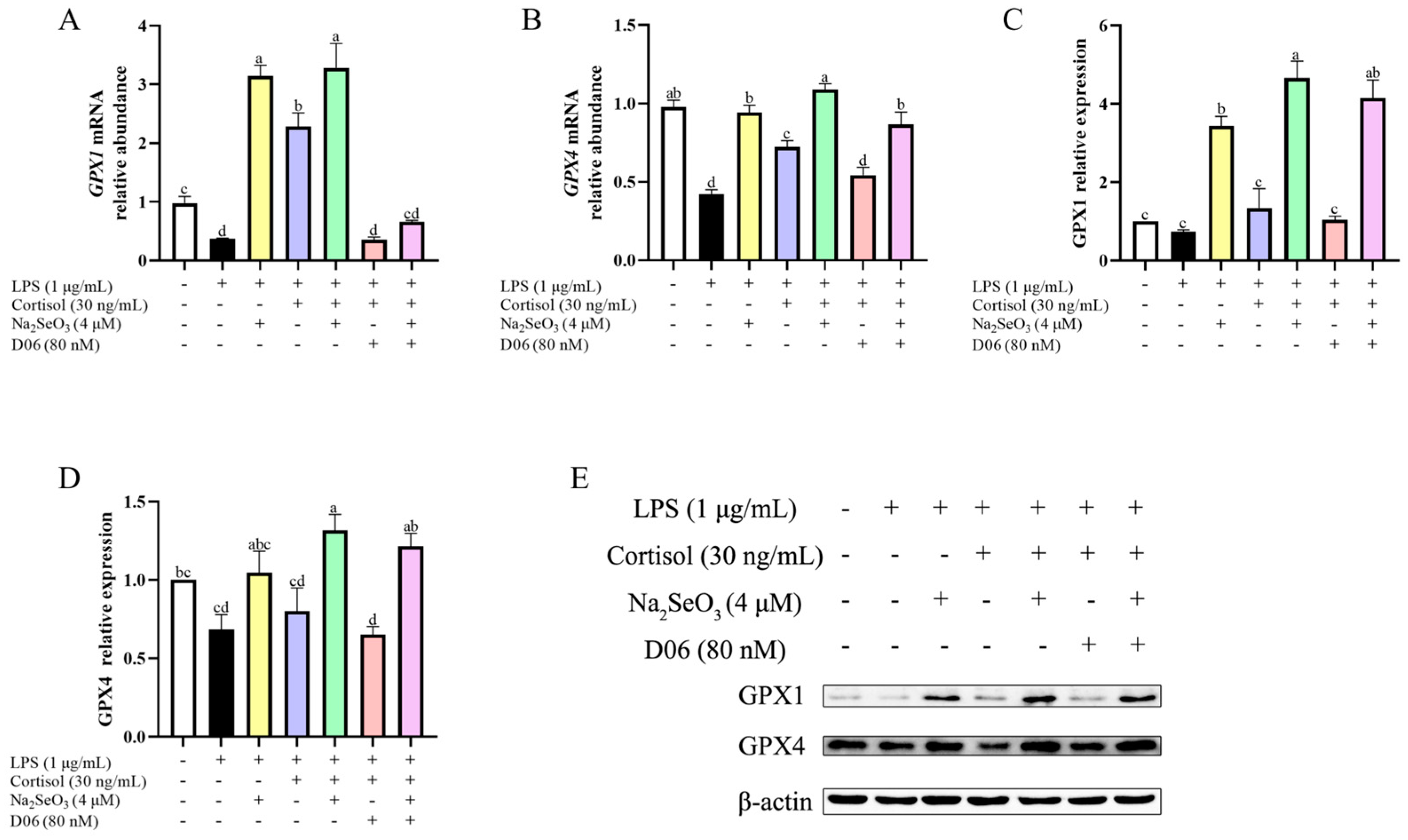
3.5. Cortisol Antioxidation Was Mediated by the Cortisol Receptor
3.6. Cortisol Affected GPX1 and GPX4 Expression in the Presence of Se
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTB | Beta-actin |
| BEEC | bovine endometrial epithelial cells |
| BMEC | bovine mammary epithelial cell |
| BESC | bovine endometrial stromal cells |
| CAT | catalase |
| D06 | AL 082D06 |
| GPX1 | glutathione peroxidase 1 |
| GPX4 | glutathione peroxidase 4 |
| GSH-Px | glutathione peroxidase |
| HO-1 | heme oxygenase |
| LDH | lactate dehydrogenase |
| LPS | lipopolysaccharide |
| MDA | malondialdehyde |
| NFE2L2 | Nuclear factor erythroid 2-related factor 2 |
| NO | reactive nitrogen |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 |
| ROS | reactive oxygen species |
| Se | selenium |
| SEM | standard error of means |
| SOD | superoxide dismutase |
| T-AOC | total antioxidant capacity |
| TRXR | thioredoxin reductase |
References
- LeBlanc, S.J.; Osawa, T.; Dubuc, J. Reproductive tract defense and disease in postpartum dairy cows. Theriogenology 2011, 76, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Omori, R.; Koyama, K.; Matsui, Y.; Sugimoto, M. Optimization of diagnostic methods and criteria of endometritis for various postpartum days to evaluate infertility in dairy cows. Theriogenology 2018, 119, 225–232. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zoldan, K.; Moellmer, T.; Schneider, J.; Fueldner, C.; Knauer, J.; Lehmann, J. Increase of CD25 expression on bovine neutrophils correlates with disease severity in post-partum and early lactating dairy cows. Dev. Comp. Immunol. 2014, 47, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Alabdullah, H.A.; Fox, L.K.; Gay, J.M.; Barrington, G.M. Interactive effects of dexamethasone and opsonized Mycoplasma bovis on bovine neutrophil function in vitro. Vet. Immunol. Immunopathol. 2018, 196, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zheng, F.; Zhang, M.; Wang, Z.; Meng, X.; Dong, J.; Liu, K.; Guo, L.; Wang, H.; Li, J. Selenium suppressed the LPS-induced oxidative stress of bovine endometrial stromal cells through NRF2 pathway with high cortisol background. J. Anim. Sci. 2024, 102, skae260. [Google Scholar] [CrossRef]
- Zhao, K.; Huo, B.; Shen, X. Studies on antioxidant capacity in selenium-deprived the Choko Yak in the Shouqu Prairie. Biol. Trace Elem. Res. 2021, 199, 3297–3302. [Google Scholar] [CrossRef]
- Cerri, R.L.A.; Rutigliano, H.M.; Lima, F.S.; Araújo, D.B.; Santos, J.E.P. Effect of source of supplemental selenium on uterine health and embryo quality in high-producing dairy cows. Theriogenology 2009, 71, 1127–1137. [Google Scholar] [CrossRef]
- Bourne, N.; Wathes, D.C.; Lawrence, K.E.; McGowan, M.; Laven, R.A. The effect of parenteral supplementation of vitamin E with selenium on the health and productivity of dairy cattle in the UK. Vet. J. 2008, 177, 381–387. [Google Scholar] [CrossRef]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef]
- Wilde, D. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 2006, 96, 240–249. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Kasper, K.; Traber, M.G.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of supranutritional organic selenium supplementation on postpartum blood micronutrients, antioxidants, metabolites, and inflammation biomarkers in selenium-replete dairy cows. Biol. Trace Elem. Res. 2014, 161, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.R.; Yasui, T. Practical applications of trace minerals for dairy cattle. J. Anim. Sci. 2014, 92, 416–426. [Google Scholar] [CrossRef]
- Thatcher, W.W.; Santos, J.E.; Silvestre, F.T.; Kim, I.H.; Staples, C.R. Perspective on physiological/endocrine and nutritional factors influencing fertility in post-partum dairy cows. Reprod. Domest. Anim. 2010, 45 (Suppl. S3), 2–14. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Dalia, A.M.; Loh, T.C.; Akit, H.; Samsudin, A.A. Effect of organic and inorganic dietary selenium supplementation on gene expression in oviduct tissues and Selenoproteins gene expression in Lohman Brown-classic laying hens. BMC Vet. Res. 2021, 17, 281. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant properties of selenium and vitamin E; their role in periparturient dairy cattle health regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, S.O.; Zheng, P.; Feng, R.; Adegoke, E.O.; Huang, F.; Ma, M.; Wang, Z.; Ifarajimi, O.O.; Li, X.; Zhang, G. The Antioxidant Role of Selenium via GPx1 and GPx4 in LPS-Induced Oxidative Stress in Bovine Endometrial Cells. Biol. Trace Elem. Res. 2022, 200, 1140–1155. [Google Scholar] [CrossRef]
- Fu, X.; Chen, D.; Ma, Y.; Yuan, W.; Zhu, L. Bovine Herpesvirus 1 Productive Infection Led to Inactivation of NRF2 Signaling through Diverse Approaches. Oxid. Med. Cell. Longevity 2019, 2019, 4957878. [Google Scholar] [CrossRef]
- Ye, Y.; Li, X.; Chen, M.; Wang, X.; Li, M.; Jiang, F.; Zhang, X.; Zhang, C.; Li, S. The extracts derived from Artemisia japonica thunb. Leaves mitigate oxidative stress and inflammatory fesponse induced by LPS in RAW264.7 cells through modulation of the NRF2/HO-1 signaling pathway. Molecules 2024, 29, 1375. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Lei, H.; Cai, Y.; Shen, J.; Zhu, P.; He, Q.; Zhao, M. The Nrf-2/HO-1 Signaling axis: A ray of hope in cardiovascular diseases. Cardiol. Res. Pract. 2020, 2020, 5695723. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, X.; Yan, S.; Zhang, B.; Shi, B. Mechanism underlying the protective effect of selenium on NO-induced oxidative damage in bovine mammary epithelial cells. Biol. Trace Elem. Res. 2019, 191, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Lin, T.; Yue, X.; Zhang, S.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal selenium-enriched yeast supplementation in sows enhances offspring growth and antioxidant status through the Nrf2/Keap1 pathway. Antioxidants 2023, 12, 2064. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Contreras, G.A.; Aitken, S.L. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Anim. Health Res. Rev. 2009, 10, 53–63. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Liu, C.; Liu, R.; Yang, C.; Wang, L.; Song, L. Cortisol modulates glucose metabolism and oxidative response after acute high temperature stress in Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 2022, 126, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.C.; Yip, T.; Lee, J.K.; Juliani, J.; Sernia, C.; Hill, A.F.; Lavidis, N.A.; Spiers, J.G. Restraint stress alters expression of glucocorticoid bioavailability mediators, suppresses Nrf2, and promotes oxidative stress in liver tissue. Antioxidants 2020, 9, 853. [Google Scholar] [CrossRef]
- Alam, M.M.; Okazaki, K.; Nguyen, L.T.T.; Ota, N.; Kitamura, H.; Murakami, S.; Shima, H.; Igarashi, K.; Sekine, H.; Motohashi, H. Glucocorticoid receptor signaling represses the antioxidant response by inhibiting histone acetylation mediated by the transcriptional activator NRF2. J. Biol. Chem. 2017, 292, 7519–7530. [Google Scholar] [CrossRef]
- Fu, Y.; Jin, Y.; Tian, Y.; Yu, H.; Wang, R.; Qi, H.; Feng, B.; Zhang, J. Zearalenone promotes LPS-induced oxidative stress, endoplasmic reticulum stress, and accelerates bovine mammary epithelial cell apoptosis. Int. J. Mol. Sci. 2022, 23, 10925. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Sun, M.; Li, B.; Li, Y.; Hua, S. Cecropin A alleviates LPS-induced oxidative stress and apoptosis of bovine endometrial epithelial cells. Animals 2024, 14, 768. [Google Scholar] [CrossRef]
- Dong, J.; Qu, Y.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Wang, H. Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells. Int. Immunopharmacol. 2018, 56, 71–77. [Google Scholar] [CrossRef]
- Riek, A.; Schrader, L.; Zerbe, F.; Petow, S. Comparison of cortisol concentrations in plasma and saliva in dairy cattle following ACTH stimulation. J. Dairy Res. 2019, 86, 406–409. [Google Scholar] [CrossRef]
- Miner, J.N.; Tyree, C.; Hu, J.; Berger, E.; Marschke, K.; Nakane, M.; Coghlan, M.J.; Clemm, D.; Lane, B.; Rosen, J. A nonsteroidal glucocorticoid receptor antagonist. Mol. Endocrinol. 2003, 17, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.; Mullford, M.; Whittlestone, W.G.; Payne, E. Bovine plasma corticoids during parturition. J. Dairy Sci. 1976, 59, 744–746. [Google Scholar] [CrossRef]
- Kindahl, H.; Kornmatitsuk, B.; Gustafsson, H. The cow in endocrine focus before and after calving. Reprod. Domest. Anim. 2004, 39, 217–221. [Google Scholar] [CrossRef]
- Rubio, C.P.; Escribano, D.; Mainau, E.; Cerón, J.J.; Navarro, E.; Manteca, X. Changes in salivary biomarkers of oxidative status in calves at weaning and grouping. BMC Vet. Res. 2021, 17, 373. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Vallejo-Mateo, P.J.; Lamy, E.; Cerón, J.J.; Rubio, C.P. Changes in salivary analytes in cows due to the in vitro presence of feed. BMC Vet. Res. 2022, 18, 275. [Google Scholar] [CrossRef]
- Zebeli, Q.; Sivaraman, S.; Dunn, S.M.; Ametaj, B.N. Intermittently-induced endotoxaemia has no effect on post-challenge plasma metabolites, but increases body temperature and cortisol concentrations in periparturient dairy cows. Res. Vet. Sci. 2013, 95, 1155–1162. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcácer, J.; Pellicer, J.A.; Salvador, R.; Cerdá, C.; Sáez, G.T.; et al. Pterostilbene decreases the antioxidant defenses of aggressive cancer cells in vivo: A physiological glucocorticoids- and Nrf2-dependent mechanism. Antioxid. Redox Signal. 2015, 24, 974–990. [Google Scholar] [CrossRef]
- Kratschmar, D.V.; Calabrese, D.; Walsh, J.; Lister, A.; Birk, J.; Appenzeller-Herzog, C.; Moulin, P.; Goldring, C.E.; Odermatt, A. Suppression of the Nrf2-dependent antioxidant response by glucocorticoids and 11β-HSD1-mediated glucocorticoid activation in hepatic cells. PLoS ONE 2012, 7, e36774. [Google Scholar] [CrossRef]
- Song, P.; Liu, C.; Sun, M.; Liu, J.; Lin, P.; Chen, H.; Zhou, D.; Tang, K.; Wang, A.; Jin, Y. Transcription factor Nrf2 modulates lipopolysaccharide-induced injury in bovine endometrial epithelial cells. Int. J. Mol. Sci. 2023, 24, 11221. [Google Scholar] [CrossRef]
- Ikeda, T.; Yang, L.; Ikenoue, T.; Mallard, C.; Hagberg, H. Endotoxin-induced hypoxic-ischemic tolerance is mediated by up-regulation of corticosterone in neonatal rat. Pediatr. Res. 2006, 59, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, M.; Zheng, F.; Yuan, C.; Wang, Z.; Qiu, S.; Meng, X.; Dong, J.; Liu, K.; Guo, L.; et al. Selenium elicited an enhanced anti-inflammatory effect in primary bovine endometrial stromal cells with high cortisol background. BMC Vet. Res. 2024, 20, 383. [Google Scholar] [CrossRef]
- Mehdi, Y.; Dufrasne, I. Selenium in cattle: A review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Pavlata, L.; Pechová, A.; Illek, J. Direct and indirect assessment of selenium status in cattle–A comparison. Acta Vet. Brno 2000, 69, 281–287. [Google Scholar] [CrossRef]
- Sun, L.; Wang, F.; Wu, Z.; Ma, L.; Baumrucker, C.; Bu, D. Comparison of selenium source in preventing oxidative stress in bovine mammary epithelial cells. Animals 2020, 10, 842. [Google Scholar] [CrossRef]
- Gladyshev, V.N.; Factor, V.M.; Housseau, F.; Hatfield, D.L. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochem. Biophys. Res. Commun. 1998, 251, 488–493. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Wei, Y. Selenium and selenoproteins in health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S.J. TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Zhao, Z.; Kim, J.; Lei, X.G. High Dietary Fat and Selenium concentrations exert tissue- and glutathione peroxidase 1–Dependent impacts on lipid metabolism of young-adult mice. J. Nutr. 2020, 150, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Bruzelius, K.; Sundler, R.; Pagmantidis, V.; Åkesson, B. Regulation of selenoprotein mRNA expression by hormones and retinoic acid in bovine mammary cells. J. Trace Elem. Med. Biol. 2010, 24, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Wu, P.; Chu, Y.; Gui, S.; Zheng, Y.; Chen, X. Dietary selenium alleviated mouse liver oxidative stress and NAFLD induced by obesity by regulating the KEAP1/NRF2 pathway. Antioxidants 2022, 11, 349. [Google Scholar] [CrossRef]
- Jin Jung, Y.; Choi, H.; Oh, E. Selenium mitigates ferroptosis-mediated dopaminergic cell death by regulating the Nrf2/GPX4 pathway. Neurosci. Lett. 2023, 810, 137314. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhang, J.; Guo, J.; Zhang, M.; Li, W.; Dong, J.; Liu, K.; Guo, L.; Li, J.; Wang, H.; et al. Selenium suppressed the LPS-induced inflammation of bovine endometrial epithelial cells through NF-κB and MAPK pathways under high cortisol background. J. Cell. Mol. Med. 2023, 27, 1373–1383. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′ → 3′) | Length (bp) | NCBI Accession |
|---|---|---|---|
| ACTB | F: CATCACCATCGGCAATGAGC | 156 | NM_173979.3 |
| R: AGCACCGTGTTGGCGTAGAG | |||
| NFE2L2 | F: CCCAGTCTTCACTGCTCCTC | 165 | NM_001011678.2 |
| R: TCAGCCAGCTTGTCATTTTG | |||
| HMOX1 | F: GGCAGCAAGGTGCAAGA | 221 | NM_001014912.1 |
| R: GAAGGAAGCCAGCCAAGAG | |||
| NQO1 | F: AACCAACAGACCAGCCAATC | 154 | NM_001034535.1 |
| R: CACAGTGACCTCCCATCCTT | |||
| GPX1 | F: CTTGCTGCTTGGCGGTCA | 139 | NM_174076.3R |
| R: AGGGGAGGCTGGGATGGAT | |||
| GPX4 | F: CACCGCCGAGATGAGCTTTA | 198 | NM_174770.4 |
| R: ACGTGGCCCCGGTACTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Zhong, J.; Duan, J.; Li, W.; Mao, P.; Dong, J.; Liu, K.; Guo, L.; Wang, H.; Li, J. The Antioxidant Effect of Selenium Is Enhanced by Cortisol Through Nrf2 Pathway in Bovine Endometrial Epithelial Cells. Animals 2025, 15, 1075. https://doi.org/10.3390/ani15081075
Cui L, Zhong J, Duan J, Li W, Mao P, Dong J, Liu K, Guo L, Wang H, Li J. The Antioxidant Effect of Selenium Is Enhanced by Cortisol Through Nrf2 Pathway in Bovine Endometrial Epithelial Cells. Animals. 2025; 15(8):1075. https://doi.org/10.3390/ani15081075
Chicago/Turabian StyleCui, Luying, Jingyi Zhong, Jiangyao Duan, Wanting Li, Peng Mao, Junsheng Dong, Kangjun Liu, Long Guo, Heng Wang, and Jianji Li. 2025. "The Antioxidant Effect of Selenium Is Enhanced by Cortisol Through Nrf2 Pathway in Bovine Endometrial Epithelial Cells" Animals 15, no. 8: 1075. https://doi.org/10.3390/ani15081075
APA StyleCui, L., Zhong, J., Duan, J., Li, W., Mao, P., Dong, J., Liu, K., Guo, L., Wang, H., & Li, J. (2025). The Antioxidant Effect of Selenium Is Enhanced by Cortisol Through Nrf2 Pathway in Bovine Endometrial Epithelial Cells. Animals, 15(8), 1075. https://doi.org/10.3390/ani15081075









