Bacterial Community Composition and Its Relationship with Environmental Factors in the Artificial Reef Area for Marine Ranching in Changhai County
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
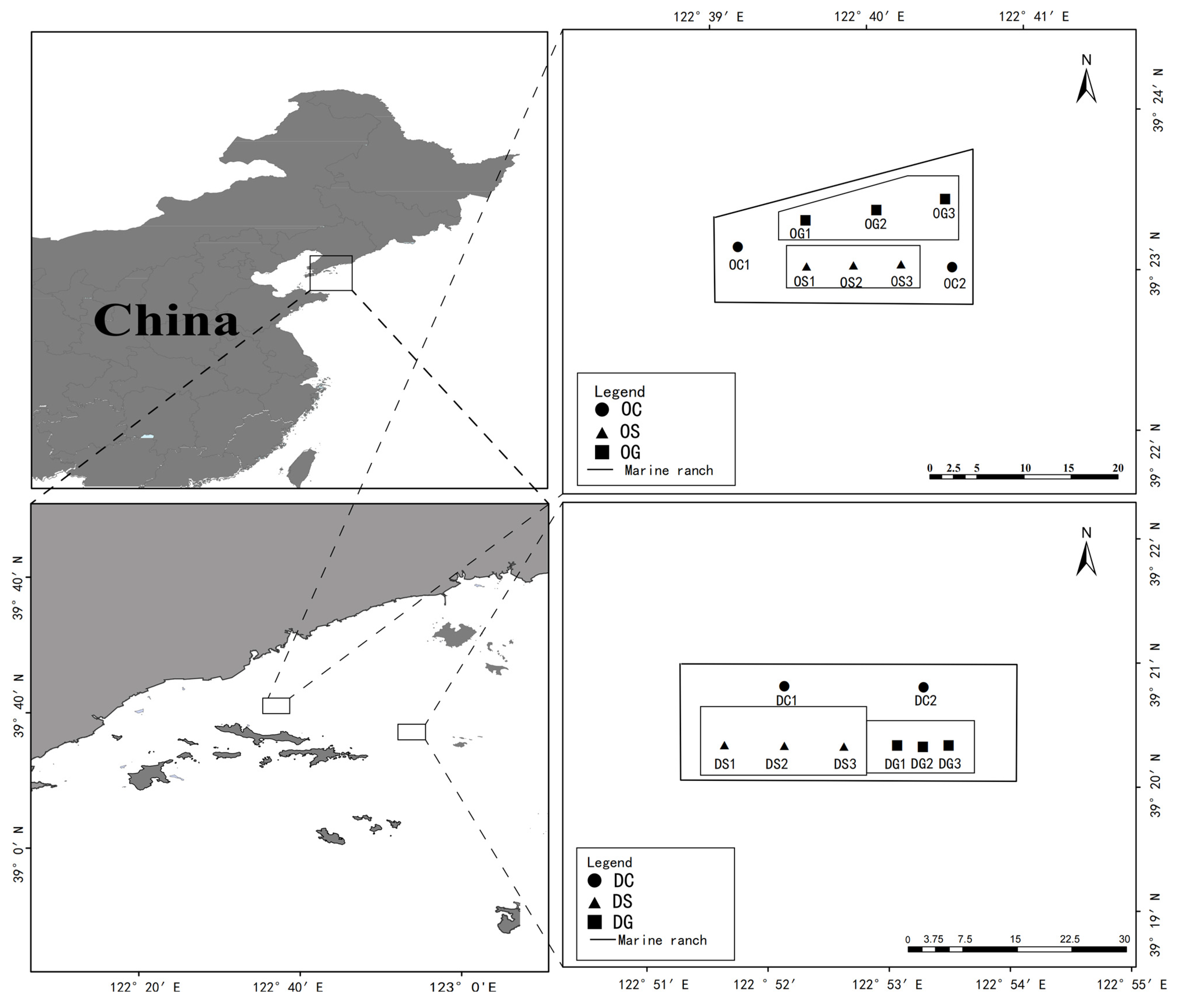
2.1. Sample Collection
2.2. Environmental Factor Measurements
2.3. DNA Extraction and PCR Amplification
2.4. Statistical Analyses
3. Results
3.1. Changes in Physicochemical Characteristics of Overlying Water and Sediment
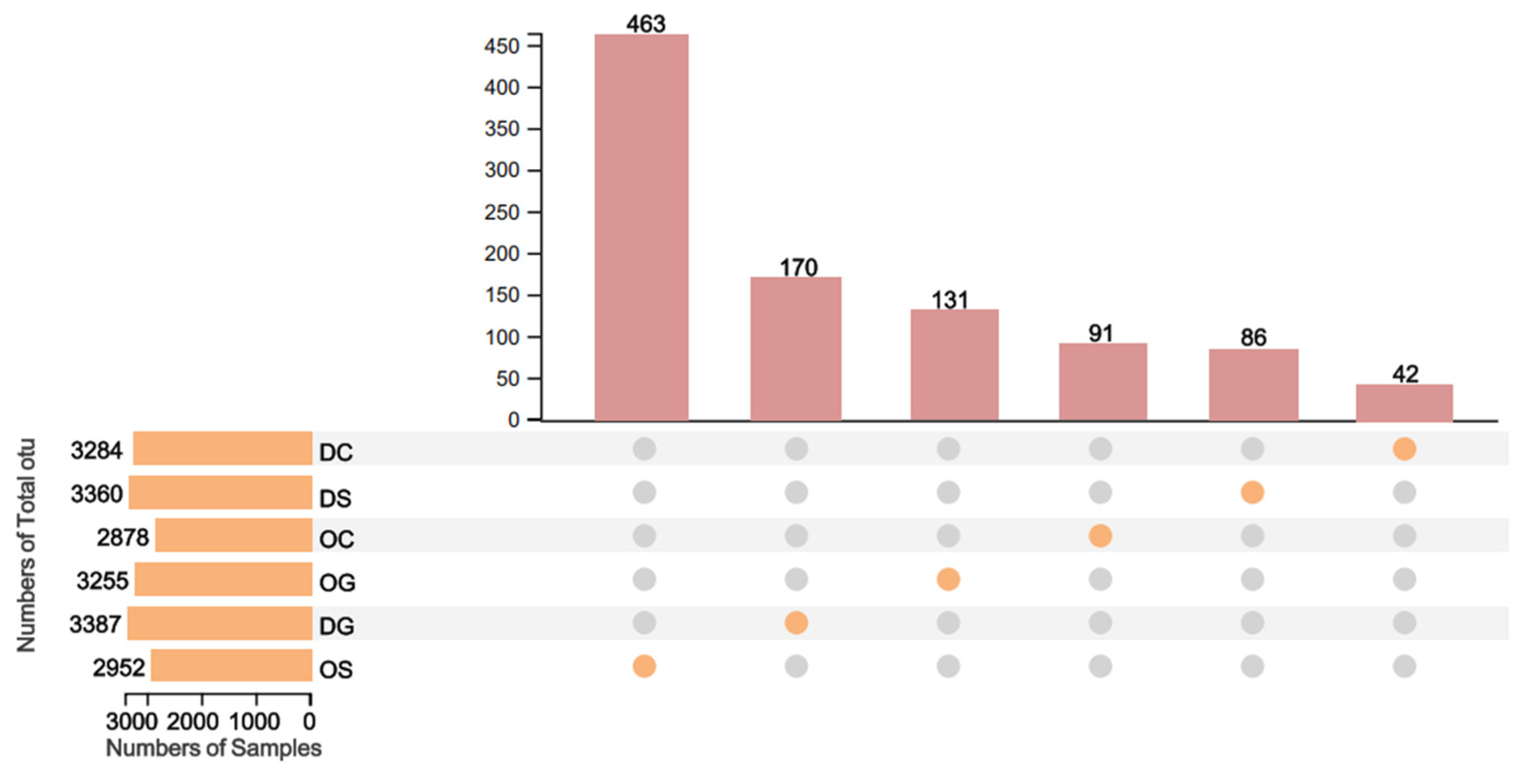
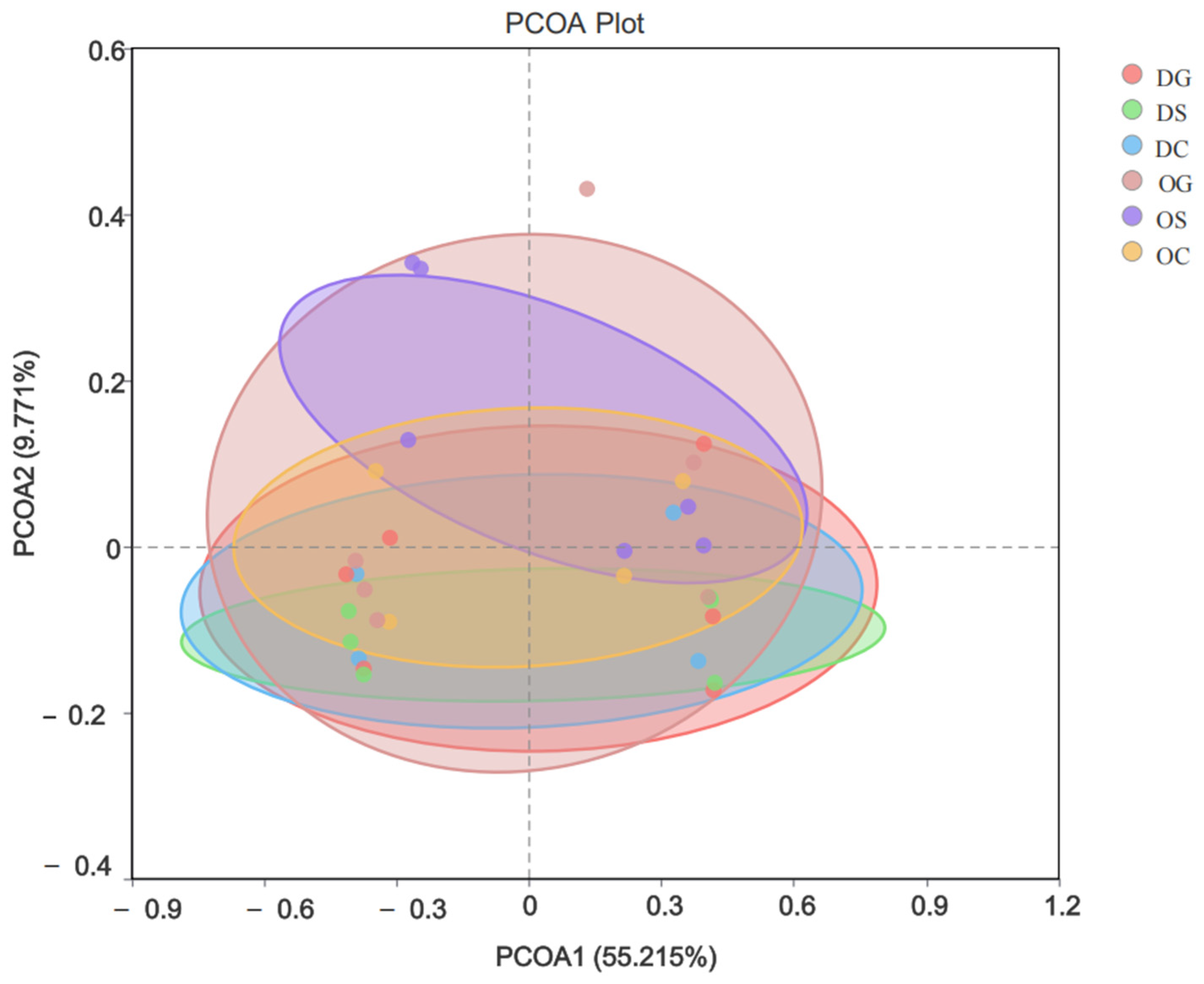
3.2. Bacterial Diversity Analysis
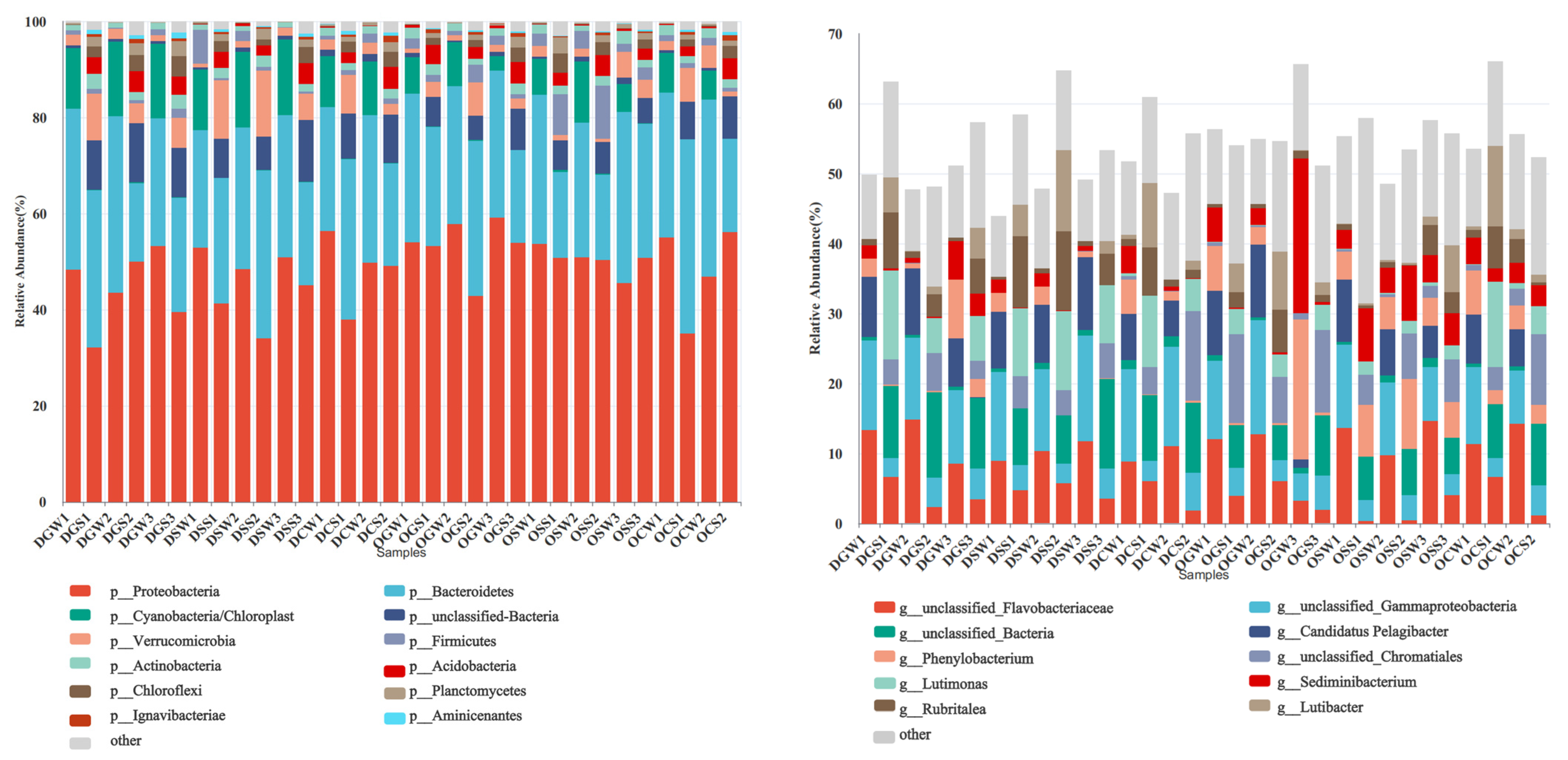
3.3. Compositional and Structural Analysis of Bacterial Communities
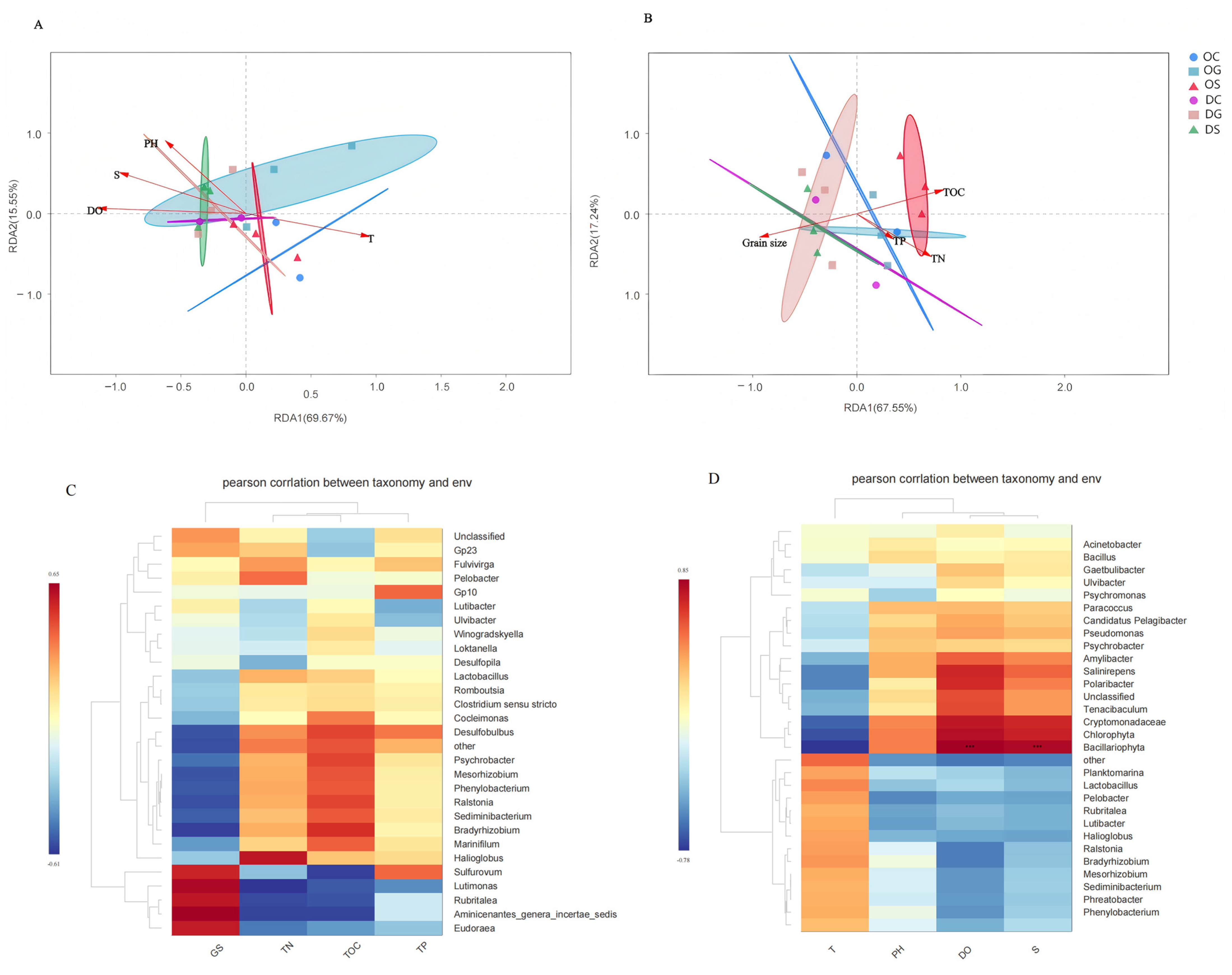
3.4. Relationship Between Bacterial Communities and Environmental Factors
4. Discussion
4.1. Marine Ranching Environmental Factors
4.2. Characteristics of Bacterial Community Composition
4.3. Main Drivers of Bacterial Community Structure Changes in Marine Environments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, H.S. Construction of marine ranching in China: Reviews and prospects. J. Fish. China 2016, 40, 1133–1140. [Google Scholar]
- Kitada, S. Economic, ecological and genetic impacts of marine stock enhancement and sea ranching: A systematic review. Fish Fish. 2018, 19, 511–532. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, P. Current Situation, Problems and Countermeasures of Marine Ranching Development in Guangdong Province, China. Asian Agric. Res. 2022, 49, 141–154. [Google Scholar]
- Plagányi, É. Climate change impacts on fisheries. Science 2019, 363, 930–931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, D.; Zhang, X.; Yan, H. Regional ecological efficiency and future sustainable development of marine ranch in China: Anempirical research using DEA and system dynamics. Aquaculture 2021, 534, 736339. [Google Scholar] [CrossRef]
- Qin, M.; Wang, X.; Du, Y.; Wan, X. Influencing factors of spatial variation of national marine ranching in China. Ocean Coast. Manag. 2021, 199, 105407. [Google Scholar] [CrossRef]
- Rouse, S.; Porter, J.S.; Wilding, T.A. Artificial reef design affects benthic secondary productivity and provision of functional habitat. Ecol. Evol. 2020, 10, 2122–2130. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, Z.Z.; Chen, P.M.; Yu, J.; Chen, G.B. Optimization of fishery resources assessment methods and ecological effects evaluation of artificial reefs. Mar. Biol. Res. 2021, 17, 72–85. [Google Scholar] [CrossRef]
- Vivier, B.; Dauvin, J.-C.; Navon, M.; Rusig, A.-M.; Mussio, I.; Orvain, F.; Boutouil, M.; Claquin, P. Marine artificial reefs, a meta-analysis of their design, objectives and effectiveness. Glob. Ecol. Conserv. 2021, 27, e01538. [Google Scholar] [CrossRef]
- Cardenas-Rojas, D.; Mendoza, E.; Escudero, M.; Verduzco-Zapata, M. Assessment of the performance of an artificial reef made of modular elements through small scale experiments. J. Mar. Sci. Eng. 2021, 9, 130. [Google Scholar] [CrossRef]
- Lemoine, H.R.; Paxton, A.B.; Anisfeld, S.C.; Rosemond, R.C.; Peterson, C.H. Selecting the optimal artificial reefs to achieve fish habitat enhancement goals. Biol. Conserv. 2019, 238, 108200. [Google Scholar] [CrossRef]
- Pickering, H.; Whitmarsh, D.; Jensen, A. ARs as a Tool to Aid Rehabilitation of Coastal Ecosystems: Investigating the Potential. Mar. Pollut. Bull. 1999, 37, 505–514. [Google Scholar] [CrossRef]
- Bai, Y.B.; Zhang, C.G.; Luo, X.F. Research on flow field effect and stability of ARs in Lusi fishing ground. Yangtze River 2018, 49, 25–30. [Google Scholar]
- Li, D.; Tang, C.; Xia, C.; Zhang, H. Acoustic mapping and classification of benthic habitat using unsupervised learning in artificial reef water. Estuar. Coast. Shelf Sci. 2016, 185, 11–21. [Google Scholar] [CrossRef]
- Schygulla, C.; Peine, F. Nienhagen reef: Abiotic boundary conditions at a large brackish water artificial reef in the baltic sea. J. Coast. Res. 2013, 29, 478–486. [Google Scholar] [CrossRef]
- Folpp, H.R.; Schilling, H.T.; Clark, G.F.; Lowry, M.B.; Maslen, B.; Gregson, M.; Suthers, I.M. Artificial reefs increase fish abundance in habitat-limited estuaries. J. Appl. Ecol. 2020, 57, 1752–1761. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, C.G.; Na, W.B.; Kim, J.K. Chemical degradation characteristics of reinforced concrete reefs in South Korea. Ocean. Eng. 2008, 35, 738–748. [Google Scholar] [CrossRef]
- Pollet, T.; Berdjeb, L.; Garnier, C.; Durrieu, G.; Poupon, C.L.; Misson, B.; Briand, J.-F. Prokaryotic community successions and interactions in marine biofilms: The key role of Flavobacteriia. FEMS Microbiol. Ecol. 2018, 94, fiy083. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Al Fahdi, D.; Muthukrishnan, T. Short-term succession of marine microbial fouling communities and the identification of primary and secondary colonizers. Biofouling 2019, 35, 526–540. [Google Scholar] [CrossRef]
- von Ammon, U.; Wood, S.A.; Laroche, O.; Zaiko, A.; Tait, L.; Lavery, S.; Inglis, G.; Pochon, X. The impact of artificial surfaces on marine bacterial and eukaryotic biofouling assemblages: A high-throughput sequencing analysis. Mar. Environ. Res. 2018, 133, 57–66. [Google Scholar] [CrossRef]
- Guo, Z.S. Characteristics of microbial community structure in typical artificial reef area of Weihai. Agric. Sci. Technol. 2022, 30. [Google Scholar] [CrossRef]
- Azam, F.; Worden, A.Z. Microbes, Molecules, and Marine Ecosystems. J. Comp. Neurol. 2004, 303, 1622–1624. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T.; Ammerman, J.W.; Van Mooy, B.A.S. Microbes and the marine phosphorus cycle. Oceanography 2007, 20, 110–116. [Google Scholar] [CrossRef]
- Lu, Z.; Deng, Y.; Van Nostrand, J.D.; He, Z.; Voordeckers, J.; Zhou, A.; Lee, Y.-J.; Mason, O.U.; Dubinsky, E.A.; Chavarria, K.L.; et al. Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J. 2012, 6, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.H.; You, G.G.; Zhao, D.Y.; Kong, C.R.; Liu, M. Research on the construction of marine ranch in Changhai County from the perspective of ecology. J. Fish. 2018, 31, 43–47. [Google Scholar]
- Gao, X.G.; Yu, Z.A.; Xia, Y.; Li, Y.L.; Li, Y.F. Current situation, problems and sustainable development countermeasures of fishery in Changhai County. Fish. Inf. Strategy 2020, 35, 257–261. [Google Scholar]
- Mohamed, H.F.; Abd-Elgawad, A.; Cai, R.; Luo, Z.; Pie, L.; Xu, C. Microbial community shift on artificial biological reef structures (ABRs) deployed in the South China Sea. Sci. Rep. 2023, 13, 3456. [Google Scholar]
- Guo, Z.; Wang, L.; Cong, W.; Jiang, Z.; Liang, Z. Comparative Analysis of the Ecological Succession of Microbial Communities on Two Artificial Reef Materials. Microorganisms 2021, 9, 120. [Google Scholar] [CrossRef]
- Fang, G.J.; Yu, H.L.; Sheng, H.X.; Tang, Y.L.; Liang, Z.L. Comparative analysis of microbial communities between water and sediment in Laoshan Bay marine ranching with varied aquaculture activities. Mar. Pollut. Bull. 2021, 173, 112990. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, PlatformIndependent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.J.; Tian, T.; Fu, W.T.; Liu, Y.H.; Yang, J. Study on the Characteristics of Summer Water Quality Change in Artificial Reef Area of Dachangshan Sea. Mod. Agric. Sci. Technol. 2017, 7, 192–196. [Google Scholar]
- Arrigo, K. Marine microorganisms and global nutrient cycles. Nature 2005, 437, 349–355. [Google Scholar] [CrossRef]
- Lee, H.; Heo, Y.M.; Kwon, S.L.; Yoo, Y.J.; Kim, D.J.; Lee, J.M.; Kown, B.O.; Khim, J.S.; Kim, J.J. Environmental drivers affecting the bacterial community of intertidal sediments in the Yellow Sea. Sci. Total Environ. 2020, 755 Pt 2, 142726. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.; Bailey-Brock, J.H. Benthic infaunal communities around two ARs in Mamala Bay, Oahu, Hawaii. Mar. Environ. Res. 2008, 65, 250–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez, A.S.; Dafforn, K.A.; Johnston, E.L.; Filippini, G.; Potts, J.; Mayer-Pinto, M. Variations in benthic fluxes of sediments near pier pilings and natural rocky reefs. Mar. Environ. Res. 2022, 177, 105640. [Google Scholar] [CrossRef]
- Heery, E.C.; Dafforn, K.A.; Smith, J.A.; Ushiama, S.; Mariana, M.P. Not all artificial structures are created equal: Pilings linked to greater ecological and environmental change in sediment communities than seawalls. Mar. Environ. Res. 2018, 142, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, H.; Zhao, Y. Bacterial distribution at different stations in the Northern Yellow Sea. Acta Microbiol. Sin. 2009, 49, 343. [Google Scholar]
- Yu, J.Y.; Jiang, Y.Y.; Sun, P.; Zhang, H.; Ling, J.Z. Bacterial community structure and diversity in different habitats in Xiangshan Port. J. Ecol. 2021, 40, 2842–2849. [Google Scholar]
- Singer, D.; Seppey, C.V.W.; Lentendu, G.; Dunthorn, M.; Bass, D.; Belbahri, L.; Blandenier, Q.; Debroas, D.; de Groot, G.A.; de Vargas, C.; et al. Protist taxonomic and functional diversity in soil, freshwater and marine ecosystems. Environ. Int. 2021, 146, 106262. [Google Scholar] [CrossRef]
- Nogales, B.; Lanfranconi, M.P.; Piña-Villalonga, J.M.; Bosch, R. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol. Rev. 2011, 35, 275–298. [Google Scholar] [CrossRef]
- Fang, L.; Chen, L.; Liu, Y.; Tao, W.; Zhang, Z.; Liu, H.; Tang, Y. Planktonic and sedimentary bacterial diversity of Lake Sayram in summer. MicrobiologyOpen 2015, 4, 814–825. [Google Scholar] [CrossRef]
- Inagaki, F. Sulfurovum lithotrophicum gen. nov. sp. nov. a novel sulfur-oxidizing chemolithoautotroph within the—Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int. J. Syst. Evol. Microbiol. 2004, 54, 1477–1482. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, T.; Liu, H.; Zhang, Y.; Ding, G.; Xin, M.L.; Nie, M. Response of artificial reef microbiome of different materials to oil pollution. Fudan J. Nat. Sci. Ed. 2021, 60, 73–83. [Google Scholar]
- Kegler, H.F.; Muhammad, L.; Mirta, T.; Jeremiah, P.J.; Hassenrück, C.; Christian, W.; Astrid, G. Bacterial Community Composition and Potential Driving Factors in Different Reef Habitats of the Spermonde Archipelago, Indonesia. Front. Microbiol. 2017, 8, 662. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.Y.; Zheng, T.L. Survival patterns of bacteria and their community characteristics in the natural environment. Chin. J. Appl. Environ. Biol. 2013, 19, 553–5602. [Google Scholar] [CrossRef]
- Fodrie, F.J.; Rodriguez, A.B.; Gittman, R.K.; Grabowski, J.H.; Lindquist, N.L.; Peterson, C.H.; Piehler, M.F.; Ridge, J.T. Oyster reefs as carbon sources and sinks. Proc. R. Soc. B-Biol. Sci. 2017, 284, 20170891. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Z.L.; Davies, I.M.; Baxter, J.M.; Diele, K.; Sanderson, W.G. A blue carbon model for the European flat oyster (Ostrea edulis) and its application in environmental restoration. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4030. [Google Scholar] [CrossRef]
- Leclair, L.L.; Eveningsong, O.; Schultz, J.M. Seasonal changes in abundance and compelling evidence of migration for 2 rockfish species (Sebastes auriculatus and S. caurinus) inhabiting a nearshore, temperate-water artificial reef. Fish. Bull. 2016, 114, 302–316. [Google Scholar] [CrossRef]
- Huggett, M.J.; Williamson, J.E.; De Nys, R.; Kjelleberg, S.; Steinberg, P.D. Larval settlement of the common Australian sea urchin Heliocidaris erythrogramma in response to bacteria from the surface of coralline algae. Oecologia 2006, 149, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bao, Y.; Li, K.; Yang, N.; He, P.; He, C.; Liu, J. The diversity of planktonic bacteria driven by environmental factors in different mariculture areas in the East China Sea. Mar. Pollut. Bull. 2024, 201, 116136. [Google Scholar] [CrossRef] [PubMed]
- Auladell, A.; Barberán, A.; Logares, R.; Garcés, E.; Gasol, J.M.; Ferrera, I. Seasonal niche differentiation among closely related marine bacteria. ISME J. 2022, 16, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Auladell, A.; Sánchez, P.; Sánchez, O.; Gasol, J.M.; Ferrera, I. Long-term seasonal and interannual variability of marine aerobic anoxygenic photoheterotrophic bacteria. ISME J. 2019, 13, 1975–1987. [Google Scholar] [CrossRef]
- Jrgensen, B.B.; Findlay, A.J.; Pellerin, A. The biogeochemical sulfur cycle of marine sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yue, Y.; Lin, S.; Gu, H.; Han, Z.; Li, Q.; Tian, T.; Wei, X.; Wu, Z. Preliminary Study on the Structure of Fungal Communities in Artificial Reef Areas in the Northern Yellow Sea. Oceans 2025, 6, 11. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, Y.B.; Choi, S.; Lee, Y.; Shin, S.G.; Unno, T.; Kim, Y.M. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea. Environ. Pollut. 2018, 233, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni-Isaksson, J.; Seymour, J.R.; Ingleton, T.; van de Kamp, J.; Bodrossy, L.; Brown, M.V. Spatial and temporal variability of aerobic anoxygenic photoheterotrophic bacteria along the east coast of Australia. Environ. Microbiol. 2016, 18, 4485–4500. [Google Scholar] [CrossRef]

| Groups | T (°C) | DO (mg/L) | Sal (‰) | pH | TOC (mg/L) | TP (mg/g) | TN (mg/g) | GS (μm) |
|---|---|---|---|---|---|---|---|---|
| DG | 10.17 ± 0.21 | 9.63 ± 0.05 | 30.63 ± 0.11 | 8.31 ± 0.16 | 0.86 ± 0.03 | 0.49 ± 0.03 | 1.42 ± 0.39 | 153.33 ± 8.11 |
| DS | 10.3 ± 0.10 | 9.64 ± 0.07 | 30.76 ± 0.18 | 8.22 ± 0.10 | 0.85 ± 0.07 | 0.46 ± 0.07 | 1.81 ± 0.12 | 157.53 ± 2.57 |
| DC | 10.3 ± 0.14 | 9.47 ± 0.25 | 30.61 ± 0.35 | 8.21 ± 0.08 | 0.92 ± 0.12 | 0.41 ± 0.08 | 1.98 ± 0.42 | 137.08 ± 8.45 |
| OG | 12.3 ± 0.10 | 9.18 ± 0.06 | 29.86 ± 0.12 | 8.16 ± 0.10 | 1.14 ± 0.07 | 0.50 ± 0.02 | 2.10 ± 0.05 | 19.36 ± 4.41 |
| OS | 12.45 ± 0.25 | 9.29 ± 0.05 | 29.78 ± 0.09 | 8.05 ± 0.01 | 1.15 ± 0.06 | 0.48 ± 0.01 | 2.03 ± 0.14 | 20.11 ± 2.03 |
| OC | 12.35 ± 0.07 | 9.21 ± 0.03 | 29.69 ± 0.11 | 8.08 ± 0.06 | 1.23 ± 0.04 | 0.46 ± 0.11 | 1.99 ± 0.10 | 32.20 ± 2.80 |
| Groups | Sample Size | R | p | Permutation Number |
|---|---|---|---|---|
| CS-CW | 8 | 1.000 | 0.026 | 999 |
| CS-SS | 10 | −0.131 | 0.829 | 999 |
| CS-GS | 10 | −0.162 | 0.885 | 999 |
| CS-GW | 10 | 1.000 | 0.009 | 999 |
| SW-CS | 10 | 1.000 | 0.007 | 999 |
| SW-CW | 10 | −0.012 | 0.400 | 999 |
| SW-SS | 12 | 0.996 | 0.002 | 999 |
| SW-GS | 12 | 1.000 | 0.005 | 999 |
| SW-GW | 12 | −0.061 | 0.784 | 999 |
| SS-CW | 10 | 0.992 | 0.007 | 999 |
| SS-GW | 12 | 0.015 | 0.350 | 999 |
| SS-GS | 12 | 0.963 | 0.006 | 999 |
| GS-CW | 10 | 1.000 | 0.005 | 999 |
| GW-CW | 10 | −0.004 | 0.422 | 999 |
| GW-GS | 12 | 0.996 | 0.006 | 999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Wei, X.; Si, L.; Zhang, Z.; Zhao, J.; Deng, L.; Tian, T.; Li, Q.; Yin, Z.; Wu, Z. Bacterial Community Composition and Its Relationship with Environmental Factors in the Artificial Reef Area for Marine Ranching in Changhai County. Animals 2025, 15, 639. https://doi.org/10.3390/ani15050639
Yan J, Wei X, Si L, Zhang Z, Zhao J, Deng L, Tian T, Li Q, Yin Z, Wu Z. Bacterial Community Composition and Its Relationship with Environmental Factors in the Artificial Reef Area for Marine Ranching in Changhai County. Animals. 2025; 15(5):639. https://doi.org/10.3390/ani15050639
Chicago/Turabian StyleYan, Jiamin, Xu Wei, Liwei Si, Zheng Zhang, Jingsi Zhao, Liyu Deng, Tao Tian, Qingxia Li, Zengqiang Yin, and Zhongxin Wu. 2025. "Bacterial Community Composition and Its Relationship with Environmental Factors in the Artificial Reef Area for Marine Ranching in Changhai County" Animals 15, no. 5: 639. https://doi.org/10.3390/ani15050639
APA StyleYan, J., Wei, X., Si, L., Zhang, Z., Zhao, J., Deng, L., Tian, T., Li, Q., Yin, Z., & Wu, Z. (2025). Bacterial Community Composition and Its Relationship with Environmental Factors in the Artificial Reef Area for Marine Ranching in Changhai County. Animals, 15(5), 639. https://doi.org/10.3390/ani15050639







