Integrating Indoor Hibernation into the Italian Outdoor Snail Farming System: A Potential Solution for Colder Climates
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Snail Gathering
2.3. Snail Hibernation
2.4. Statistical Analysis
3. Results
3.1. Snail Gathering
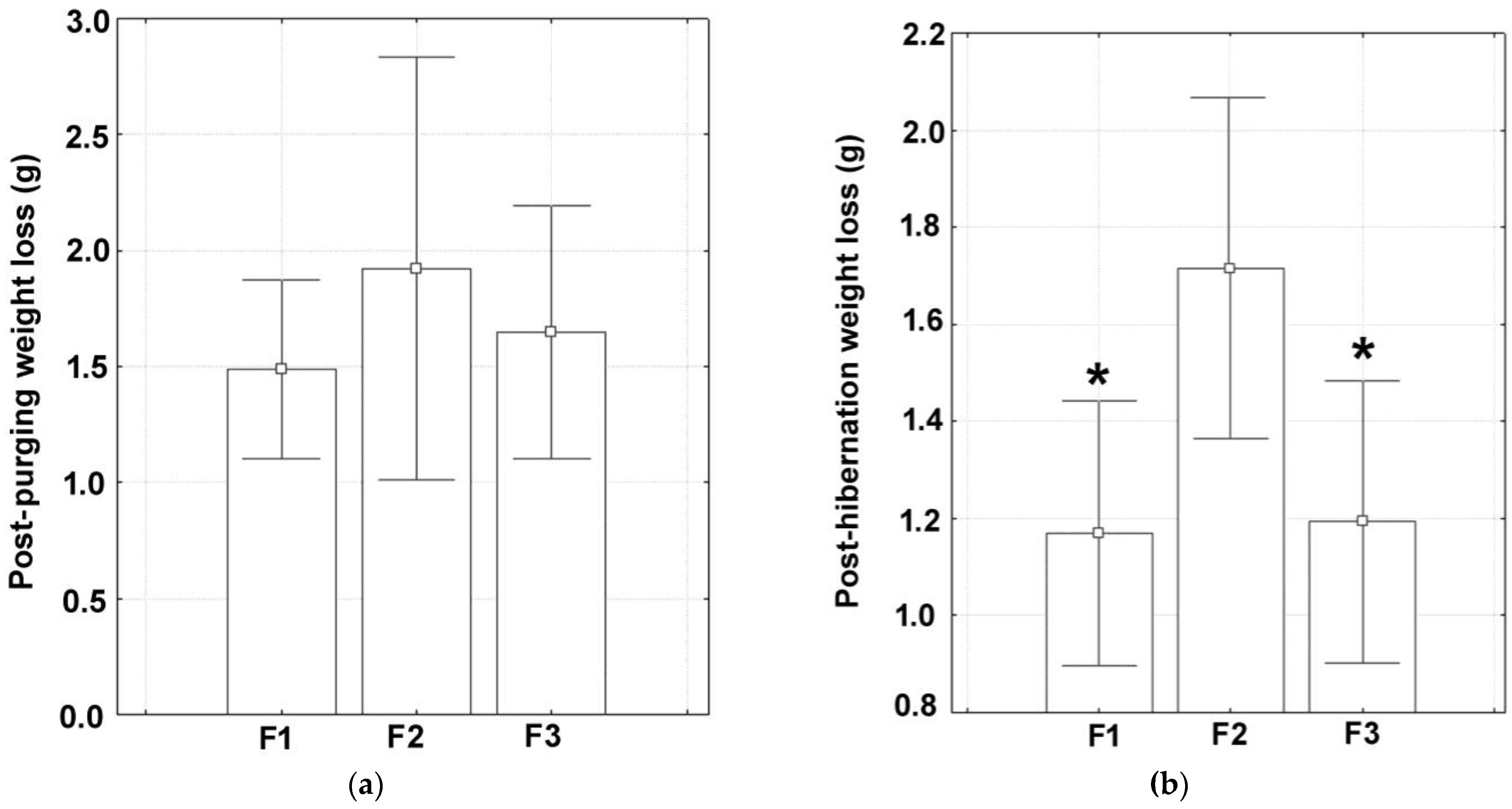
3.2. Body Weight Loss
3.3. Overwintering Survival
3.4. Mortality Factors
4. Discussion
4.1. Snail Gathering
4.2. Body Weight Loss
4.3. Overwintering Survival
4.4. Mortality Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gheoca, V. Can heliciculture act as a tool for edible land snails’natural populations’management in Romania? Manag. Sustain. Dev. 2013, 5, 21–25. [Google Scholar] [CrossRef]
- Nica, D. Researches Regarding the Morphophysiology of the Gastropods and the Farming of the Little Grey Snail (Helix aspersa Muller) in the Pedoclimatic Conditions of Our Country. Ph.D. Thesis, University of Life Sciences “King Mihai I” from Timișoara, Timişoara, Romania, 2009. [Google Scholar]
- Mvodo Meyo, E.S.; Nkemasong, Z.A.; Shu, G.; Ngono, J.P.N.; Ngosong, C. Snail farming as an alternative profitable livestock system for sustainable development. In Sustainable Development in Africa: Fostering Sustainability in One of the World’s Most Promising Continents, 1st ed.; Filho, W.L., Pretorius, R., de Sousa, L.O., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 477–490. [Google Scholar]
- Avagnina, G. Snail Breeding, 4th ed.; International Snail Breeding Institute: Cherasco, Italy, 2012; pp. 12–70. [Google Scholar]
- Rygało-Galewska, A.; Zglińska, K.; Niemiec, T. Edible snail production in Europe. Animals 2022, 12, 2732. [Google Scholar] [CrossRef] [PubMed]
- Manea, D.; Ienciu, A.A.; Ștef, R.; Peț, I.; Șmuleac, L.; Grozea, I.; Cărăbeț, A.; Drăghici, G.A.; Nica, D.V. The “sandwich” system: A potential solution for protecting overwintering Cornu aspersum snails reared in semi-intensive heliciculture farms in colder climates. Animals 2021, 11, 1420. [Google Scholar] [CrossRef]
- Apostolou, K.; Staikou, A.; Sotiraki, S.; Hatziioannou, M. An assessment of snail-farm systems based on land use and farm components. Animals 2021, 11, 272. [Google Scholar] [CrossRef]
- Drăghici, G.A.; Dehelean, C.; Susan, R.; Berceanu-Văduva, D.; Nica, D. Indoor Hibernation of Helix aspersa Juveniles. In Invertebrates-Ecophysiology and Management; Ray, S., Diarte-Plata, G., Escamilla-Montes, R., Eds.; IntechOpen: London, UK, 2020; p. 3. [Google Scholar]
- Ansart, A.; Vernon, P. Cold hardiness abilities vary with the size of the land snail Cornu aspersum. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 139, 205–211. [Google Scholar] [CrossRef]
- Baur, A.; Baur, B. The effect of hibernation position on winter survival of the rock-dwelling land snails Chondrina clienta and Balea perversa on Öland, Sweden. J. Molluscan Stud. 1991, 57, 331–336. [Google Scholar] [CrossRef]
- Lazaridou-Dimitriadou, M.; Alpoyanni, E.; Baka, M.; Brouziotis, T.H.; Kifonidis, N.; Mihaloudi, E.; Vellis, G. Growth, mortality and fecundity in successive generations of Helix aspersa Müller cultured indoors and crowding effects on fast-, medium-, and slow-growing snails of the same clutch. J. Molluscan Stud. 1998, 64, 67–74. [Google Scholar] [CrossRef]
- Gomot, P.; Gomot, L. Étude exploratoire de la spermatogenèse induite par la chaleur chez l’escargot Helix aspersa en hibernation: Rôle du cerveau. Invertebr. Reprod. Dev. 1989, 16, 23–32. [Google Scholar] [CrossRef]
- Daguzan, J.; Bonnet, J.C.; Perrin, Y.; Perrin, E.; Rouet, H. Contribution à l’élevage de l’escargot Petit-Gris: Helix aspersa Müller (Mollusque Gastéropode Pulmoné Stylommatophore). I. - Reproduction et éclosion des jeunes, en bâtiment et en conditions thermohygrométriques contrôlées. Ann. Zootech. 1981, 30, 249–272. [Google Scholar] [CrossRef]
- Dupont-Nivet, M.; Mallard, J.; Bonnet, J.C.; Blanc, J.M. Quantitative genetics of growth traits in the edible snail, Helix aspersa Müller. Genet. Sel. Evol. 1997, 29, 571–587. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Dernekbaşi, S.; Sariipek, M.; Karayücel, S. The reproductive response of Cornu aspersum to different hibernation conditions. Molluscan Res. 2022, 42, 253–259. [Google Scholar] [CrossRef]
- Nicolai, A. The Impact of Diet Treatment on Reproduction and Thermophysiological Processes in the Land Snails Cornu aspersum and Helix pomatia. Ph.D. Thesis, Université Rennes 1a, Rennes, France, 2010. [Google Scholar]
- Drozd, Ł.; Ziomek, M.; Szkucik, K.; Paszkiewicz, W.; Maćkowiak-Dryka, M.; Bełkot, Z.; Gondek, M. Selenium, copper, and zinc concentrations in the raw and processed meat of edible land snails harvested in Poland. J. Vet. Res. 2017, 61, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Danilova, I.S.; Danilova, T.M. Veterinary and sanitary requirements for snail farms. Sci. Messenger LNU Vet. Med. Biotechnol. Ser. Vet. Sci. 2023, 25, 26–31. [Google Scholar] [CrossRef]
- Charrier, M.; Daguzan, J. Food consumption, production, and energy evaluation in Helix aspersa Müller (a terrestrial pulmonated gastropod). Proc. Ann. Nutr. Aliment. 1980, 34, 147–166. [Google Scholar]
- Bonnet, J.C.; Aupinel, P.; Vrillon, J.L. L’escargot Helix aspersa Biologie—Élevage; Editions Quae: Versailles, France, 2019; pp. 8–45. [Google Scholar]
- Bura, M. Creşterea Melcilor o Activitate Profitabilă, 1st ed.; Eurobit: Timişoara, Romania, 2004; pp. 5–50. [Google Scholar]
- Romano, G. Manuale di Elicicoltura; Edizioni Coclè: Piana di Monteverna, Italy, 2017; pp. 27–88. [Google Scholar]
- Grilla, A.; LaJeunesse, C.; McMaster, D.; Morgan, D. Feasibility of Snail Farming as a Model for Small Urban Farms to Expand into Niche Markets for Increased Profitability. Bachelor’s Thesis, Worchester Polytechnic Institute, Worchester, UK, 2016. [Google Scholar]
- Hatziioannou, M.; Issari, A.; Neofitou, C.; Aifadi, S.; Matsiori, S. Economic analysis and production techniques of snail farms in southern Greece. World J. Agric. Res. 2014, 2, 276–279. [Google Scholar]
- Tanyitiku, M.N.; Nicholas, G.; Sullivan, J.J.; Njombissie Petcheu, I.C.; On, S.L. Snail meat consumption in Buea, Cameroon: The methodological challenges in exploring its public health risks. Int. J. Qual. Methods 2022, 21, 16094069221078132. [Google Scholar] [CrossRef]
- Nica, D.V.; Draghici, G.A.; Andrica, F.M.; Popescu, S.; Coricovac, D.E.; Dehelean, C.A.; Herman, H.; Tsatsakis, A. Short-term effects of very low-dose cadmium feeding on copper, manganese, and iron homeostasis: A gastropod perspective. Environ. Toxicol. Pharmacol. 2019, 65, 9–13. [Google Scholar] [CrossRef]
- Popețiu, R.O.; Donath-Miklos, I.; Borta, S.M.; Rus, L.A.; Vîlcea, A.; Nica, D.V.; Pușchiță, M. Serum YKL-40 levels, leukocyte profiles, and acute exacerbations of advanced COPD. J. Clin. Med. 2023, 12, 6106. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Duman, M.B.; Sarıipek, M.; Gören, G.U.; Öztürk, D.K.; Karayücel, S. Growth and mortality rates of Cornu aspersum: Organic snail culture system, Black Sea region. J. Agric. Sci. 2018, 25, 189–196. [Google Scholar] [CrossRef]
- Ligaszewski, M.; Lysak, A. Growth of Helix aspersa in laboratory culture. Folia Malacol. 2000, 8, 291–298. [Google Scholar]
- Şereflişan, H.; Duysak, Ö. Hibernation period in some land snail species (Gastropoda: Helicidae): Epiphragmal structure and hypometabolic behaviors. Turk. J. Agric. Food Sci. Technol. 2021, 9, 166–171. [Google Scholar] [CrossRef]
- Tkachenko, I.; Tkachenko, S.; Dedkov, V. How diets and the environment influence the weight of Roman snails in captivity. E3S Web Conf. 2020, 222, 02046. [Google Scholar] [CrossRef]
- Massari, S.; Pastore, S. Heliciculture and snail caviar: New trends in the food sector. In Commodity in Research and Practice—Future Trends and Challenges in the Food Sector; Polish Society of Commodity Science: Cracow, Poland, 2014; pp. 79–90. [Google Scholar]
- Elmslie, L.J. Snail collection and small-scale production in Africa and Europe. In Ecological Implications of Minilivestock: Potential of Insects, Rodents, Frogs, and Zsnails, 1st ed.; Paolletti, M.G., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 93–121. [Google Scholar]
- Daguzan, J. Contribution à l’élevage de l’escargot Petit-Gris: Helix aspersa Müller (Mollusque Gastéropode Pulmoné Stylommatophore). III—Élevage mixte (reproduction en bâtiment contrôlé et engraissement en parc extérieur), activité des individus et évolution de la population juvénile selon la charge biotique du parc. Ann. Zootech. 1985, 34, 127–148. [Google Scholar]
- Daguzan, J. Contribution à l’élevage de l’escargot Petit-Gris: Helix aspersa Müller (Mollusque Gastéropode Pulmoné Stylommatophore). II—Évolution de la population juvénile de l’éclosion à l’âge de 12 semaines, en bâtiment et en conditions d’élevage contrôlées. Ann. Zootech. 1982, 31, 87–110. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Ge, H.; Fazio, P.; Chen, G. Determination of optimum insulation thickness of exterior wall with moisture transfer in hot summer and cold winter zone of China. Procedia Eng. 2015, 121, 1008–1015. [Google Scholar] [CrossRef]
- Hebbal, B.; Marif, Y.; Hamdani, M.; Belhadj, M.M.; Bouguettaia, H.; Bechki, D. The geothermal potential of underground buildings in hot climates: Case of Southern Algeria. Case Stud. Therm. Eng. 2021, 28, 101422. [Google Scholar]
- Ansart, A.; Vernon, P.; Daguzan, J. Elements of cold hardiness in a littoral population of the land snail Helix aspersa (Gastropoda: Pulmonata). J. Comp. Physiol. B 2002, 172, 619–625. [Google Scholar] [CrossRef]
- Elmslie, L.J. Snail farming in field pens in Italy. Br. Crop Prot. Counc. Monogr. 1989, 41, 19–25. [Google Scholar]
- Penazzi, G. Helix aspersa: Potenziali proprietà ed usi in cosmesi del secreto di Helix aspersa o bava di lumaca. Cosmet. Technol. 2010, 13, 25–28. [Google Scholar]
- Murgia, G.; Loi, F.; Cappai, S.; Cogoni, M.P. Entomological investigation of the main entomatic adversities for terrestrial gastropods Helix aspersa Müller (Mollusca Gastropoda Pulmonata): A preliminary study in Sardinian heliciculture farms. Insects 2022, 13, 660. [Google Scholar] [CrossRef]
- Symondson, W.O. Coleoptera (Carabidae, Staphylinidae, Lampyridae, Drilidae and Silphidae) as predators of terrestrial gastropods. In Natural Enemies of Terrestrial Molluscs; CABI: Wallingford, UK, 2004; pp. 37–84. [Google Scholar]
- Potts, D. Persistence and extinction of local populations of the garden snail Helix aspersa in unfavorable environments. Oecologia. 1975, 21, 313–334. [Google Scholar] [CrossRef] [PubMed]
- Ligaszewski, M.; Pol, P. Reproduction of the Roman snail (Helix pomatia L.) from a local natural population in farm conditions and in a natural habitat. Ann. Anim. Sci. 2021, 21, 693–708. [Google Scholar] [CrossRef]
- Němec, T.; Horsák, M. Specific damage recognized on land snail shells as a tool for studying predation intensity: Differences related to habitat and predator types. Contrib. Zool. 2019, 88, 277–296. [Google Scholar] [CrossRef]
- Baalbergen, E.; Helwerda, R.; Schelfhorst, R.; Castillo Cajas, R.F.; van Moorsel, C.H.M.; Kundrata, R.; Welter-Schultes, F.W.; Giokas, S.; Schilthuizen, M. Predator-prey interactions between shell-boring beetle larvae and rock-dwelling land snails. PLoS ONE 2014, 9, e100366. [Google Scholar] [CrossRef]

| Breeding Pen | Snail Number | Work Hours | |
|---|---|---|---|
| Population | Harvesting | ||
| BP1 * | 1250 | 1054 (84.32%) | 14 |
| BP2 * | 1267 | 1083 (85.48%) | 18 |
| BP3 * | 1287 | 1120 (87.02%) | 20 |
| BP4 | 1234 | 1082 (87.68%) | 23 |
| BP5 | 1289 | 1104 (85.65%) | 28 |
| BP6 | 1271 | 1081 (85.05%) | 25 |
| F1 Farm | F2 Farm | F3 Farm | |||
|---|---|---|---|---|---|
| Shell Height | Body Weight | Shell Height | Body Weight | Shell Height | Body Weight |
| 25 | 8.23 | 27 | 9.15 | 31 | 11.57 |
| 26 | 10.94 | 28 | 9.73 | 29 | 12.12 |
| 32 | 11.27 | 25 | 8.43 | 27 | 12.45 |
| 29 | 8.58 | 24 | 11.72 | 29 | 10.94 |
| 22 | 11.22 | 31 | 11.54 | 22 | 12.13 |
| 29 | 10.31 | 33 | 9.04 | 27 | 9.05 |
| 27 | 10.82 | 28 | 12.33 | 29 | 12.35 |
| 26 | 11.41 | 32 | 12.86 | 33 | 9.47 |
| 30 | 9.02 | 30 | 10.65 | 26 | 8.56 |
| 32 | 8.77 | 26 | 8.29 | 25 | 11.29 |
| 31 | 10.26 | 30 | 10.91 | 26 | 11.41 |
| 25 | 11.38 | 34 | 9.06 | 31 | 10.02 |
| 27 | 8.42 | 28 | 10.11 | 29 | 10.27 |
| 29 | 11.28 | 34 | 12.03 | 26 | 9.33 |
| 31 | 10.03 | 22 | 11.08 | 30 | 9.29 |
| 28.07 (2.94) | 10.13 (1.20) | 28.80 (3.65) | 10.46 (1.47) | 28.01 (2.80) | 10.68 (1.33) |
| F1 Farm | F2 Farm | F3 Farm | |
|---|---|---|---|
| Temperature (°C) | 3 °C (±2 °C) | 3 °C (±5 °C) | 4 °C (±2 °C) |
| Photoperiod (dark hours: light hours) | 24 h:0 h | natural photoperiod | 16 h:8 h |
| Relative humidity (%) | 70% (±5%) | 50% (±20%) | 65% (±8%) |
| Number of snails (pre-purging) | 6524 | 8602 | 2485 |
| Mortalities (post-purging) | 78 | 96 | 29 |
| Post-purging mortalities (%) | 1.20% | 1.12% | 1.17% |
| Number of snails alive post-purging | 6446 | 8506 | 2456 |
| Post-hibernation mortalities | 1418 | 2637 | 509 |
| Number of snails alive post-hibernation | 5106 | 5965 | 1976 |
| Overwinter survival | 78.26% | 69.34% | 79.52% |
| Overwintering duration (days) | 116 | 110 | 105 |
| F1 Farm | F2 Farm | F3 Farm | |
|---|---|---|---|
| Intact shell | 1023 | 2522 | 353 |
| Smashed shell | 254 | 32 | 34 |
| Small holes | 141 | 83 | 122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștef, R.; Manea, D.; Ienciu, A.A.; Onișan, E.; Nica, D.V.; Cărăbeț, A. Integrating Indoor Hibernation into the Italian Outdoor Snail Farming System: A Potential Solution for Colder Climates. Animals 2025, 15, 914. https://doi.org/10.3390/ani15070914
Ștef R, Manea D, Ienciu AA, Onișan E, Nica DV, Cărăbeț A. Integrating Indoor Hibernation into the Italian Outdoor Snail Farming System: A Potential Solution for Colder Climates. Animals. 2025; 15(7):914. https://doi.org/10.3390/ani15070914
Chicago/Turabian StyleȘtef, Ramona, Dan Manea, Anișoara Aurelia Ienciu, Emilian Onișan, Dragoș Vasile Nica, and Alin Cărăbeț. 2025. "Integrating Indoor Hibernation into the Italian Outdoor Snail Farming System: A Potential Solution for Colder Climates" Animals 15, no. 7: 914. https://doi.org/10.3390/ani15070914
APA StyleȘtef, R., Manea, D., Ienciu, A. A., Onișan, E., Nica, D. V., & Cărăbeț, A. (2025). Integrating Indoor Hibernation into the Italian Outdoor Snail Farming System: A Potential Solution for Colder Climates. Animals, 15(7), 914. https://doi.org/10.3390/ani15070914








