Seasonal Spatial Distribution Patterns of Abralia multihamata in the East China Sea Region: Predictions Under Various Climate Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Survey Procedures
2.2. Ensemble Model, Selection of Environmental Variables, and Calibrations
3. Results
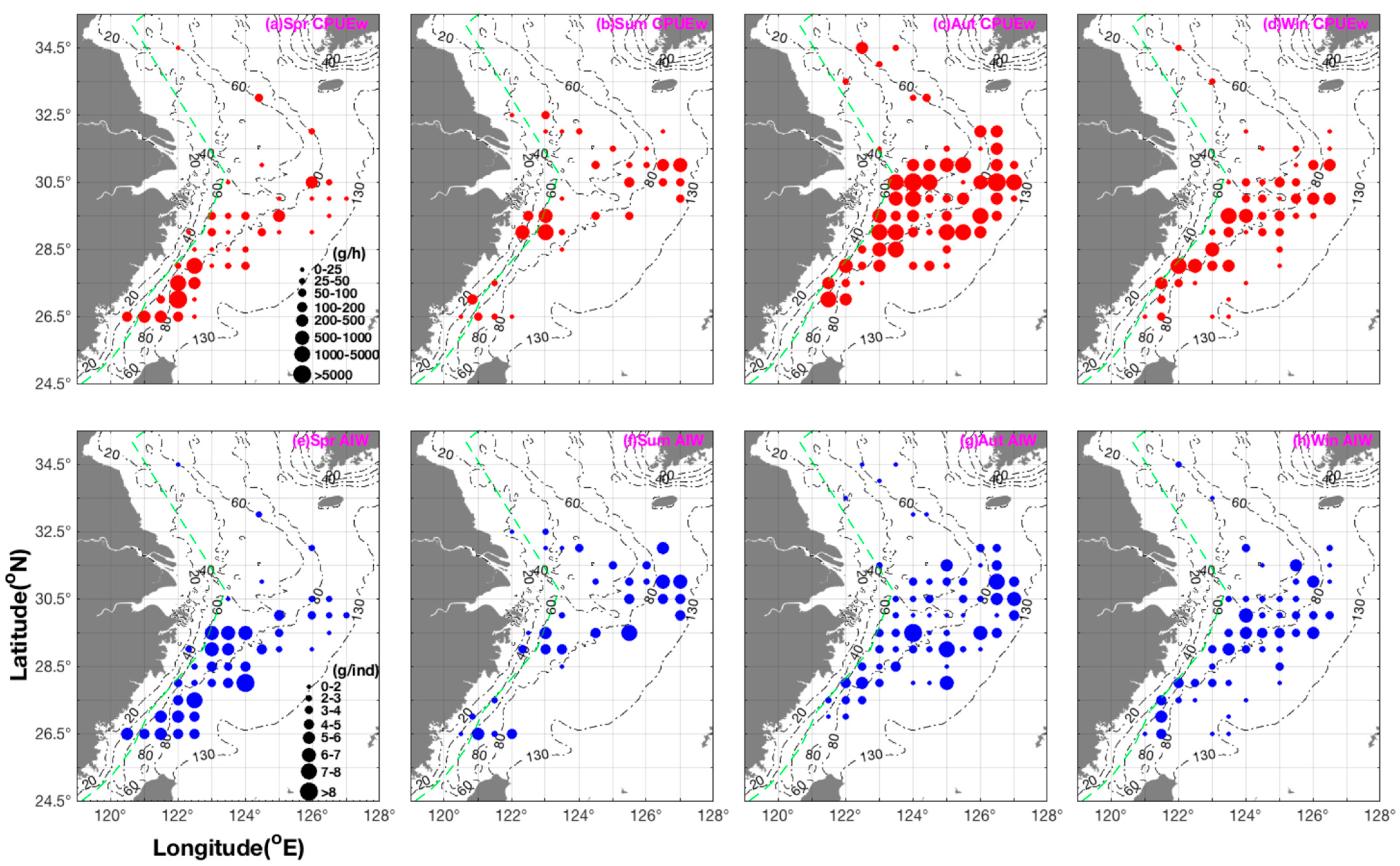
3.1. Seasonal Spatial Distribution Characteristics of Biomass, Number, and Size
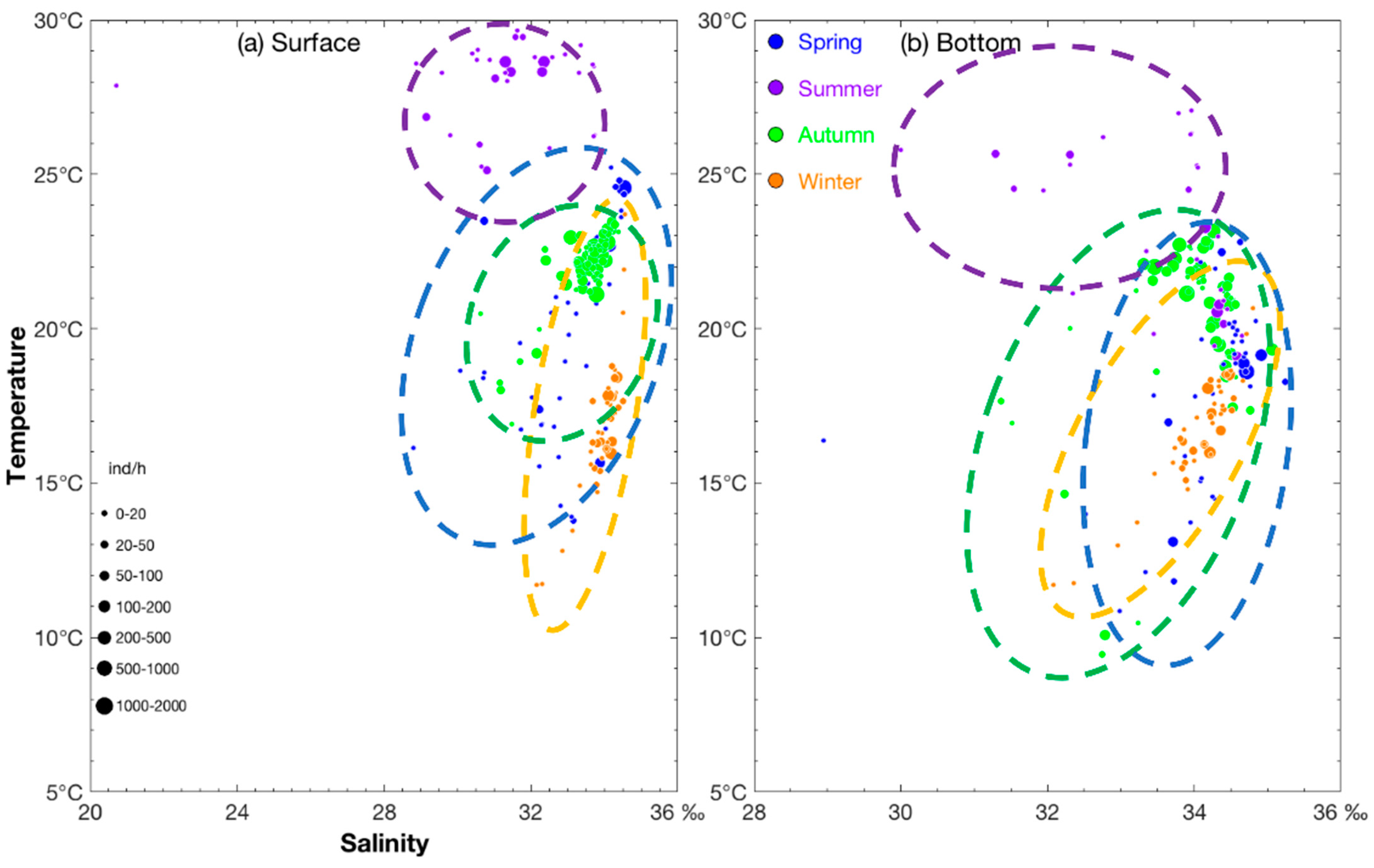
3.2. Seasonal Variations of Measured Environmental Factors
3.3. Calibration of Algorithms, and Predictions Under Climate Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Quere, C. Trends in the land and ocean carbon uptake. Curr. Opin. Environ. Sustain. 2010, 2, 219–224. [Google Scholar] [CrossRef]
- Keeling, R. The Keeling Curve. Univ. Calif., San Diego. 2016. Available online: https://scripps.ucsd.edu/programs/keelingcurve (accessed on 10 January 2025).
- IPCC (Intergov. Panel Clim. Change). Summary for policymakers. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 1–30. [Google Scholar]
- Zhu, Y.; Lin, Y.; Chu, J.; Kang, B.; Reygondeau, G.; Zhao, Q.; Zhang, A.; Wang, Y.; Cheung, W.W.L. Modelling the variation of demersal fish distribution in Yellow Sea under climate change. J. Oceanol. Limnol. 2022, 40, 1544–1555. [Google Scholar] [CrossRef]
- Dawe, E.G.; Warren, W.G. Recruitment of short-finned squid in the Northwest Atlantic Ocean and some environmental relationships. J. Cephalopod Biol. 1993, 2, 1–21. [Google Scholar]
- Ma, S.; Liu, Y.; Li, J.; Fu, C.; Ye, Z.; Sun, P.; Yu, H.; Cheng, J.; Tian, Y. Climate-induced long-term variations in ecosystem structure and atmosphere-ocean-ecosystem processes in the Yellow Sea and East China Sea. Prog. Oceanogr. 2019, 175, 183–197. [Google Scholar] [CrossRef]
- Fernandes Guerreiro, M.; Oliveira Borges, F.; Pereira Santos, C.; Rosa, R. Future distribution patterns of nine cuttlefish species under climate change. Mar. Biol. 2023, 170, 159. [Google Scholar] [CrossRef]
- Rubaie, Z.M.; Idris, M.H.; Abu Hena, M.K.; King, W.S. Diversity of Cephalopod from Selected Division of Sarawak, Malaysia. Int. J. Adv. Sci. Eng. Inf. Technol. 2012, 2, 279–281. [Google Scholar] [CrossRef]
- Anusha, J.R.; Fleming, A.T. Cephalopod: Squid Biology, Ecology and Fisheries in Indian waters. Int. J. Fish. Aquat. Stud. 2014, 1, 41–50. [Google Scholar]
- Norman, M. Cephalopods: A WorldGuide; ConchBooks: Hackenheim, Germany, 2003; 320p. [Google Scholar]
- Santo, M.B.; Clarke, M.R.; Pierce, G.J. Assessing the importance of cephalopods in the diets of marine mammals and other top predators: Problems and solutions. Fish. Res. 2001, 52, 121–139. [Google Scholar] [CrossRef]
- Guerra, A.; Allcock, L.; Pereira, J. Cephalopod life history, ecology and fisheries: An introduction. Fish. Res. 2010, 106, 117–124. [Google Scholar] [CrossRef]
- Young, R.E.; Tsuchiya, K. Abralia Gray 1849. In The Tree of Life Web Project. Available online: http://tolweb.org/Abralia/19642 (accessed on 16 March 2025).
- Reid, S.B.; Hirota, J.; Young, R.E.; Hallacher, L.E. Mesopelagic-boundary community in Hawaii: Micronekton at the interface between neritic and oceanic ecosystems. Mar. Biol. 1991, 109, 427–440. [Google Scholar] [CrossRef]
- Guerra-Marrero, A.; Hernandez-García, V.; Sarmiento-Lezcano, A.; Jimenez-Alvarado, D.; Pino, A.S.D.; Castro, J.J. Migratory patterns, vertical distributions and diets of Abralia veranyi and Abraliopsis morisii (Cephalopoda: Enoploteuthidae) in the eastern North Atlantic. J. Molluscan Stud. 2020, 86, 27–34. [Google Scholar]
- Li, S.F.; Yan, L.P.; Li, H.Y.; Li, J.S.; Cheng, J.H. Spatial distribution of cephalopod assemblages in the region of the East China Sea. J. Fish. Sci. China 2006, 13, 936–944. (In Chinese) [Google Scholar]
- Dawe, E.G.; Colbourne, E.B.; Drinkwater, K.F. Environmental effects on recruitment of short-finned squid (Illex illecebrosus). ICES J. Mar. Sci. 2000, 57, 1002–1013. [Google Scholar] [CrossRef]
- Rodhouse, P.G. Managing and forecasting squid fisheries in variable environments. Fish. Res. 2001, 54, 3–8. [Google Scholar]
- Waluda, C.M.; Rodhouse, P.G.; Podesta, G.P.; Trathan, P.N.; Pierce, G.P. Surface oceanography of the inferred hatching grounds of Illex argentinus (Cephalopoda: Ommastrephidae) and influences on recruitment variability. Mar. Biol. 2001, 139, 671–679. [Google Scholar]
- Pierce, G.J.; Boyle, P.R. Empirical modelling of interannual trends in abundance of squid (Loligo forbesi) in Scottish waters. Fish. Res. 2003, 59, 305–326. [Google Scholar]
- Robin, J.P.; Denis, V. Squid stock fluctuations and water temperature: Temporal analysis of English Channel Loliginidae. J. Appl. Ecol. 1999, 36, 101–110. [Google Scholar] [CrossRef]
- Xavier, J.C.; Raymond, B.; Jones, D.C.; Griffiths, H. Biogeography of Cephalopods in the Southern Ocean Using Habitat Suitability Prediction Models. Ecosystems 2015, 19, 220–247. [Google Scholar] [CrossRef]
- Boavida-Portugal, J.; Guilhaumon, F.; Rosa, R.; Araújo, M.B. Global patterns of coastal cephalopod diversity under climate change. Front. Mar. Sci. 2022, 8, 740781. [Google Scholar]
- Alabia, I.D.; Molinos, J.G.; Saitoh, S.I.; Hirata, T.; Hirawake, T.; Mueter, F.J. Multiple facets of marine biodiversity in the Pacific Arctic under future climate. Sci. Total Environ. 2020, 744, 140913. [Google Scholar]
- Hodapp, D.; Roca, I.T.; Fiorentino, D.; Garilao, C.; Kaschner, K.; Kesner-Reyes, K.; Schneider, B.; Segschneider, J.; Kocsis, Á.T.; Kiessling, W.; et al. Climate change disrupts core habitats of marine species. Glob. Change Biol. 2023, 29, 3304–3317. [Google Scholar]
- Liu, S.; Liu, Y.; Alabia, I.D.; Tian, Y.; Ye, Z.; Yu, H.; Li, J.; Cheng, J. Impact of Climate Change on Wintering Ground of Japanese Anchovy (Engraulis japonicus) Using Marine Geospatial Statistics. Front. Mar. Sci. 2020, 7, 604. [Google Scholar]
- Liu, S.; Liu, Y.; Teschke, K.; Hindell, M.A.; Downey, R.; Woods, B.; Kang, B.; Ma, S.; Zhang, C.; Li, J.; et al. Incorporating mesopelagic fish into the evaluation of conservation areas for marine living resources under climate change scenarios. Mar. Life. Sci. Technol. 2024, 6, 68–83. [Google Scholar] [CrossRef]
- Araujo, M.; New, M. Ensemble forecasting of species distributions. Trends. Ecol. Evol. 2007, 22, 42–47. [Google Scholar]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackere Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II 2023, 207, 105227. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling; R Package Version; 2024; Available online: https://biomodhub.github.io/biomod2/ (accessed on 3 March 2019).
- Alabia, I.D.; Saitoh, S.I.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Ensemble squid habitat model using three-dimensional ocean data. ICES J. Mar. Sci. 2016, 73, 1863–1874. [Google Scholar]
- Liu, S.; Liu, Y.; Xing, Q.; Li, Y.; Tian, H.; Luo, Y.; Ito, S.; Tian, Y. Climate change drives fish communities: Changing multiple facets of fish biodiversity in the Northwest Pacific Ocean. Sci. Total Environ. 2024, 955, 176854. [Google Scholar]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geoscientific Model Development. 2015, 9, 1937–1958. [Google Scholar]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar]
- Räty, O.; Räisänen, J.; Ylhäisi, J.S. Evaluation of delta change and bias correction methods for future daily precipitation: Intermodel cross-validation using ENSEMBLES simulations. Clim. Dyn. 2014, 42, 2287–2303. [Google Scholar]
- Beyer, R.; Krapp, M.; Manica, A. An empirical evaluation of bias correction methods for palaeoclimate simulations. Clim. Past. 2020, 16, 1493–1508. [Google Scholar]
- Cheung, W.W.L.; Brodeur, R.D.; Okey, T.A.; Pauly, D. Projecting future changes in distributions of pelagic fish species of Northeast Pacific shelf seas. Prog. Oceanogr. 2015, 130, 19–31. [Google Scholar]
- Berry, S.S. A note on the occurrence and habits of a luminous squid (Abralia veranyi) at Madeira. Biol. Bull. 1926, 51, 257–268. [Google Scholar]
- Sasaki, M. Observations on Hotaru-ika Watasenia scintillans. J. Coll. Agric. Tohoku Imp. Univ. Sapporo Jpn. 1914, 6, 75–107. [Google Scholar]
- Roper, C.F.E.; Sweeney, M.J.; Nauen, C.E. Cephalopods of the world. In FAO Fisheries Synopsis; FAO Species Catalogue; FAO: Rome, Italy, 1984; Volume 125, p. 273. [Google Scholar]
- Bigelow, K.A. Age and growth in paralarvae of the mesopelagic squid Abralia trigonura based on daily growth increments in statoliths. Mar. Ecol. Prog. Ser. 1992, 82, 31–40. [Google Scholar]
- Silas, E.G. Cephalopoda of the west coast of India collected during the cruises of the research vessel Varuna, with a catalogue of the species known from the Indian Ocean. In Proceedings of the Symposium on Mollusca, Bangalore, India, 12–16 January 1968; Marine Biological Association of India: Bangalore, India, 1968; pp. 277–359. [Google Scholar]
- Cabanellas-Reboredo, M.; Calvo-Manazza, M.; Palmer, M.; Hernández-Urcera, J.; Garci, M.E.; González, Á.F.; Guerra, Á.; Morales-Nin, B. Using artificial devices for identifying spawning preferences of the European squid: Usefulness and limitations. Fish. Res. 2014, 157, 70–77. [Google Scholar]
- Roberts, M.J. Chokka squid (Loligo vulgaris reynaudii) abundance linked to changes in South Africa’s Agulhas Bank ecosystem during spawning and the early life cycle. ICES J. Mar. Sci. 2005, 62, 33–55. [Google Scholar]
- Pecl, G.T.; Jackson, G.D. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Rev. Fish. Biol. Fish. 2008, 18, 373–385. [Google Scholar]
- Moriwaki, S. Ecology and fishing condition of Kensakisquid, Photololigo edulis, in the southwestern Japan Sea. Bull. Shimane Pref. Fish. Exp. Stat. 1994, 8, 1–111, (In Japanese with English Abstract). [Google Scholar]
- Young, R.E.; Mangold, K.M. Growth and reproduction in the mesopelagic-boundary squid Abralia trigonura. Mar. Biol. 1994, 119, 413–421. [Google Scholar]
- Miyahara, K.; Ota, T.; Kohno, N.; Ueta, Y.; Bower, J.R. Catch fluctuations of the diamond squid Thysanoteuthis rhombus in the Sea of Japan and models to forecast CPUE based on analysis of environmental factors. Fish. Res. 2005, 72, 71–79. [Google Scholar]
- Pang, Y.; Tian, Y.; Fu, C.; Wang, B.; Li, J.; Ren, Y.; Wan, R. Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 2018, 208, 22–33. [Google Scholar]
- Boavida-Portugal, J.; Rosa, R.; Calado, R.; Pinto, M.; Boavida-Portugal, I.; Araújo, M.B.; Guilhaumon, F. Climate change impacts on the distribution of coastal lobsters. Mar. Biol. 2018, 165, 186. [Google Scholar]
- Borges, F.O.; Guerreiro, M.; Santos, C.P.; Paula, J.R.; Rosa, R. Projecting future climate change impacts on the distribution of the ‘Octopus vulgaris species complex’. Front. Mar. Sci. 2022, 9, 1018766. [Google Scholar]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar]
- Xu, M.; Yang, L.; Liu, Z.; Zhang, Y.; Zhang, H. Seasonal and spatial distribution characteristics of Sepia esculenta in the East China Sea Region: Transfer of the central distribution from 29° N to 28° N. Animals 2024, 14, 1412. [Google Scholar] [CrossRef]
- Xu, M.; Feng, W.J.; Liu, Z.; Li, Z.G.; Song, X.J.; Zhang, H.; Zhang, C.L.; Yang, L.L. Seasonal-spatial distribution variations and predictions of Loliolus beka and Loliolus uyii in the East China Sea region: Implications from climate change scenarios. Animals 2024, 14, 2070. [Google Scholar] [CrossRef]
- Xu, M.; Liu, S.; Zhang, H.; Li, Z.; Song, X.; Yang, L.; Tang, B. Seasonal analysis of spatial distribution patterns and characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region. Animals 2024, 14, 2716. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Cui, Y.; Liu, S. Seasonal spatial distribution patterns of AmphiOctopus ovulum in the East China Sea: Current status future projections under various climate change scenarios. Front. Mar. Sci. 2025, 12. [Google Scholar]
- Yang, L.; Xu, M.; Liu, Z.; Zhang, Y.; Cui, Y.; Li, S. Seasonal and spatial distribution of AmphiOctopus fangsiao and Octopus variabilis in the southern Yellow and East China Seas: Fisheries management implications based on climate scenario predictions. Reg. Stud. Mar. Sci. 2025, 83, 104072. [Google Scholar]
- Torrejón-Magallanes, J.; Ángeles-González, L.E.; Csirke, J.; Bouchon, M.; Morales-Bojórquez, E.; Arreguín-Sánchez, F. Modeling the Pacific chub mackerel (Scomber japonicus) ecological niche and future scenarios in the northern Peruvian Current System. Prog. Oceanogr. 2021, 197, 102672. [Google Scholar] [CrossRef]

| Factor | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Depth (m) | 23.00–118.00 | 16.00–97.00 | 21.00–107.00 | 39.00–145.00 |
| SST (°C) | 13.77–25.23 | 25.14–29.67 | 16.91–23.66 | 11.68–23.71 |
| SBT (°C) | 10.85–22.79 | 18.87–27.06 | 9.47–23.15 | 11.68–20.67 |
| SSS (‰) | 28.80–34.53 | 20.72–33.74 | 30.64–34.38 | 32.16–34.55 |
| SBS (‰) | 28.95–35.25 | 30.00–34.65 | 31.37–35.07 | 32.08–34.80 |
| SSDO (mg/L) | 7.84–8.43 | 4.51–10.53 | / | 6.92–8.62 |
| SBDO (mg/L) | 7.76–8.98 | 2.73–6.60 | / | 7.30–8.58 |
| Mean CPUEw at collection stations (g/h) | 362.55 | 171.08 | 791.69 | 174.37 |
| Value range of CPUEw (g/h) | 1.34–10,184.0 | 10.80–1405.76 | 1.62–6064.59 | 0.60–1914.40 |
| Mean CPUEn at collection stations (ind/h) | 78.31 | 41.06 | 248.39 | 50.65 |
| Value range of CPUEn (ind/h) | 1.00–1920.00 | 3.00–285.00 | 1.00–1764.00 | 1.00–496.00 |
| Mean AIW (g/ind) | 3.99 | 3.71 | 3.64 | 3.04 |
| Value range of AIW (g/ind) | 1.16–8.34 | 1.20–7.80 | 0.90–8.00 | 0.60–6.50 |
| Case | Loss% | Gain% | Gain% − Loss% |
|---|---|---|---|
| SSP126–2050 | −2.89% | 3.61% | 0.72% |
| SSP126–2100 | −5.56% | 2.60% | −2.96% |
| SSP245–2050 | −1.87% | 3.59% | 1.72% |
| SSP245–2100 | −4.09% | 9.15% | 5.06% |
| SSP370–2050 | −0.77% | 10.50% | 9.72% |
| SSP370–2100 | −6.79% | 9.69% | 2.90% |
| SSP585–2050 | −1.96% | 3.97% | 2.00% |
| SSP585–2100 | −16.95% | 7.81% | −9.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, S.; Yang, C.; Yang, L. Seasonal Spatial Distribution Patterns of Abralia multihamata in the East China Sea Region: Predictions Under Various Climate Scenarios. Animals 2025, 15, 903. https://doi.org/10.3390/ani15070903
Xu M, Liu S, Yang C, Yang L. Seasonal Spatial Distribution Patterns of Abralia multihamata in the East China Sea Region: Predictions Under Various Climate Scenarios. Animals. 2025; 15(7):903. https://doi.org/10.3390/ani15070903
Chicago/Turabian StyleXu, Min, Shuhao Liu, Chunhui Yang, and Linlin Yang. 2025. "Seasonal Spatial Distribution Patterns of Abralia multihamata in the East China Sea Region: Predictions Under Various Climate Scenarios" Animals 15, no. 7: 903. https://doi.org/10.3390/ani15070903
APA StyleXu, M., Liu, S., Yang, C., & Yang, L. (2025). Seasonal Spatial Distribution Patterns of Abralia multihamata in the East China Sea Region: Predictions Under Various Climate Scenarios. Animals, 15(7), 903. https://doi.org/10.3390/ani15070903





