The Effect of G0S2 Gene Knockout on the Proliferation, Apoptosis, and Differentiation of Chicken Preadipocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Establishment of G0S2 Knockout Chicken Preadipocyte Cell Line
2.3. Cell Proliferation Assay
2.4. Cell Cycle Assay
2.5. Cell Apoptosis Assay
2.6. Oil Red O Staining and Extraction Assay
2.7. RNA Extraction and RT-qPCR
2.8. Western Blotting Assay
2.9. RNA-Seq
2.10. Functional and Enrichment Analysis
2.11. Statistical Analysis
3. Results
3.1. Comparison of HITI, HMEJ, and HDR Donor Integration Efficiency and Construction of G0S2 Knockout Preadipocyte Cell Line in Chickens
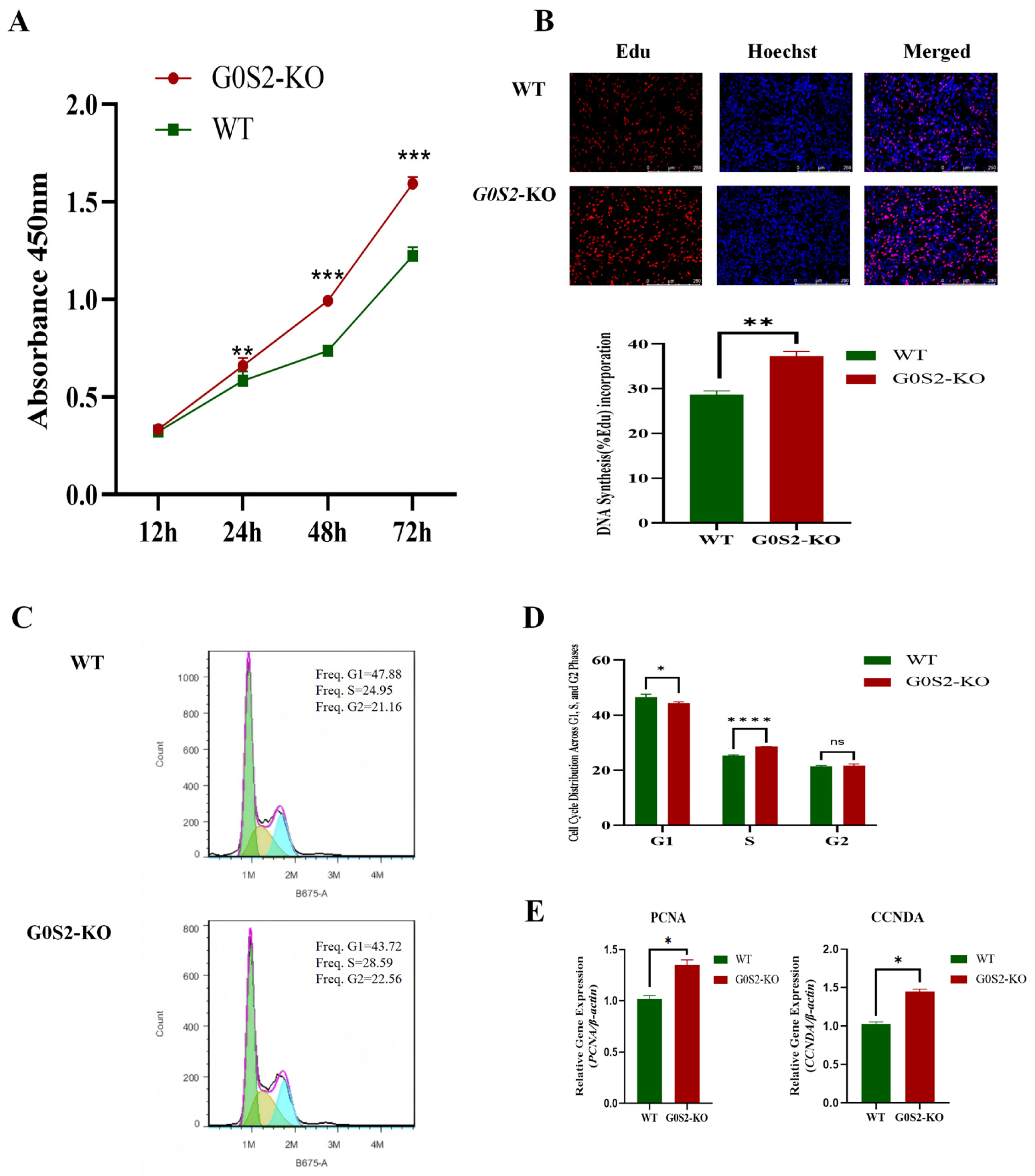
3.2. G0S2 Knockout Promotes the Proliferation of Chicken Preadipocytes
3.3. G0S2 Knockout Mildly Promotes Early Apoptosis in Chicken Preadipocytes
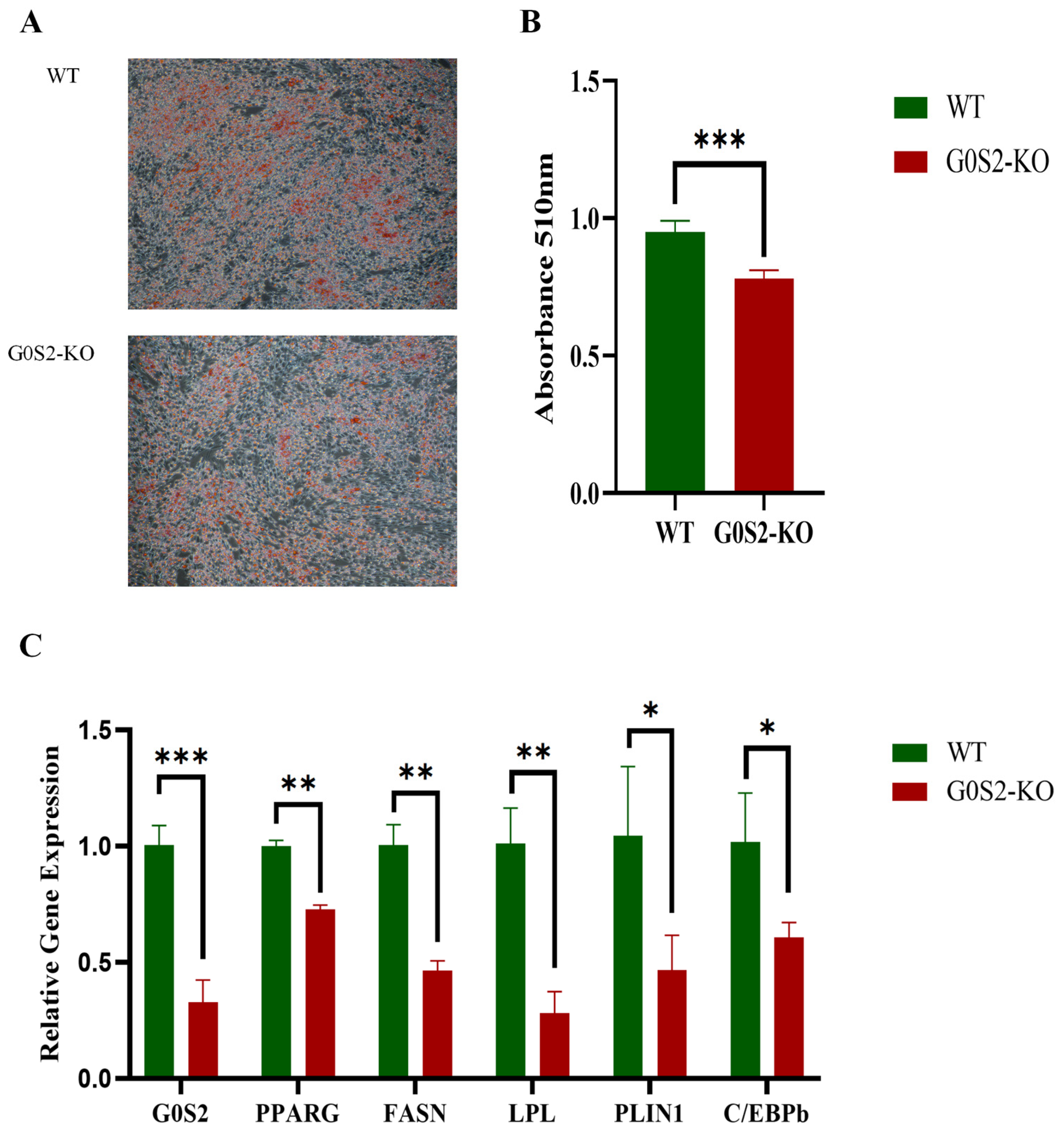
3.4. G0S2 Knockout Inhibits Differentiation of Chicken Preadipocytes
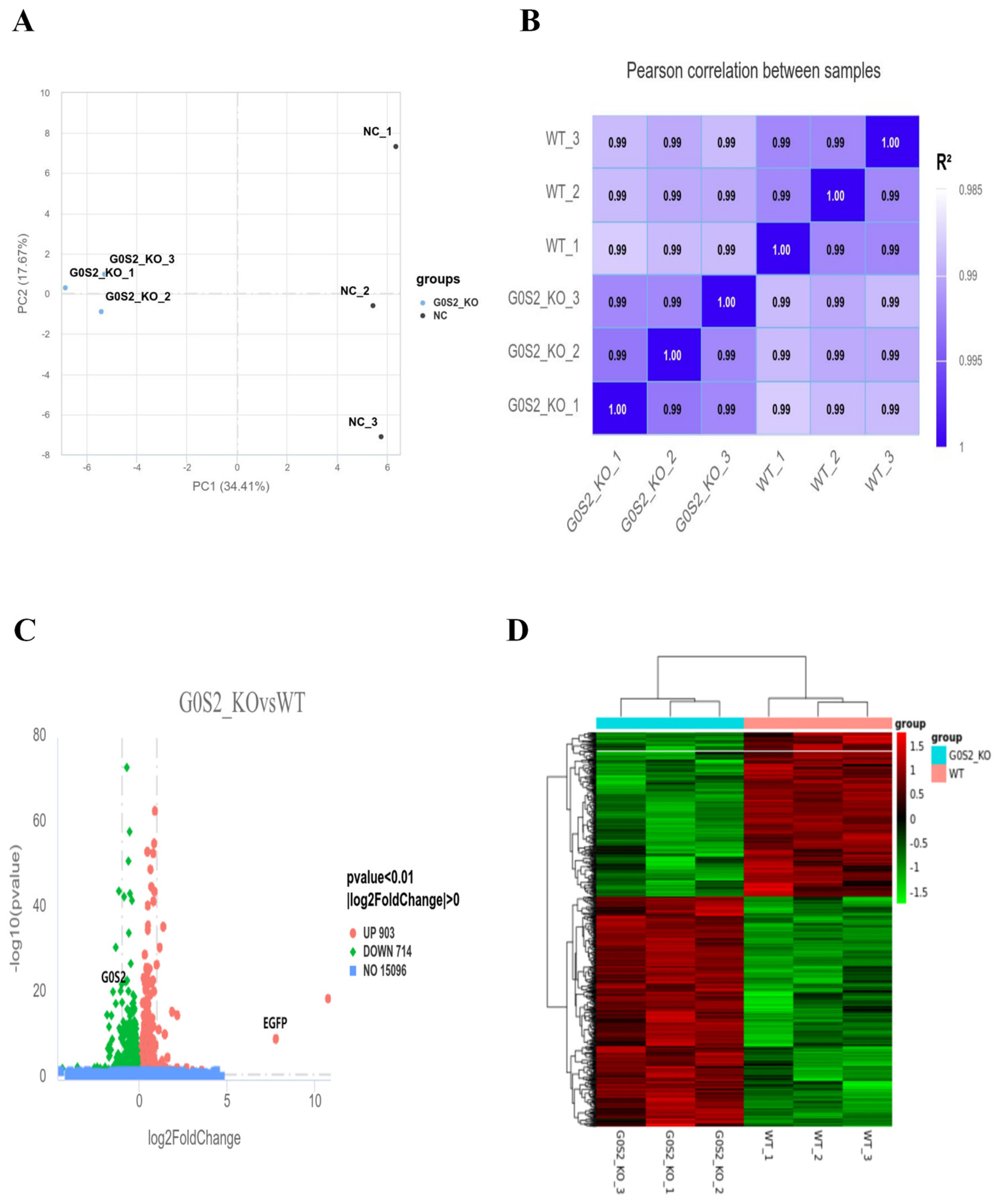
3.5. Transcriptome Sequencing and DEGs Analysis
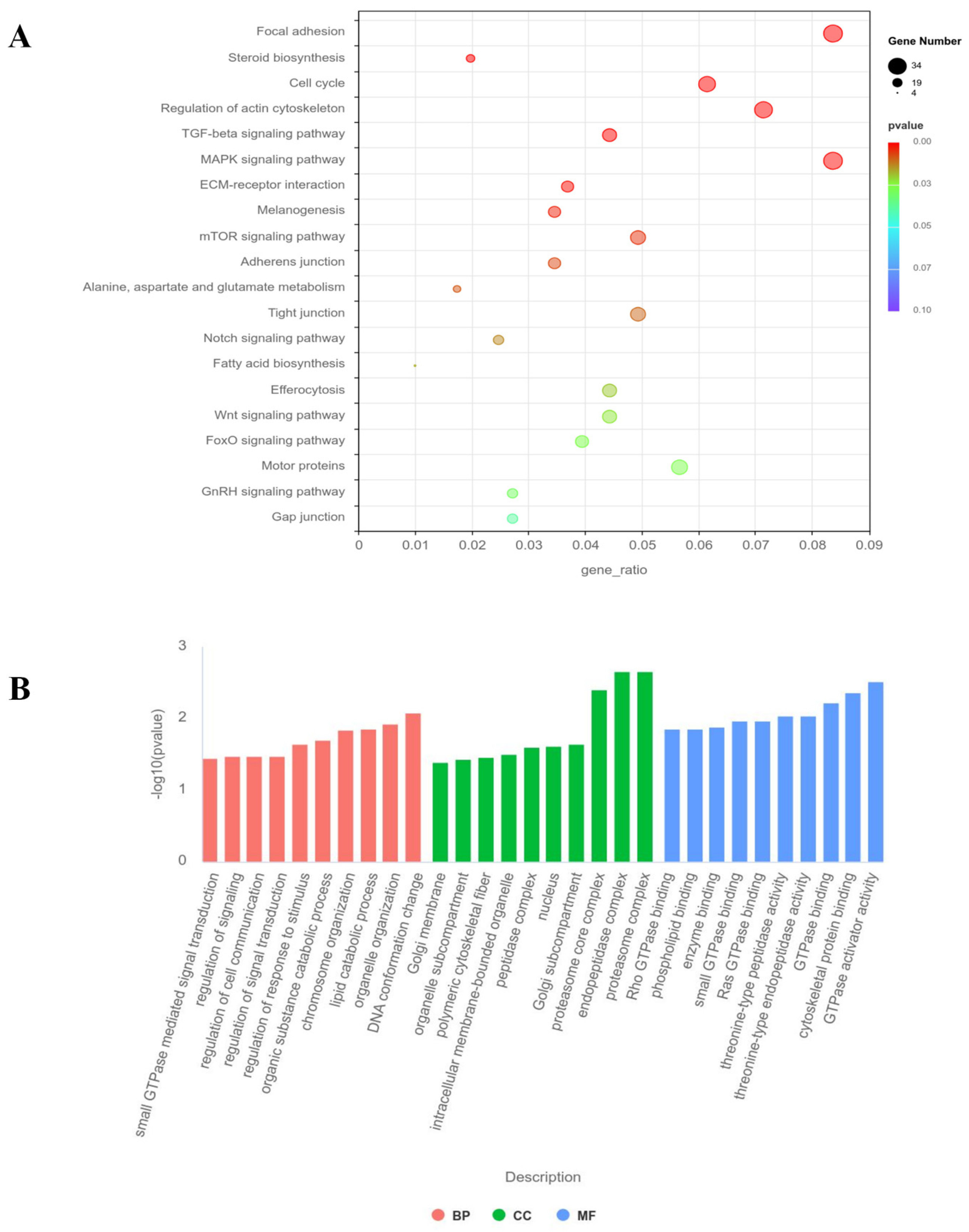
3.6. GO and KEGG Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sell-Kubiak, E.; Wimmers, K.; Reyer, H.; Szwaczkowski, T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: A review. J. Appl. Genet. 2017, 58, 487–498. [Google Scholar] [PubMed]
- Moreira, G.C.M.; Boschiero, C.; Cesar, A.S.M.; Reecy, J.M.; Godoy, T.F.; Pertille, F.; Ledur, M.C.; Moura, A.S.A.M.; Garrick, D.J.; Coutinho, L.L. Integration of genome wide association studies and whole genome sequencing provides novel insights into fat deposition in chicken. Sci. Rep. 2018, 8, 16222. [Google Scholar]
- Zhang, X.Y.; Wu, M.Q.; Wang, S.Z.; Zhang, H.; Du, Z.Q.; Li, Y.M.; Cao, Z.P.; Luan, P.; Leng, L.; Li, H. Genetic selection on abdominal fat content alters the reproductive performance of broilers. Animal 2018, 12, 1232–1241. [Google Scholar] [PubMed]
- Li, D.; Zhang, K.; Pan, Z.; Yu, M.; Lu, Y.; Wang, G.; Wu, J.; Zhang, J.; Zhang, K.; Du, W. Antibiotics promote abdominal fat accumulation in broilers. Anim. Sci. J. 2020, 91, e13326. [Google Scholar]
- Milicevic, D.; Vranic, D.; Masic, Z.; Parunovic, N.; Trbovic, D.; Nedeljkovic-Trailovic, J.; Petrovic, Z. The role of total fats, saturated/unsaturated fatty acids and cholesterol content in chicken meat as cardiovascular risk factors. Lipids Health Dis. 2014, 13, 42. [Google Scholar]
- Ailhaud, G. Cell surface receptors, nuclear receptors and ligands that regulate adipose tissue development. Clin. Chim. Acta 1999, 286, 181–190. [Google Scholar]
- Cereijo, R.; Gallego-Escuredo, J.M.; Moure, R.; Villarroya, J.; Domingo, J.C.; Fontdevila, J.; Martínez, E.; Gutiérrez, M.D.M.; Mateo, M.G.; Giralt, M.; et al. The molecular signature of HIV-1-associated lipomatosis reveals differential involvement of brown and beige/brite adipocyte cell lineages. PLoS ONE 2015, 10, e136571. [Google Scholar]
- Magun, R.; Boone, D.L.; Tsang, B.K.; Sorisky, A. The effect of adipocyte differentiation on the capacity of 3t3-l1 cells to undergo apoptosis in response to growth factor deprivation. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 567–571. [Google Scholar]
- Prins, J.B.; O’Rahilly, S. Regulation of adipose cell number in man. Clin. Sci. 1997, 92, 3–11. [Google Scholar]
- Papineau, D.; Gagnon, A.; Sorisky, A. Apoptosis of human abdominal preadipocytes before and after differentiation into adipocytes in culture. Metabolism 2003, 52, 987–992. [Google Scholar]
- Chen, Y.; Harris, R.A.; Hatahet, Z.; Chou, K. Ablation of xp-v gene causes adipose tissue senescence and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 2015, 112, E4556–E4564. [Google Scholar] [CrossRef] [PubMed]
- Siderovski, D.P.; Blum, S.; Forsdyke, R.E.; Forsdyke, D.R. A set of human putative lymphocyte G0/G1 switch genes includes genes homologous to rodent cytokine and zinc finger protein-encoding genes. DNA Cell Biol. 1990, 9, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Forsdyke, D.R. A human putative lymphocyte G0/G1 switch gene containing a cpg-rich island encodes a small basic protein with the potential to be phosphorylated. DNA Cell Biol. 1991, 10, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, X.; Lombès, M.; Rha, G.B.; Chi, Y.; Guerin, T.M.; Smart, E.J.; Liu, J. The G0/G1 switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010, 11, 194–205. [Google Scholar] [CrossRef]
- Zandbergen, F.; Mandard, S.; Escher, P.; Tan, N.S.; Patsouris, D.; Jatkoe, T.; Rojas-Caro, S.; Madore, S.; Wahli, W.; Tafuri, S.; et al. The G0/G1 switch gene 2 is a novel ppar target gene. Biochem. J. 2005, 392, 313–324. [Google Scholar] [CrossRef]
- Welch, C.; Santra, M.K.; El-Assaad, W.; Zhu, X.; Huber, W.E.; Keys, R.A.; Teodoro, J.G.; Green, M.R. Identification of a protein, G0S2, that lacks bcl-2 homology domains and interacts with and antagonizes bcl-2. Cancer Res. 2009, 69, 6782–6789. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhu, Y.; Zhang, P. Lipolytic inhibitor G0/G1 switch gene 2 inhibits reactive oxygen species production and apoptosis in endothelial cells. Am. J. Physiol. Cell Physiol. 2015, 308, C496–C504. [Google Scholar] [CrossRef]
- Shin, S.; Choi, Y.M.; Han, J.Y.; Lee, K. Inhibition of lipolysis in the novel transgenic quail model overexpressing G0/G1 switch gene 2 in the adipose tissue during feed restriction. PLoS ONE 2014, 9, e100905. [Google Scholar] [CrossRef]
- Park, T.S.; Park, J.; Lee, J.H.; Park, J.; Park, B. Disruption of G0/G1 switch gene 2 (G0S2) reduced abdominal fat deposition and altered fatty acid composition in chicken. FASEB J. 2019, 33, 1188–1198. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crrna ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [PubMed]
- Yao, X.; Wang, X.; Hu, X.; Liu, Z.; Liu, J.; Zhou, H.; Shen, X.; Wei, Y.; Huang, Z.; Ying, W.; et al. Homology-mediated end joining-based targeted integration using crispr/cas9. Cell Res. 2017, 27, 801–814. [Google Scholar] [PubMed]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via crispr/cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [PubMed]
- Wang, B.; Li, K.; Wang, A.; Reiser, M.; Saunders, T.; Lockey, R.F.; Wang, J. Highly efficient crispr/hdr-mediated knock-in for mouse embryonic stem cells and zygotes. Biotechniques 2015, 59, 201–208. [Google Scholar] [PubMed]
- Xie, L.; Sun, J.; Mo, L.; Xu, T.; Shahzad, Q.; Chen, D.; Yang, W.; Liao, Y.; Lu, Y. Hmej-mediated efficient site-specific gene integration in chicken cells. J. Biol. Eng. 2019, 13, 90. [Google Scholar]
- Wang, W.; Zhang, T.; Wu, C.; Wang, S.; Wang, Y.; Li, H.; Wang, N. Immortalization of chicken preadipocytes by retroviral transduction of chicken tert and tr. PLoS ONE 2017, 12, e177348. [Google Scholar] [CrossRef]
- Yamada, T.; Park, C.S.; Shen, Y.; Lacorazza, H.D. Role of DNA methylation of the G0/G1 switch gene 2(G0S2) in the proliferation of myeloid leukemia cells. Blood 2012, 120, 3520. [Google Scholar]
- Yamada, T.; Park, C.S.; Burns, A.; Nakada, D.; Lacorazza, H.D. The cytosolic protein g0s2 maintains quiescence in hematopoietic stem cells. PLoS ONE 2012, 7, e38280. [Google Scholar]
- Choi, H.; Lee, H.; Kim, T.; Kim, H.J.; Lee, Y.J.; Lee, S.J.; Yu, J.H.; Kim, D.; Kim, K.; Park, S.W.; et al. G0/G1 switch gene 2 has a critical role in adipocyte differentiation. Cell Death Differ. 2014, 21, 1071–1080. [Google Scholar]
- Morrison, D.K. Map kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar]
- Dalton, S. Linking the cell cycle to cell fate decisions. Trends Cell Biol. 2015, 25, 592–600. [Google Scholar]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [PubMed]
- Huang, Y.; Yang, X.; Wu, Y.; Jing, W.; Cai, X.; Tang, W.; Liu, L.; Liu, Y.; Grottkau, B.E.; Lin, Y. Gamma-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of notch and ppar-gamma. Cell Prolif. 2010, 43, 147–156. [Google Scholar] [PubMed]
- Kang, S.; Bajnok, L.; Longo, K.A.; Petersen, R.K.; Hansen, J.B.; Kristiansen, K.; MacDougald, O.A. Effects of wnt signaling on brown adipocyte differentiation and metabolism mediated by pgc-1alpha. Mol. Cell. Biol. 2005, 25, 1272–1282. [Google Scholar] [PubMed]
- Urbich, C.; Knau, A.; Fichtlscherer, S.; Walter, D.H.; Brühl, T.; Potente, M.; Hofmann, W.K.; de Vos, S.; Zeiher, A.M.; Dimmeler, S. Foxo-dependent expression of the proapoptotic protein bim: Pivotal role for apoptosis signaling in endothelial progenitor cells. FASEB J. 2005, 19, 974–976. [Google Scholar]


| Name | Oligonucleotide Sequence (5′-3′) |
|---|---|
| G0S2-1e-sgRNA1 | GCGTTCTTCGGCGTGGTCATCGG |
| G0S2-1e-sgRNA2 | CTTCGGCGTGGTCATCGGTCTGG |
| G0S2-1e-sgRNA3 | CGGCGTGGTCATCGGTCTGGTGG |
| Primer Name | Primer Sequences |
|---|---|
| G0S2-F | 5′-AGAAGCCCAACAGGAAGATG-3′ |
| G0S2-R | 5′-CTTGCTCTGCTCCAACACC-3′ |
| EGFP-F | 5′-CCAGCAGAACACCCCC-3′ |
| EGFP-R | 5′-CTCGTCCATGCCGAGA-3′ |
| PPARγ-F | 5′-GTGCAATCAAAATGGAGCC-3′ |
| PPARγ-R | 5′-CTTACAACCTTCACATGCA-3′ |
| LPL-F | 5′-ATGCTGATGCCCCTATC -3′ |
| LPL-R | 5′-TTCTGAATCCCAATGCT-3′ |
| FASN-F | 5′-ATGGGTATTGTCGCTCT-3′ |
| FASN-R | 5′-CACCTTGCTCCTTAAAG-3′ |
| C/EBPβ-F | 5′-ACCTGTCCACCTCGTCC-3′ |
| C/EBPβ-R | 5′-GCAGCCTCTCGTTCTCG-3′ |
| PLIN1-F | 5′-GGGGTGACTGGCGGTTGTA-3′ |
| PLIN1R | 5′-GCCGTAGAGGTTGGCGTAG-3′ |
| β-actin-F | 5′-CCAGCCATCTTTCTTGGGTA-3′ |
| β-actin-R | 5′-ATGCCAGGGTACATTGTGGT-3′ |
| caspas3-F | 5′-ATAAGAACTTCCACCGA-3′ |
| caspas3-R | 5′-GCAACACACAAACAAAA-3′ |
| FAS-F | 5′-AGATGTTGACCTGACCC-3′ |
| FAS-R | 5′-CTCCCATTCCATGTTTT-3′ |
| PCNA-F | 5′-CGTTGGCTCTAGTGTTT-3′ |
| PCNA-R | 5′-GCTTCTTCCTCTTTGTC-3′ |
| CCNDA-F | 5′-CTTGGATGCTGGAGGTC-3′ |
| CCNDA-R | 5′-GCTTTTCTTGAGGGGTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, B.; Wang, Z.; Wen, J.; Zhou, T.; Tang, J.; Li, Z. The Effect of G0S2 Gene Knockout on the Proliferation, Apoptosis, and Differentiation of Chicken Preadipocytes. Animals 2025, 15, 951. https://doi.org/10.3390/ani15070951
Li Y, Wang B, Wang Z, Wen J, Zhou T, Tang J, Li Z. The Effect of G0S2 Gene Knockout on the Proliferation, Apoptosis, and Differentiation of Chicken Preadipocytes. Animals. 2025; 15(7):951. https://doi.org/10.3390/ani15070951
Chicago/Turabian StyleLi, Yantao, Boyu Wang, Zhaochuan Wang, Jintian Wen, Tianle Zhou, Jiahao Tang, and Zhenhui Li. 2025. "The Effect of G0S2 Gene Knockout on the Proliferation, Apoptosis, and Differentiation of Chicken Preadipocytes" Animals 15, no. 7: 951. https://doi.org/10.3390/ani15070951
APA StyleLi, Y., Wang, B., Wang, Z., Wen, J., Zhou, T., Tang, J., & Li, Z. (2025). The Effect of G0S2 Gene Knockout on the Proliferation, Apoptosis, and Differentiation of Chicken Preadipocytes. Animals, 15(7), 951. https://doi.org/10.3390/ani15070951





